Magnesium-fortified phosphate fertilizers improve nutrient uptake and plant growth without reducing phosphorus availability
2022-11-01ZhenyaLUYanyanWANGFienDEGRYSEChengdongHUANGCuihongHOULiangquanWURongfengJIANGMichaelMCLAUGHLINandFusuoZHANG
Zhenya LUYanyan WANGFien DEGRYSEChengdong HUANGCuihong HOULiangquan WURongfeng JIANGMichael J.MCLAUGHLIN and Fusuo ZHANG
1College of Resources and Environmental Sciences, National Academy of Agriculture Green Development, Key Laboratory of Plant-Soil Interactions,Ministryof Education,China Agricultural University,Beijing 100193(China)
2Department of Pomology,College of Horticulture,China Agricultural University,Beijing 100193(China)
3Fertilizer TechnologyResearch Centre,School of Agriculture,Food and Wine,Universityof Adelaide,PMB 1,Glen Osmond SA 5064(Australia)
4School of Chemical Engineering,Zhengzhou University,Zhengzhou 450001(China)
5International Magnesium Institute,Fujian Agriculture and ForestryUniversity,Fuzhou 350002(China)
ABSTRACT Magnesium(Mg)deficiency can significantly limit crop yield and quality.Separate application of straight Mg fertilizer is unattractive because of additional labor costs.Meanwhile,bulk blending Mg with other macronutrient fertilizers is also a suboptimal solution because bulk blended fertilizers often yield poor nutrient distributions.One rapid and economical alternative to alleviating Mg deficiency is to co-granulate macronutrient fertilizers with Mg.However,few commercial products have implemented this approach.One of the barriers hindering the production of Mg-fortified phosphorus(P)fertilizers is the assumption that precipitation of P with Mg will reduce P solubility.In this study,four Mg compounds,anhydrous magnesium sulfate(MgSO4),magnesium oxide(MgO),anhydrous magnesium chloride(MgCl2),and dolomite(CaMg(CO3)2),were co-granulated with mono-ammonium phosphate(MAP),and their granule strength,Mg and P availabilities,and agronomic effectiveness were evaluated.Results showed that there were no significant differences in P solubility between Mg-fortified MAP and MAP treatments.X-ray diffraction(XRD)indicated that the Mg species after co-granulation were boussingaultite(Mg(NH4)2(SO4)2·6H2O),schertelite(Mg(NH4)2H2(PO4)2·4H2O),magnesium hydrogen phosphate(Mg(H2PO4)2),and dolomite(CaMg(CO3)2).A pot experiment using an acidic soil demonstrated an average 9.6-fold increase in shoot Mg uptake,3.0-fold increase in shoot P uptake,and 3.2-fold increase in soybean shoot dry matter in Mg-fortified MAP treatments,compared to those in MAP treatment.The current study provides a simple,effective,and low-cost approach for the addition of Mg to macronutrient fertilizers,to minimize Mg deficiency.
Key Words: agronomic effectiveness, crop growth, magnesium deficiency, mono-ammonium phosphate, macronutrient fertilizer, P solubility, X-ray diffraction(XRD)
INTRODUCTION
Magnesium(Mg)is essential for crop growth and development.It plays an important role in a number of key functions in plants,such as photosynthesis,energy metabolism,carbon allocation,protein synthesis,and resistance to environmental stress(Hermanset al.,2005;Cakmak and Kirkby,2008; Boseet al., 2011; Marschner, 2012; Farhatet al.,2016;Chenet al.,2018).However,Mg availability is often a neglected aspect of crop production,partially due to the ignorance of farmers and agronomists for the essentiality of this element(Cakmak and Yazici,2010;Guoet al.,2016).As a result,Mg deficiency in crops is becoming more common worldwide(Peacock and Christensen,1996;Gerendás and Führs,2013),limiting crop yield and quality,especially in the frequently acidic soils of southern China(Baiet al.,2004).
