In situ stabilization of arsenic in soil with organoclay,organozeolite,birnessite,goethite and lanthanum-doped magnetic biochar
2022-11-01SaeedBAGHERIFAMTrevorBROWNChristopherFELLOWSRaviNAIDUandSridharKOMARNENI
Saeed BAGHERIFAMTrevor C.BROWNChristopher M.FELLOWSRavi NAIDU and Sridhar KOMARNENI
1Chemistry-School of Science and Technology,Universityof New England,Armidale NSW 2351(Australia)
2Global Centre for Environmental Remediation,Facultyof Science and Information Technology,Universityof Newcastle,UniversityDrive,Callaghan NSW 2308(Australia)
3Department of Ecosystem Science and Management and Materials Research Institute,204 Energyand the Environment Laboratory,Pennsylvania State University,UniversityPark PA 16802(USA)
ABSTRACT Arsenic(As)is a known carcinogen and naturally occurring semi-metal in soils and in the Earth’s crust.Contamination of soils and water with As poses a serious threat to millions of people worldwide due to its health hazards and toxicological properties.Hence,devising novel and efficient methods for remediation of contaminated areas has attracted a great deal of interest across the globe.In this study,we investigated the usefulness of synthetic birnessite,goethite,hexadecylpyridinium chloride-modified montmorillonite(HDPC-M),hexadecylpyridinium bromide-modified zeolite(HDPB-Z),and lanthanum(La)-doped magnetic biochar produced from eucalyptus bark(La-Euchar)as adsorbents at 10%dosage for As stabilization in a soil spiked with 1 000 mg kg-1 As.The effectiveness of the above adsorbents in As immobilization in soil was assessed using single-step extractions with 2 mol L-1 HNO3 and deionized water,the simplified bioaccessibility extraction test(SBET)method,and sequential extraction with the modified Community Bureau of Reference(BCR)method.Application of the adsorbents shifted the exchangeable fraction of As to more recalcitrant fractions and dramatically reduced the exchangeable fraction by 6%–99%and the extractable amounts with HNO3,deionized water,and SBET method by 30%–92%,17%–95%,and 12%–90%,respectively,compared to the unamended control.The immobilizing effects of adsorbents on As decreased in the sequence of birnessite >La-Euchar >goethite >HDPB-Z >HDPC-M.Birnessite exhibited great affinity for As and drastically reduced As extractability by more than 90%in all single extractions.The results revealed that HDPC-M,HDPB-Z,La-Euchar,birnessite,and goethite are promising immobilizing agents for in situ stabilization of As in terrestrial environments.
KeyWords: adsorbent,bioaccessibility,bioavailability,environmental contamination,immobilizing agent,modified biochar,modified clay,remediation technique
INTRODUCTION
Arsenic(As)is a ubiquitous metalloid whose environmental contamination has attracted a lot of attention because of its harmful effects on both humans and ecological receptors.Contamination of environmental systems with As compounds is sourced from natural geological processes and anthropogenic activities(Bagherifamet al.,2019).However,anthropogenic activities such as the application of As pesticides and wood preservation chemicals,processing of glassware,ceramics,electronics,cosmetics,and fireworks,burning of fossil fuels, and mining and smelting of Asbearing minerals are considered the major sources of As contamination(Bagherifamet al.,2019;Liet al.,2020).Arsenic is predominantly associated with sulfidic minerals such as arsenopyrite and pyrite,and dissolution of As-containing minerals may result in elevated levels of As in groundwater,which has affected more than 70 countries and approximately 150 million people worldwide(Shankaret al.,2014).
Owing to the toxicological properties of As in humans and terrestrial environments, numerous efforts have been made to design techniques and strategies for the remediation of As-impacted soils and water.There is a range of methods used for the remediation of polluted soils,including bioremediation, phytoremediation, soil leaching, and chemical stabilization(Yaoet al.,2012).In situstabilization or immobilization is an environmental remediation technology used to reduce the environmental bioavailability, bioaccessibility,and mobility of metal(loids)by trapping,adsorbing,or immobilizing environmental contaminants in terrestrial environments(Sparks,2003).Numerous immobilizing amendments have been used for stabilization of As in contaminated soils,including iron(Fe)-,manganese(Mn)-,and aluminum(Al)-modified zeolites (Bagherifamet al., 2014b), zerovalent iron(ZVI)(Lianget al.,2017;Yueet al.,2020;Liet al.,2021),sodium dodecyl sulfate-coated synthetic ZVI(Kimet al.,2012),sulfidated ZVI(Qiaoet al.,2021),modified ZVI(Lianget al.,2017),magnetite nanoparticles(Kimet al.,2012),layered double oxides,red mud,ferrihydrite(Sunet al., 2015), maleic anhydride-styrene-acrylic acid copolymer(Mansouriet al.,2017),schwertmannites(Chaiet al.,2016),and biogenic Mn oxide(Wanget al.,2020).