Many previous studies have shown that applying Mg fertilizer to Mg-deficient soils improves crop yield and quality(Tinker,1967;Grzebisz,2013;Senbayramet al.,2015;Chenet al., 2017; Sunet al., 2018; Wanget al., 2020).The application of Mg fertilizer is an effective and rapid way to improve the concentration of readily available Mg in soil,to ensure that crops have adequate access to Mg for normal growth(White and Broadley,2009).Unfortunately,the direct application of Mg fertilizer is not always practical owing to the lack of knowledge and added cost and labor for smallholders (Abatet al., 2015). In consideration of such costs,the bulk blending of fertilizers is widely adopted owing to its convenience and low cost(Park,2001).However,bulk blending can result in the segregation of granules during transportation and poor nutrient distribution in the field(Hoffmeisteret al.,1964;Virket al.,2013;Abatet al.,2015). Co-granulating Mg fertilizer with other macronutrient fertilizers may be a better way to overcome these problems,especially in China,where compound fertilizers are commonly used,with NPK(nitrogen,phosphorus,and potassium)compound fertilizer being the primary fertilizer product,accounting for 40.1%of the total chemical fertilizer consumption(National Bureau of Statistics of China,2019).
However,it is commonly observed that co-granulating Mg fertilizer with phosphate fertilizers, such as monoammonium phosphate(MAP),would cause nutrient limitations.One possible reason for this is that the orthophosphate ion may react with Mg2+to produce magnesium ammonium phosphate or magnesium phosphate(Soudée and Péra,2000;Liuet al.,2013),and hence convert P from a water-soluble form to a sparingly soluble form.In wastewater treatment,magnesium salts are often used to recover ammonium and phosphate from wastewater by precipitating struvite(Morseet al.,1998;Prattet al.,2012;Rahmanet al.,2014;Songet al.,2014).Although struvite is poorly soluble in water,it dissolves in soil over time(depending on soil pH and particle size of struvite)and can therefore be used as a slow-release fertilizer(El Diwaniet al.,2007;Latifianet al.,2012;Everaertet al.,2016;Talboyset al.,2016;Degryseet al.,2017).Previous studies have proposed the use of humic-metal(Mg and Zn)-phosphate(HMP)complexes,formed through metal bridges,in strategies to develop phosphate-based fertilizers which reduce nutrient losses and maintain P bioavailability(Erroet al.,2007;Urrutiaet al.,2014).
MATERIALS AND METHODS
Fertilizer sources
Raw materials for the fertilizers used in this study were anhydrous magnesium sulfate (MgSO4, analytic reagent(AR),≥99%),magnesium oxide(MgO,AR,≥98%),anhydrous magnesium chloride(MgCl2,AR,≥99%),dolomite(CaMg(CO3)2mineral, 123 g Mg kg-1), and industrialgrade mono-ammonium phosphate(MAP,266 g P kg-1and 118 g N kg-1).
Co-granulation of Mg sources with MAP
Four Mg sources were co-granulated with MAP at a rate of 20 g Mg kg-1. Granulation was performed using the procedure described by Abatet al. (2015). The raw materials were sieved to<250 μm and mixed evenly.Part of the mixture(ca.50 g)was transferred into a laboratoryscale disc granulator,and deionized water was sprayed on the rotating mixture using a nebulizer until granules with a diameter of 0.1–1 mm were formed. Then, more of the powdered mixture was gradually added to the pan, while spraying with deionized water continued, to further build up the granules.Under-and over-sized granules(<1 or>4 mm, respectively) were removed by screening, ground,and returned to the granulator. The whole process lasted 50–60 min, and approximately 100 g of deionized water was used in total.In addition,MAP without addition of Mg source was granulated as a control.The granules produced were moved to a stainless-steel plate and dried in an oven at 70°C for 6–7 h.The granules were kept in a resealable bag and placed in a closed container before analysis.
Fertilizer propertydetermination
Concentrations of citric acid-and water-extractable Mg were measured by dissolving 2 g ground sample in 150 mL citric acid(20 g L-1)and deionized water,respectively.The suspensions were oscillated in a shaker at 180 r min-1for 30 min at 25°C, diluted to 250 mL, filtered using a 0.45 μm syringe filter,and analyzed using inductively coupled plasma optical emission spectrometry(ICP-OES,Avio200,PerkinElmer,USA).A gravimetric method using quinoline phosphomolybdate was used to measure citric acid and waterextractable P.Precisely 0.2 g ground samples were dissolved in 150 mL of 20 g L-1citric acid or deionized water.The suspensions were oscillated in a thermostatic water bath for 1 h at 60°C,diluted to 250 mL,and filtered using filter papers(9 cm in diameter,No.102,Newstar,China).Following this,25 mL of the filtered solution was transferred to a beaker,and 10 mL of a nitric acid solution (1:1, volume:volume,nitric acid:deionized water)was added.The solutions were diluted to 100 mL with deionized water and boiled for 3 min,after which 35 mL quinoline molybdate was added,with boiling continuing for another 1 min,followed by an additional filtering step.The solution was then dried in an oven for 45 min at 180°C,cooled,and weighed,following the protocol established by the National Technical Committee for Standardization of Fertilizers and Soil Conditioners(2010).
Granule strength was measured with 30 randomly selected 2-4 mm granules using a digital granule strength tester(KY-20,Leici,China).Phase analysis of the ground fertilizer was performed using an X-ray diffractometer(XRD,X’Pert Pro, PANalytical, The Netherlands)with Cu as the anode material, at a scan step size of 0.05°from 2θ of 5°–90°,using Jade 6.5.
Pot experiment
A pot experiment was performed in a greenhouse using soybean (Glycine max(L.) Merr., Williams 82), which is highly sensitive to Mg deficiency.The soil(0–10 cm)used,an acid soil,was obtained from Pinghe County,Fujian Province,China,air-dried,sieved to<2 mm,and analyzed before use.Exchangeable Mg concentration was 0.07 cmol(+)kg-1,well below the concentration considered deficient(<0.25 cmol(+)kg-1)(Wanget al.,2020).Other selected properties of the soil are listed in Table I.

TABLE I Selected chemical and physical properties of the soil used in this study
Spun linen cloth was placed at the bottom of plastic pots(210 mm in height×210 mm in diameter)to prevent soil loss during watering.Approximately 5 kg of air-dried soil was placed in the plastic pots and moistened to field capacity. Fertilizers were added, placed equidistantly at 5 cm below the soil surface,to supply 20 mg Mg kg-1and 228 mg P kg-1, corresponding to 28.9 kg Mg ha-1and 330 kg P ha-1,respectively.Six treatments were included in this experiment:control(no fertilizer),MAP,and MAP co-granulated with MgSO4(Co-MgSO4),MgO(Co-MgO),MgCl2(Co-MgCl2),and dolomite(Co-dolomite)(Table II).Pots were assigned in a randomized complete block design,with five replicates of each treatment.In each pot,five plump and uniformly sized soybean seeds were lightly covered with topsoil.All pots except the control treatment received basal macro- and micro-nutrients (100 mg N kg-1, 51.2 mg K kg-1, 66.2 mg Ca kg-1, 21 mg S kg-1, 3.6 mg Fe kg-1,2.8 mg Zn kg-1,1.2 mg B kg-1,1.2 mg Mn kg-1,0.08 mg Cu kg-1,and 0.08 mg Mo kg-1),added as solutions before the seeds were planted.Soils were watered to maintain field capacity every 3 d by weighing each pot with an electronic scale.The seedlings were thinned to 2 plants per pot after 1 week,and the plants were harvested 6 weeks after sowing by cutting at the cotyledon node.The soybean shoots were dried,weighed,and analyzed for Mg and P concentrations by ICP-OES after acid digestion(0.3 g ground samples digested with 5 mL nitric acid and 2 mL hydrogen peroxide).
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics 20. One-way analysis of variance was used to evaluate the effects of Mg sources on granule strength, P solubility, shoot dry matter, and nutrient uptake using apost-hocDuncan test for multiple comparisons.
RESULTS
Solubilities of P and Mg in co-granulated fertilizers
Concentrations of citric acid-extractable P in the cogranulated Mg fertilizers ranged from 224 to 250 g kg-1(Table II).Addition of Mg fertilizers to MAP had no significant effect on P solubility,compared to the MAP treatment (P >0.05). The level of Mg solubility, expressed as the percentage of water-extractable Mg relative to citric acid-extractable Mg, followed the order: Co-MgSO4and Co-MgCl2(>90%)>Co-MgO (69.5%)>Co-dolomite(8.72%).
Without a word, I opened the gate, and for the first time since her birth, she led me outside into the cool morning air, where she began to bounce and kick and just be the baby that she was.