Organoclays are hydrophobic,organically modified phyllosilicates, which have been widely used for the remediation of organic compounds,heavy metal(loid)s,pesticides,and anionic contaminants. Organoclays are mostly synthesized by exchanging the original interlayer cations for cationic surfactants or other organic compounds, which gives the modified clay hydrophobic properties. Organoclays are thus a suitable option for the removal of organic pollutants from aqueous solutions owing to the formation of an organophilic surface (Guégan, 2019). Montmorillonite((Ca,Na,H)(Al,Mg,Fe,Zn)2(Si,Al)4O10(OH)2·nH2O)belongs to the smectite group of clays,which exhibits a 2:1 layer structure and expandable interlayers,and is one of the most used naturally occurring minerals for the synthesis of organoclays(Lee Y Cet al.,2011;Parket al.,2011;Bagherifamet al.,2014a).Various types of organominerals have been used to remove As and contaminants with anionic properties from solutions(Wingenfelderet al.,2006;Lee Y Cet al.,2011;Suet al.,2011;Mudzielwanaet al.,2019;Mukhopadhyayet al., 2019). However, the As-immobilizing effects of hexadecylpyridinium bromide (HDPB)-modified zeolite(HDPB-Z)and hexadecylpyridinium chloride(HDPC)-modified montmorillonite(HDPC-M)in soils have not been investigated so far.Lanthanum(La)is a rare earth element that has been used for the adsorption of phosphates due to its multi-point coordination and high affinity for phosphate(Tokunagaet al., 1999; Wanget al., 2016; Mudzielwanaet al., 2019). In addition, La is in plentiful supply in the Earth’s crust, environmentally friendly, and inexpensive relative to other rare earth elements. The element can be loaded into the biochar porous structure and onto the external surfaces,where it reduces the negative surface charges of the biochar and consequently improves its effectiveness for the adsorption of phosphate(Mudzielwanaet al.,2019)and other anionic contaminants.Eucalyptus trees are iconic Australian trees that grow in many parts of Australia.One of the most distinctive features of eucalyptus trees is that they shed old bark as they grow,which can be used as a source for production of biochar.However,the stabilizing effect of La-doped magnetic biochar for reducing the bioavailability of As in soils has not been previously examined.Although numerous Fe and Mn oxide-based compounds have been used for As stabilization in soils and removal of As from aqueous solutions,to the best of our knowledge,the effectiveness of synthetic birnessite and goethite in immobilizing As and reducing its bioaccessibility in contaminated soils has not been systematically examined.
Therefore,the aims of this study were to compare the effectiveness of a wide variety of synthetic adsorbents,including HDPC-M,HDPB-Z,birnessite,goethite,and Ladoped magnetic biochar derived from eucalyptus bark(La-Euchar),in As stabilization and As bioavailability reduction in soils by means of a series of single and sequential extraction experiments.The information presented in this paper may assist in designing better management strategies for reducing the bioavailability of As in terrestrial environments.
MATERIALS AND METHODS
Synthesis of adsorbents
The novel organominerals were synthesized according to a previously described procedure(Chitrakaret al.,2012).Natural montmorillonite(Kunipia-F)with a cation exchange capacity(CEC)of 1.05 cmol kg-1was procured from Kunimine Industries Co.Ltd.,Japan.Natural zeolite was obtained from Zeolite Australia Pty Ltd., New South Wales(NSW), Australia. The studied zeolite contained tabular clinoptilolite (heulandite 31.3%) crystals, the main crystalline component, quartz (22.1%), mordenite (6.67%),smectite,and mica,with a total CEC of 1.19 cmol kg-1.The HDPC-M and HDPB-Z were prepared through the exchange process of inorganic cations on the surfaces of montmorillonite and zeolite with the quaternary ammonium cations of HDPC(C21H38NCl)and HDPB(C21H38NBr),respectively.Briefly,2.00 g Kunipia-Fand natural zeolite were added to two separate 500-mL beakers containing 200 mL deionized water,and the suspensions were stirred on a magnetic stirrer for 1 h.Calculated amounts of HDPC and HDPB based on the CEC of Kunipia-Fand natural zeolite,respectively,were dissolved in deionized water and then added dropwise to the Kunipia-Fand zeolite suspensions,respectively.The resulting slurries were mixed for 24 h on a magnetic stirrer,and the organominerals were then separated by centrifugation,washed with deionized water,and oven-dried at 60°C.The biochar used in this study was produced from eucalyptus bark and grass feedstock through slow pyrolysis at 400°C for 2 h under dinitrogen(N2)flow conditions(referred to as Euchar).The La-doped magnetic Euchar (La-Euchar) was synthesized using a procedure modified from the literature(Mohanet al., 2014; Wanget al., 2018; Xuet al., 2019). Briefly,10.00 g Euchar were added to 250 mL solution containing 0.05 mol L-1FeSO4·7H2O,0.05 mol L-1Fe2(SO4)3,and 0.03 mol L-1LaCl3under vigorous magnetic stirring.After the addition of 30 mL ethyl alcohol,the mixture was agitated on a magnetic stirrer for 1 h. The pH of the suspension was then adjusted to 11 by dropwise addition of ammonia solution.The resulting slurry was aged for 24 h at 80°C.Subsequently, the synthesized La-Euchar was isolated by centrifugation,washed several times with deionized water,oven-dried at 60°C overnight,and stored for further use.To synthesize birnessite,1 L 1.00 mol L-1KMnO4solution was heated to 90°C,and 66 mL concentrated HCl was added dropwise to the solution. The suspension was cooled for 30 min prior to separation by filtration.The product was then washed several times with deionized water,oven-dried,and stored for further use(McKenzie,1971;Yinget al.,2012).Goethite was synthesized according to a previously described procedure(Jaiswalet al.,2013),with slight modifications,by dissolving 50 g Fe(NO3)3·9H2O in 0.5 L sterilized deionized water. The resulting solution was stirred with a magnetic stirrer for 2 h,followed by dropwise addition of 2.5 mol L-1KOH until the pH of the solution reached 12.The suspension was aged for 24 h at 80°C,and the goethite was separated by centrifugation of the resulting thick paste,washed several times, oven-dried at 60°C, and stored for thein situAs stabilization experiment.
Adsorbent characterization and As stabilization experiment
Unpolluted soil sample was collected from a dryland in Armidale, NSW, Australia, air-dried, and passed through a 2-mm mesh sieve for use in the As stabilization experiment.Soil physical and chemical properties are presented in Table I.The five synthetic adsorbents were used as immobilizing agents in the As stabilization experiment,i.e.,PDHC-M,PDHB-Z,La-Euchar,goethite,and birnessite.Xray diffraction(XRD)was used to obtain XRD patterns of the absorbents by implementing CuKα radiation at 35 kV and 30 mA.The microscopic features,elemental composition,and surface morphology of the synthetic adsorbents were investigated using a scanning electron microscope(SEM;JEOL JSM-6010LA,JEOL Ltd.,Japan)equipped with an energy dispersive X-ray (EDX) detector. To prepare Ascontaminated soil, sodium arsenite (NaAsO2) was mixed with the soil sample,and the resulting As-contaminated soil(1 000 mg kg-1As) was aged for one month prior to the As stabilization experiment. To elucidate the distribution patterns of As and major elements in the soil,a thin section of the aged soil was prepared by embedding the soil in epoxy resin.Then,micro-characterization and elemental mapping were performed using SEM-EDX at 15 selected points.Each adsorbent was mixed thoroughly with the As-contaminated soil at 10%by weight.The obtained amended soils were then incubated for three months at 70%water holding capacity before conducting As extraction.Three replicates were used for each treatment.At the end of the incubation,elemental mapping of Mn and Fe was performed on a thin section of the birnessite-amended soil using SEM-EDX.
Bioaccessibilityof As and single and sequential extractions
The simplified bioaccessibility extraction test(SBET)isa single-step extraction method that has been widely used for the estimation of As availability in ingested soil to the human gastrointestinal tract(Rubyet al.,1996;Ettleret al.,2012). In this study, As bioaccessibility was estimated in the<250 μm soil fraction with a simulated gastric juice using the SBET method (Rubyet al., 1996; Ettleret al.,2012).Briefly,0.500 g soil was mixed with 50 mL simulated gastric juice(0.4 mol L-1glycine;pH 1.50±0.05,adjusted using trace metal grade HCl) and shaken for 1 h at 30 r min-1and 37°C on an incubator shaker. The resulting extracts were filtered by syringe filtration using 0.45-μm nitrocellulose membrane filters and analyzed for As concentration by inductively coupled plasma mass spectrometry(ICP-MS).Changes in the association of As with different soil fractions were investigated using a modified Community Bureau of Reference(BCR)sequential extraction method(Rauretet al.,1999;Zhanget al.,2018),by which the total As pool was sequentially extracted into four different fractions:acid-soluble or exchangeable(extracted with 0.11 mol L-1acetic acid for 16 h),reducible(extracted with 0.5 mol L-1NH3OHCl acidified with HNO3for 16 h),oxidizable(extracted first with 8 mol L-1H2O2for 1 h at ambient temperature and for another 1 h at 85°C in a water bath and then with 1 mol L-1pH 2 NH4OAc for 16 h), and residual(extracted with aqua regia).The As-immobilizing potential of adsorbents was evaluated by two single-step extraction procedures using deionized water and 2.00 mol L-1HNO3(Kiekens and Cottenie,1985;Szákováet al.,2009).To determine the deionized water-extractable fraction,3.00 g soil was shaken with 30 mL deionized water overnight.The HNO3-extractable fraction was collected by shaking 3.00 g soil with 30 mL HNO3for 6 h.The resulting soil extracts were centrifuged,and the supernatants were passed through 0.45-μm membrane filters using syringe filtration prior to As analysis.The concentrations of As in soil extracts were determined using ICP-MS and atomic absorption spectroscopy(AAS).