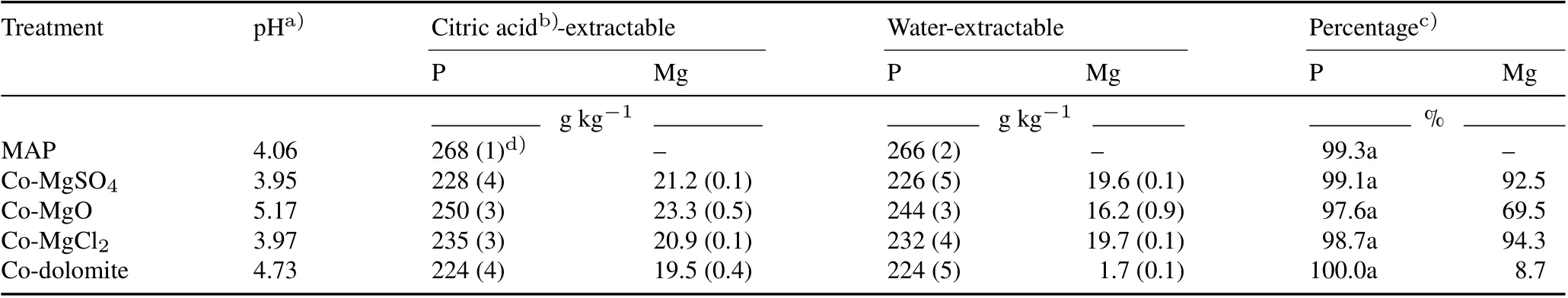
TABLE II pH and citric acid-and water-extractable concentrations of P and Mg of mono-ammonium phosphate(MAP)without co-granulation or co-granulated with MgSO4 (Co-MgSO4),MgO(Co-MgO),MgCl2 (Co-MgCl2)or dolomite(Co-dolomite)
Granule strength of co-granulated fertilizers
Granule strength is important for the mechanical stability of fertilizers during handling, transportation, and application(Xi,2013).Therefore,the granule strength of the co-granulated Mg products was also evaluated(Fig.1).Addition of MgO and dolomite increased the granule strength(>2 kg force)compared to the control(1.10 kg force),while the addition of MgSO4and MgCl2decreased the granule strength to 0.85 and 0.36 kg force,respectively.The strong negative impact of MgCl2on granule strength may be related to the hygroscopicity of MgCl2.
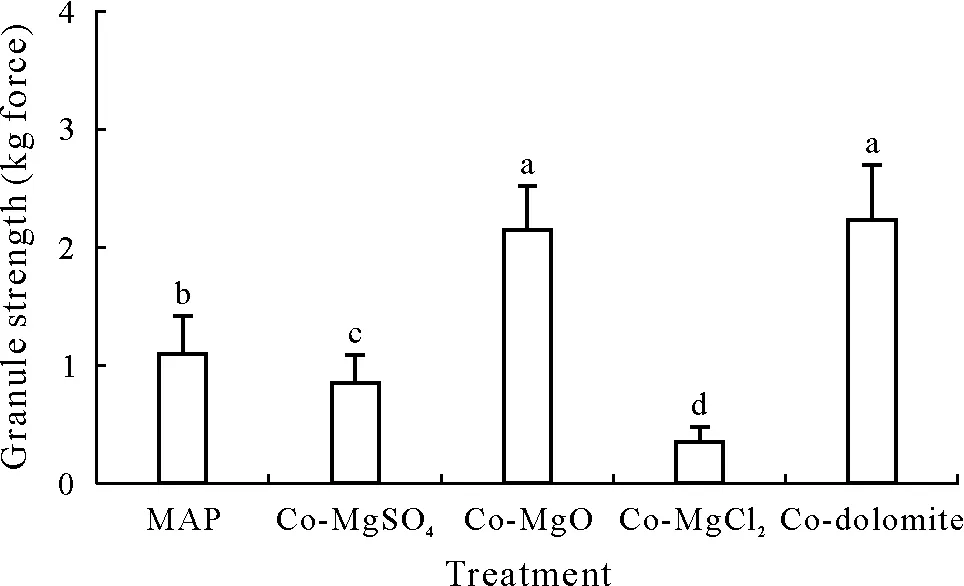
Fig.1 Granule strength of mono-ammonium phosphate(MAP)without co-granulation or co-granulated with MgSO4 (Co-MgSO4), MgO (Co-MgO),MgCl2 (Co-MgCl2),or dolomite(Co-dolomite).Bars represent the standard errors of 30 randomly selected granules.Different letters above the bars indicate significant differences(P ≤0.05).
Species of Mg in co-granulated fertilizers
The XRD patterns indicated that the main Mg species in the Co-MgSO4, Co-MgO, and Co-MgCl2treatments were boussingaultite (Mg(NH4)2(SO4)2·6H2O) (Fig. S1,see Supplementary Material for Fig. S1), schertelite(Mg(NH4)2H2(PO4)2·4H2O)(Fig.S2,see Supplementary Material for Fig.S2),and magnesium hydrogen phosphate(Mg(H2PO4)2) (Fig. S3, see Supplementary Material for Fig.S3),respectively(Fig.2).No new component was detected in the Co-dolomite treatment,in which Mg species was still present as dolomite(CaMg(CO3)2)(Fig.S4,see Supplementary Material for Fig.S4).
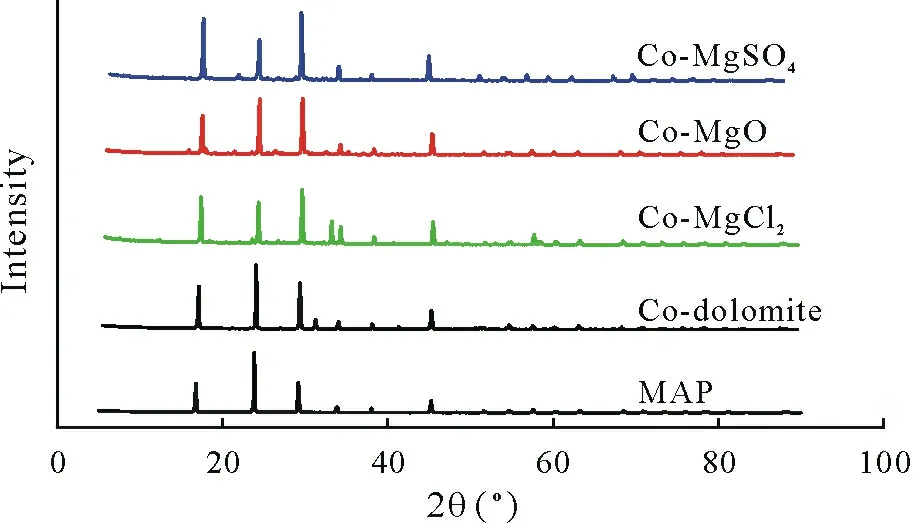
Fig.2 X-ray diffractometer(XRD)patterns of mono-ammonium phosphate(MAP)without co-granulation or co-granulated with MgSO4(Co-MgSO4),MgO(Co-MgO),MgCl2 (Co-MgCl2),or dolomite(Co-dolomite).
Plant growth and nutrient uptake
Soybean shoot dry matter increased strongly in response to the application of MAP co-granulated with Mg fertilizers(Fig.3),which indicated that the addition of Mg alleviated Mg deficiency in this soil with low ammonium acetateextractable Mg.Shoot dry matter yield of Mg-fortified MAP treatments was 3.2-fold higher than the MAP treatment,with no significant differences among the treatments of MAP co-granulated with Mg fertilizers(P >0.05).