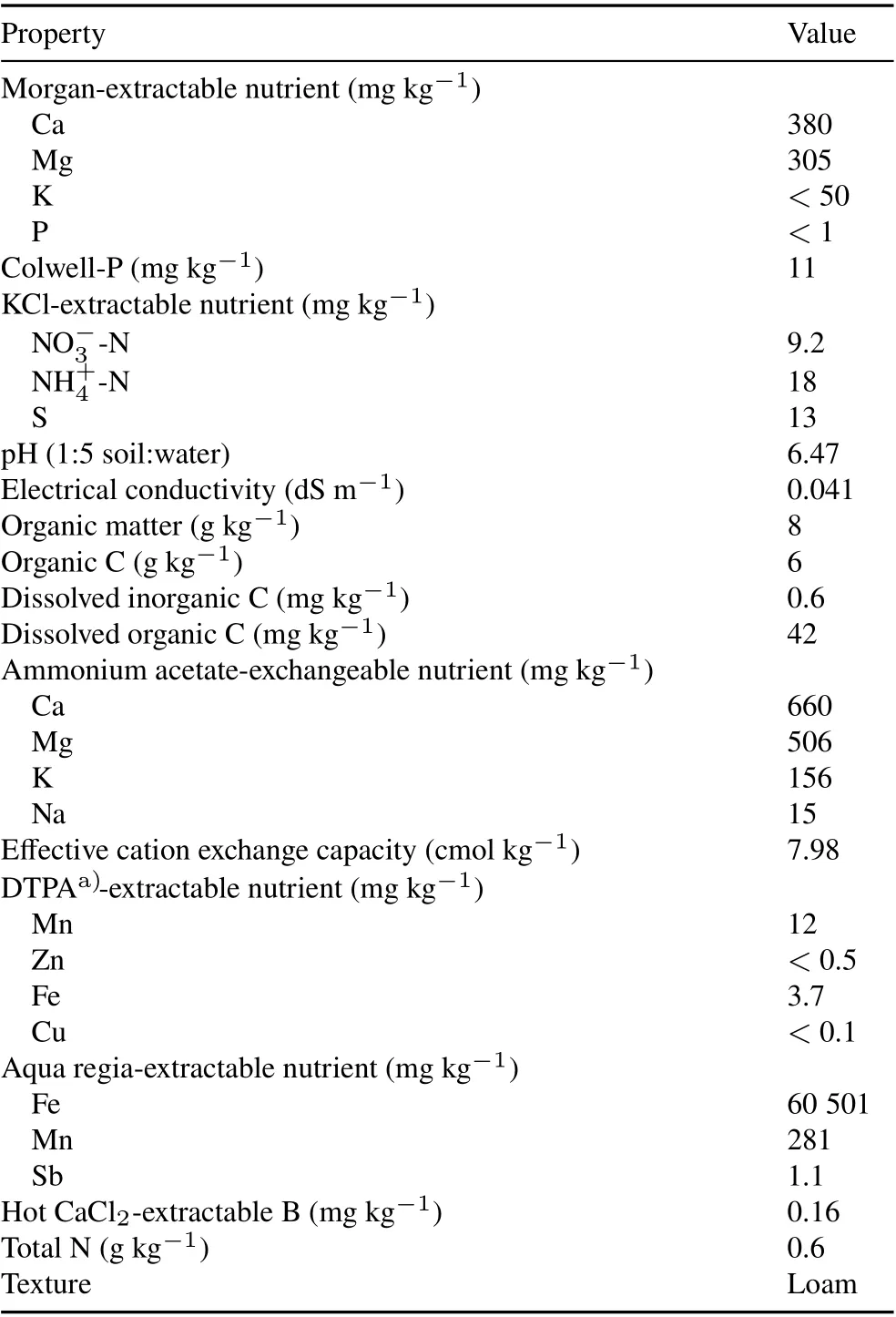
TABLE I Physical and chemical properties of the test soil collected from a dryland in Armidale,New South Wales,Australia and used in the As stabilization experiment
Statistical analysis
Statistical analysis was performed using the GenStat 12 statistical software package(VSNI,UK),and statistical differences were calculated by analysis of variance(ANOVA),using Duncan’s multiple range test for means comparison(P <0.05).