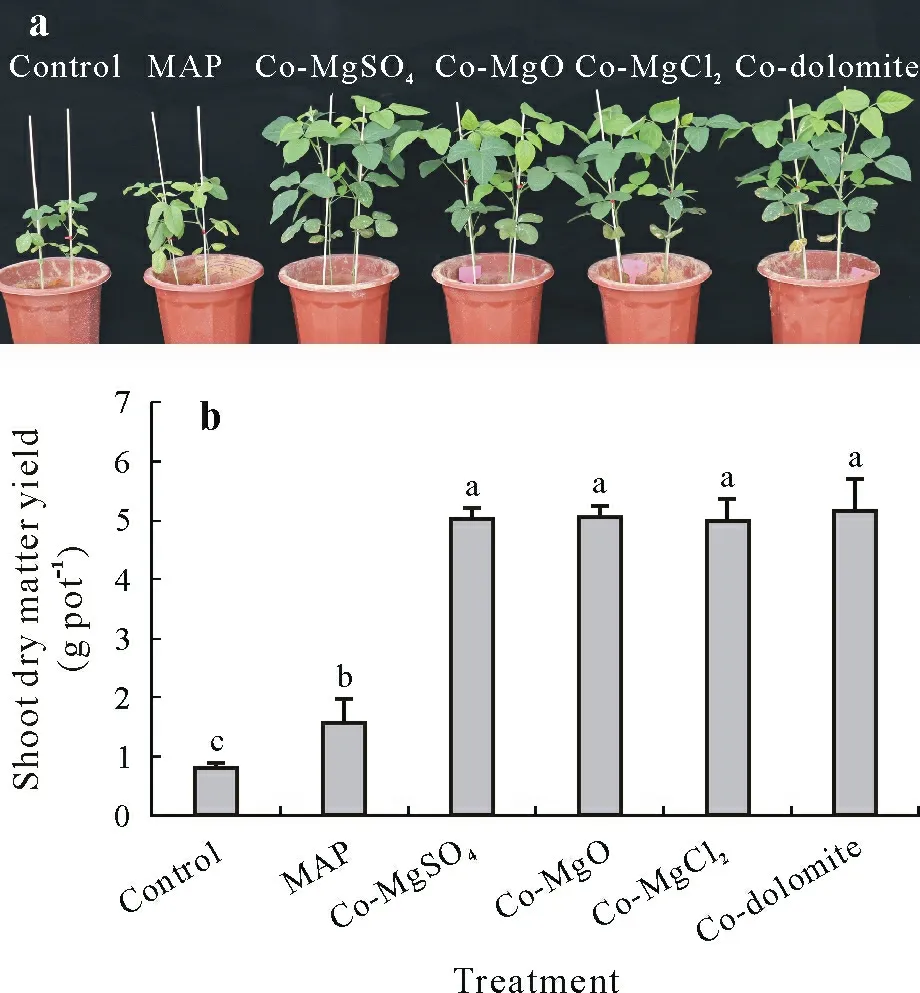
Fig.3 Shoot growth(a)and dry matter yield(b)of soybean on un-fertilized soil(control)or soil fertilized with mono-ammonium phosphate(MAP)without co-granulation or co-granulated with MgSO4 (Co-MgSO4),MgO(Co-MgO),MgCl2(Co-MgCl2),or dolomite(Co-dolomite).Bars represent the standard errors of 5 replicated pots. Different letters above the bars indicate significant differences(P ≤0.05).
Concentrations and uptake of P and Mg in soybean shoots were also measured to assess the availability of nutrients in the treatments of MAP co-granulated with Mg fertilizers(Fig. 4). Addition of Mg significantly improved nutrient concentration and uptake.Concentrations of Mg in soybean shoots in the treatments of MAP co-granulated with Mg fertilizers were within the optimal range of 1.5–3.5 g kg-1for plant growth(Marschner,2012),but were slightly lower in the Co-dolomite treatment than other treatments.In the control and MAP treatments,Mg concentrations in soybean shoots were less than 1.5 g kg-1.The uptake of Mg in the treatments of MAP co-granulated with Mg fertilizers was 9.0-to 10.0-fold higher than that in the MAP treatment.
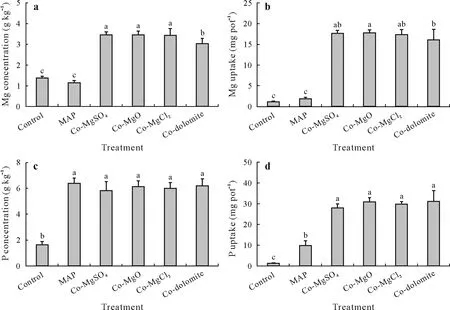
Fig.4 Concentrations(a and c)and uptake(b and d)of Mg and P in soybean shoots on un-fertilized soil(control)or soil fertilized with mono-ammonium phosphate(MAP)without co-granulation or co-granulated with MgSO4 (Co-MgSO4),MgO(Co-MgO),MgCl2 (Co-MgCl2),or dolomite(Co-dolomite).Bars represent the standard errors of 5 replicated pots.Different letters above the bars indicate significant differences(P ≤0.05).