RESULTS AND DISCUSSION
Crystalline structure and morphological features of the adsorbents
The XRD patterns of natural montmorillonite and HDPC-M are shown in Fig.1a.As previously reported in our paper(Bagherifamet al.,2014a),the XRD pattern of natural montmorillonite exhibited a broad 001 reflection at 1.229 nm,whereas HDPC-M showed sharp and intense reflections at lower 2θvalues.The XRD pattern of HDPCM showed four sharp peaks at 4.027, 2.045, 1.369, and 1.03 nm,corresponding tod-spacing valuesd001,d002,d003,andd004,respectively(Chitrakaret al.,2012;Bagherifamet al.,2014a).Furthermore,the XRD patterns showed that the intercalation of cationic surfactant into the interlayer of natural montmorillonite resulted in a marked interlayer expansion from 1.229 to 4.027 nm.Hence,given the chain length of HDPC(2.31 nm)and the basal spacing of HDPC-M(4.027 nm)in this study,the observed interlayer expansion could be attributed to the formation of a paraffin-type bilayer arrangement of HDPC in the interlayer space (Chitrakaret al.,2012;Bagherifamet al.,2014a).
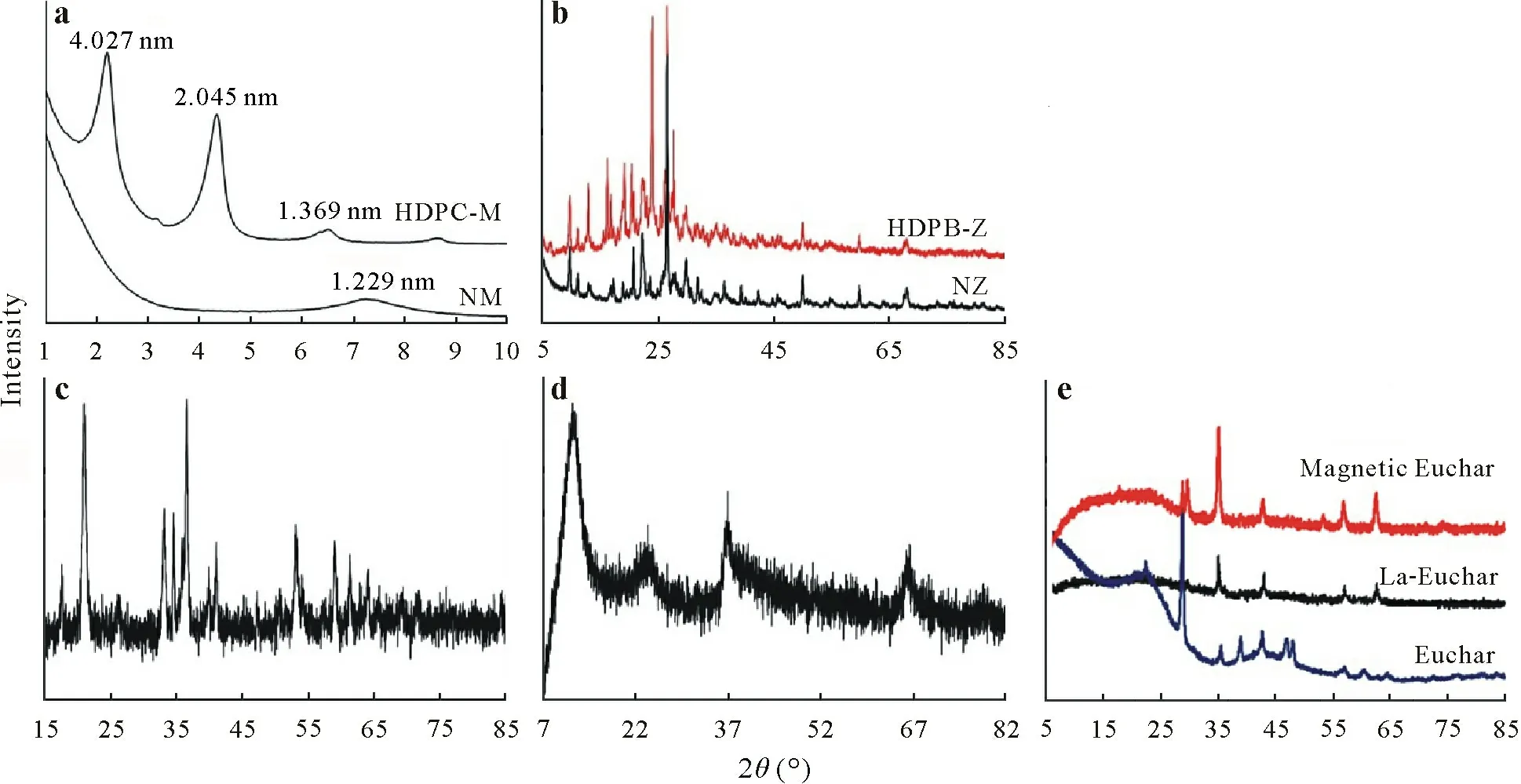
Fig.1 X-ray diffraction patterns of the natural montmorillonite(NM)and hexadecylpyridinium chloride-modified montmorillonite(HDPC-M)(a),natural zeolite(NZ)and hexadecylpyridinium bromide-modified zeolite(HDPB-Z)(b),goethite(c),birnessite(d),eucalyptus bark biochar(Euchar),magnetic Euchar,and La-doped magnetic Euchar(La-Euchar)(e)used as adsorbents in the As stabilization experiment.
Figure 1b shows the XRD patterns of the zeolite before and after modified with the HPDB surfactant.The commercial zeolite used in this study was composed mostly of tabular clinoptilolite crystals,and minor components were quartz,mordenite,smectite,and mica,with molecular channel sizes of 0.79 nm×0.35 nm and 0.44 nm×0.30 nm in clinoptilolite. Both clinoptilolite and mordenite have silica-rich frameworks.Clinoptilolite exhibits voids connected by two eight-ring windows (0.26 nm× 0.47 nm and 0.33 nm×0.46 nm) and one 10-ring window (0.30 nm× 0.76 nm),whereas mordenite shows eight- and 12-ring windows of 0.26 nm×0.57 nm and 0.65 nm×0.70 nm,respectively(Dyer,2001).There were no major alterations in the crystalline structure of organozeolite,although there were some fluctuations and changes in the intensity of major peaks between the 2θrange of 13°–27.78°, demonstrating that HPDB adsorption and ion exchange may have resulted in slight changes to peaks in this 2θrange.The zeolite framework structures contain channels and/or interconnected voids in the range of 0.3–2 nm, which are sufficiently large for exchangeable cations such as K+, Na+, Ca2+, and Mg2+(Apreuteseiet al.,2008;Liet al.,2014).Nevertheless,the molecular sizes of HPDB and other cationic surfactants are greater than those of the internal cavities of the zeolite(Apreuteseiet al.,2008;Liet al.,2014,2017).Because the large cationic surfactants cannot penetrate into the internal pores of the zeolite,they can be loaded only on the external surface of zeolite(Liet al.,2017).Therefore,there were no major changes in the crystalline structure of HDPB-Z.
The XRD diffractogram of synthesized goethite exhibited sharp and strong reflection peaks at 2θof approximately 21°,33°,34°,36°,40°,41°,53°,61°,and 64°(Fig.1c),with lattice constants ofa=0.460 8 nm,b=0.995 6 nm,andc=0.302 nm,which are in agreement with those reported by Jaiswalet al. (2013). The XRD pattern of synthesized birnessite revealed four broad asymmetric peaks at 12.31°,24.18°,37.06°,and 66.51°(Fig.1d),which are characteristic of the main peaks of birnessite,as previously reported(Händelet al.,2013).The XRD pattern of pristine Euchar showed a weak and broad peak between 20°and 29°(Fig.1e),which is mainly ascribed to the aromatization and graphitization of organic matter in eucalyptus biomass at high pyrolysis temperatures (Wanget al., 2016, 2018).In addition, the XRD pattern of Euchar showed a strong and sharp reflection around 29°and several peaks between 36°and 49°,which are assigned to CaCO3;this might be attributed to the abundance of CaCO3in eucalyptus wood biochar (Limwikranet al., 2019). Furthermore, the XRD pattern of magnetic Euchar exhibited several sharp and strong reflections at 30.10°, 35.45°, 43.08°, 56.98°, and 62.57°,indicating that Fe3O4had been successfully crystallized in the pristine biochar (Wanget al., 2018, 2019). Although La-Euchar also presented Fe3O4characteristic peaks, the diffraction intensity of the peaks decreased considerably,which indicates that La atoms were successfully doped into the structure of the magnetic biochar (Wanget al., 2018,2019).