Soybean shoot P concentration was 3.5- to 4.0-fold higher in the P-fertilized treatments than that in the control treatment (no P fertilizer), but there were no significant differences among P-fertilized treatments (Fig. 4c). The P uptake was 2.8- to 3.2-fold higher in the treatments of MAP co-granulated with Mg fertilizers than that in the MAP treatment,owing to the improved plant growth in the Mgfertilized treatments.There was no significant difference in P concentration or P uptake among the treatments of MAP co-granulated with Mg fertilizers, indicating that the Mg source used had no effect on P availability.
DISCUSSION
Addition of Mg to MAP did not reduce P solubility
Adding Mg compounds to a macronutrient fertilizer is regarded as an easy and effective approach to apply Mg fertilizer. However, there is a popular presumption that adding Mg to P fertilizers will decrease water-extractable P concentration through P-Mg precipitation (Soudée and Péra,2000;Liuet al.,2013).We tested this by adding Mg to MAP,which is a major phosphate fertilizer and an important P source in the production of compound fertilizers.
This study demonstrated that co-granulating Mg with MAP did not decrease P solubility, regardless of the Mg source used (Table II). Zhanget al. (2019) evaluated the effect of adding MgSO4·7H2O at different rates to water extracts from different P fertilizers and found that waterextractable P was not affected for the MAP-based fertilizers,but decreased with increasing addition rate of MgSO4·7H2O for the diammonium phosphate(DAP)-based fertilizers.
The XRD results explained why the Mg addition to MAP did not affect P solubility. For the Co-MgSO4treatment, the Mg compound formed was boussingaultite(Mg(NH4)2(SO4)2·6H2O),and for the Co-dolomite treatment,Mg persisted as dolomite.For the Co-MgO and Co-MgCl2treatments,the added Mg reacted with P,but the compounds formed (schertelite (Mg(NH4)2H2(PO4)2·4H2O)and magnesium hydrogen phosphate (Mg(H2PO4)2)) are highly soluble in water.The fact that struvite did not form as we initially hypothesized, is likely related to the low pH of the MAP fertilizer. It has been reported that struvite (MgNH4PO4·6H2O) formation usually occurs in the pH range of 7–11(Nelsonet al.,2003; Songet al.,2007;Tanselet al.,2018).Thus,although no effect of Mg addition on P solubility was observed for MAP-based fertilizers,co-granulating Mg with DAP fertilizers may decrease P solubility,as DAP has a higher pH than MAP.
Addition of Mg to MAP improved plant growth and nutrient uptake
Magnesium concentrations in wheat,fruit,and vegetables have declined over the past several decades in various parts of the world(Rosanoff,2013),which has adverse effects on crop yield and quality. It had been reported that Mg fertilization can increase plant yield,especially in soils with low Mg concentration(Ceylanet al.,2016;Grzebisz,2013).A meta-analysis of 99 research articles indicated that there was a 6.9%–12.5%increase in the production of multiple crops following Mg fertilization,and that there was no significant difference in yield improvement between rapidrelease fertilizers(such as MgSO4·7H2O)and slow-release fertilizers(such as MgO and dolomite)(Wanget al.,2020).In this study,the shoot dry matter yield for the treatments of MAP co-granulated with Mg was on average 3.2-fold higher than the MAP treatment(Fig.3),indicating that Mg addition can greatly improve dry matter yield in soils with low concentration of exchangeable Mg.However,there was no significant difference in dry matter yield among the treatments of MAP co-granulated with Mg,indicating that all the Mg compounds tested in this study were equally effective in increasing yields when co-granulated with MAP.
Phosphorus concentration in soybean shoots were similar in the treatments of MAP with and without co-granulation,but P uptake was higher in the treatments of MAP cogranulated with Mg sources owing to the improved growth.There was no significant difference in shoot P concentration and P uptake among the treatments of MAP co-granulated with Mg sources,in agreement with the lack of effect of Mg on the P solubility of the fertilizers.
Effects of granulation on soybean yield and nutrient uptake were also evaluated (Fig. S5, see Supplementary Material for Fig.S5),showing no significant differences in yield and Mg uptake between treatments with Mg sources and MAP co-granulated with Mg sources, except for the MgO treatment.The powdered MgO probably had a larger contact area with soil than the Co-MgO treatment, which could enhance MgO dissolution in acidic soil. However,no significant difference in P uptake was found between granulated and non-granulated treatments.This suggests that the addition of Mg to the MAP fertilizer did not adversely affect the P availability of the MAP fertilizer.