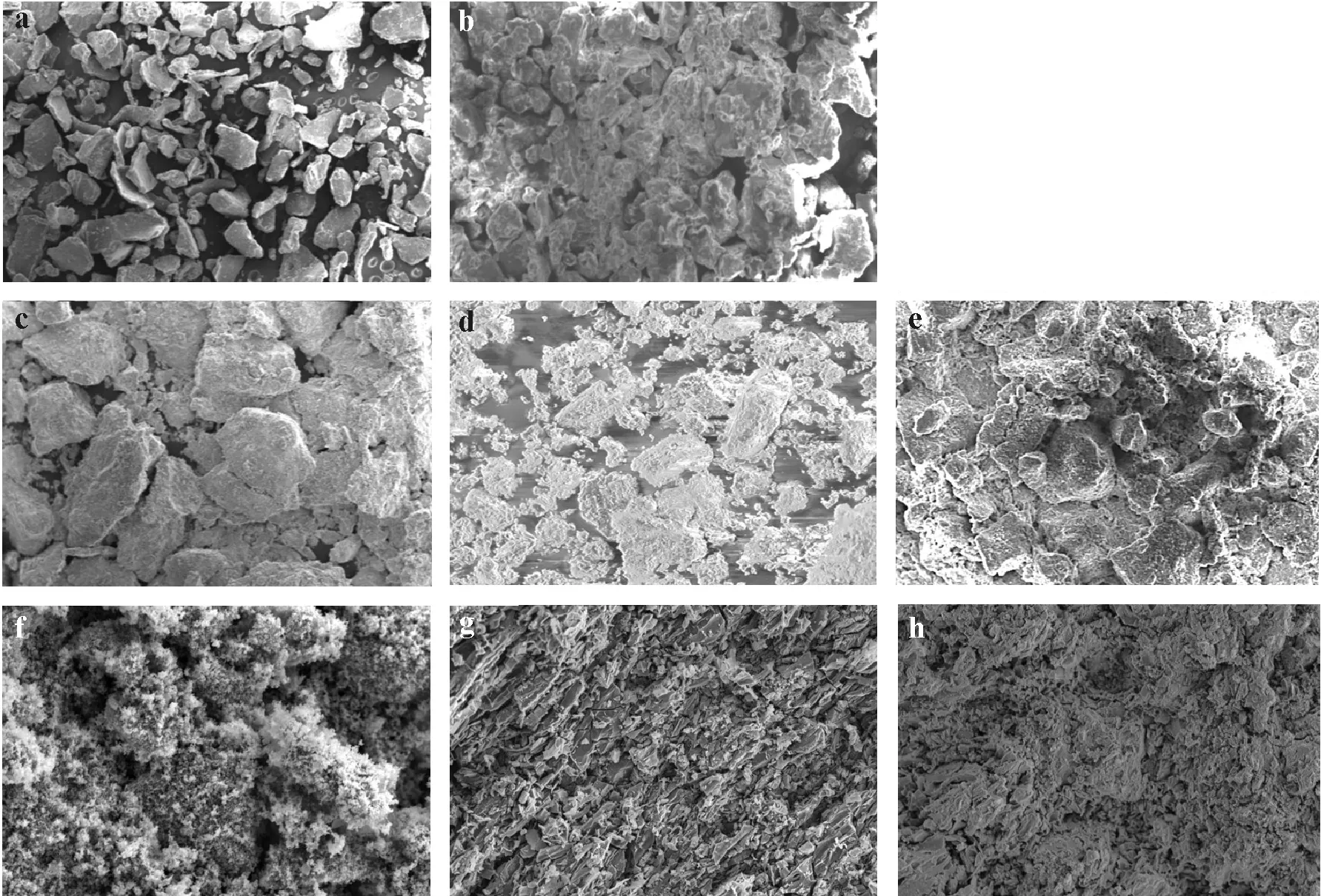
Fig.2 Scanning electron microscope(SEM)images(magnification:100×)of the natural montmorillonite(a),hexadecylpyridinium chloride-modified montmorillonite(HDPC-M)(b),natural zeolite(c),hexadecylpyridinium bromide-modified zeolite(HDPB-Z)(d),goethite(e),birnessite(f),eucalyptus bark biochar(Euchar)(g),and La-doped magnetic Euchar(La-Euchar)(h)used as adsorbents in the As stabilization experiment.