Challenges and potential of co-granulated Mg fertilizers
In this study,there were no significant differences in nutrient uptake and shoot dry matter yield among the treatments of MAP co-granulated with Mg fertilizers,except for slightly lower Mg concentration in the Co-dolomite treatment than other treatments(Fig.4).This suggests that the tested Mg sources exhibited similar Mg availability in this acidic soil.
Costs and effects on physical quality may be the main factors in selecting the most suitable Mg source,and granule strength is an important metric for the mechanical stability of fertilizers.In general, fertilizers with granule strengths exceeding 0.82 kg force can meet the requirements of fertilizer storage and transportation(Xiet al.,2013).In this study,Co-MgO,Co-dolomite,and Co-MgSO4treatments exhibited granule strengths in line with such requirements, but the co-MgCl2treatment did not(Fig.1).It should be noted that fertilizer granules made at a small bench-scale are generally less robust than commercial fertilizers because of the lower compressive forces in a pan granulator with a shallow bed depth of the product.Nevertheless,it seems that MgCl2may be a less suitable source than the others tested,owing to its high hygroscopicity.
Magnesium has a relatively high mobility in soil because of its smaller hydrated radius and weaker binding to soil charges than other cations, and it is hence easily subject to leaching in low-pH soils under high-rainfall or overirrigated conditions (Maguire and Cowan, 2002; Shaul,2002;Gerendás and Führs,2013).Thus,Mg leaching from fertilizers is highly dependent on Mg fertilizer solubility.A slow-release Mg fertilizer such as dolomite may alleviate leaching loss(Härdteret al.,2004;Loganathanet al.,2005).Senbayramet al.(2015)assessed the leaching of dolomite and magnesium sulfate in an acidic sandy soil and found no significant differences in Mg leaching between the dolomite and control (no fertilizer) treatments, while the MgSO4treatment showed a 20%–40% Mg leaching loss. In this study, we also found that when co-granulated with MAP,MgSO4had very high solubility, while dolomite had a low solubility(Table II).Hence,MAP co-granulated with dolomite may be a better choice in areas with low pH and high rainfall,while MAP co-granulated with MgSO4may be more suitable in areas with low rainfall.However,more studies are needed to verify these hypotheses.
CONCLUSIONS
Co-granulating Mg compounds with MAP did not reduce the solubility of P,regardless of the source used,as Mg2+did not react with orthophosphate ions or form a highly soluble substance.Adding Mg sources to MAP significantly improved soybean yield and Mg and P uptake in Mg-deficient soil.There was no significant difference in shoot dry matter yield between the different Mg sources investigated. Our results suggest that MgSO4,MgO,and dolomite could be added to MAP,and MgO is preferred due to its higher granule strength and facilitation of Mg uptake compared to MgSO4and dolomite.
ACKNOWLEDGEMENTS
This work received financial supports from the National Key R&D Program (Nos. 2016YFD0200401 and 2016YFE0103100) and Science and Technology Plan of Yunnan Province,China(No.202102AE090053).The authors also thank Dr.Wei Qin from China Agricultural University for helpful suggestions on the manuscript and appreciate the assistance provided by all staffand students in the lab of Zhengzhou University and Quzhou Experimental Station of China Agricultural University.
SUPPLEMENTAL MATERIAL
Supplementary material for this article can be found in the online version.
杂志排行
Pedosphere的其它文章
- Effects of silver nanoparticle size,concentration and coating on soil quality as indicated by arylsulfatase and sulfite oxidase activities
- Comparing the Soil Conservation Service model with new machine learning algorithms for predicting cumulative infiltration in semi-arid regions
- Time effects of rice straw and engineered bacteria on reduction of exogenous Cu mobility in three typical Chinese soils
- Carbon nanomaterial addition changes soil nematode community in a tall fescue mesocosm
- In situ stabilization of arsenic in soil with organoclay,organozeolite,birnessite,goethite and lanthanum-doped magnetic biochar
- Improvement of phosphorus uptake,phosphorus use efficiency,and grain yield of upland rice(Oryza sativa L.)in response to phosphate-solubilizing bacteria blended with phosphorus fertilizer