The SEM-EDX technique was employed to investigate the morphological features of the synthesized adsorbents.The morphologies of montmorillonite and zeolite changed significantly after modification with surfactants (Fig. 2a–d).Natural montmorillonite exhibited a rather flat surface morphology with small particles(Fig.2a),while the modification using surfactant resulted in the formation of a more porous surface with a noticeable non-uniform texture and a rough and wrinkled morphology(Fig.2b).The crystalline structure of the natural zeolite was clearly apparent in the SEM micrograph(Fig.2c).Nevertheless,HDPB-Z exhibited a rougher and more porous surface morphology than that of natural zeolite(Fig.2d),presumably due to the formation of a layered structure of cationic surfactant on the external surface of natural zeolite because the cationic surfactant cannot penetrate into the internal voids of zeolite owing to its large molecular size. The SEM micrograph of synthesized goethite displayed round or semi-round shapes and irregular aggregation without any specific morphological features(Fig.2e),while birnessite showed a highly porous structure with tiny pores on its surface (Fig. 2f). Similar SEM images were reported by Händelet al.(2013).The pyrolysis of eucalyptus bark led to the formation of micro-and mesopores as well as microchannel-type structures in Euchar(Fig.2g)and La-Euchar(Fig.2h).Although no noticeable morphological alterations were observed for La-Euchar,there were slight variations in the volume and diameter of pores,and La-Euchar exhibited smoother and more eroded surface microtopography,probably due to the erosive effects of chemical compounds on the biochar surface.
Elemental compositions of the adsorbents and soil
The elemental compositions of natural and modified montmorillonite and zeolite were determined using EDX.The results showed that Al and silicon(Si)were the main components(Fig. S1a–d, see Supplementary Material for Fig. S1). After the modification of montmorillonite and zeolite with cationic surfactants, however, the amount of carbon (C) significantly increased in both HDPC-M and HDPB-Z,which was accompanied by the appearance of Cl and Br peaks in the EDX spectra(Fig.S1b,d),demonstrating the successful sorption of cationic surfactants on the internal or external surfaces of organominerals. The EDX spectra of goethite and birnessite revealed that Fe,Mn,and oxygen(O)were the major identified elements(>90%by weight)and K was present to a much lesser extent in both synthesized oxides(Fig.S1e,f).Euchar was mainly composed of C and O,accounting for almost 80%of its composition,while Ca,Fe,and Mg were the other identified major components(Fig.S1g).The magnetization of Euchar and modification of it with La,however,resulted in a drastic alteration in the chemical composition of La-Euchar,leading to a dramatic increase in Fe as a new major phase along with the reduction of C content and the appearance of La peaks(Fig.S1h).
Fig.3 Correlation between Fe and As contents determined using energy dispersive X-ray(EDX)analysis at selected points of the soil aggregates spiked with 1 000 mg kg-1 As(a)and the EDX spectrum of a selected point(b).
The EDX chemical analysis of As in the control soil spiked with 1 000 mg kg-1As on 15 selected points distributed on As-containing aggregates showed strong positive correlations between As,Fe(Fig.3a),Mn,and K.Arsenic weathering in contaminated soils may result in the formation of secondary minerals(Materaet al.,2003).Furthermore,the result of semi-qualitative EDX elemental analysis revealed that the investigated soil was mostly composed of Si,Al,O,Fe,and C(Fig.3b),and that the average size of the studied soil particles was<25 or>100 μm.Moreover,the As spatial variation at the selected points ranged 0.4–2.8 g kg-1,showing substantial variations and unequal distribution patterns of As in the contaminated soil particles.After the discharge of As into environmental systems,various Asbearing minerals,such as hornesite(Mg3(AsO4)2·8H2O),calcium arsenates,calcium-magnesium arsenates,scorodite(FeAsO4·2H2O),jarosite(KFe3(SO4)2(OH)6),and arsenic phosphates(Daviset al.,1996;Fosteret al.,1998;Savageet al.,2000; Materaet al.,2003),can form in the ecosystems. Soil speciation and theoretical studies have confirmed the formation of secondary As-and K-bearing minerals such as pharmacosiderite(KFe4(AsO4)3(OH)4·6H2O and K2Fe4(AsO4)3(OH)5·6H2O)under certain environmental conditions(Morinet al.,2002;Sisret al.,2007;Haffertet al.,2010;Das,2019).There are reports suggesting that As solubility might be determined by Ca3(AsO4)2,Mn3(AsO4)2,or Pb3(AsO4)2phases under oxidizing conditions,whereas under reducing conditions,sulfides are predicted to be stable mineral phases(Hess and Blanchar,1976;Livesey and Huang,1981;Sadiqet al.,1983;Masscheleynet al.,1991).It should be noted that the results of regression analysis revealed that the As-Fe correlation at the selected points was statistically significant (R2= 0.707 1,P <0.05), while the As-Mn(R2=0.63)and As-K(R2=0.53)correlations(data not shown) were not statistically significant at the investigated points in the control soil.
Changes in As fractions
The results of the sequential extraction of the control soil showed that 21%and 12%of the As pool were exchangeable(F1)and reducible(F2),respectively,whereas the residual As fraction(F4)accounted for 65%of the total As in soils,indicating that a very large proportion of As was nonexchangeable and specifically adsorbed(Fig.4a).
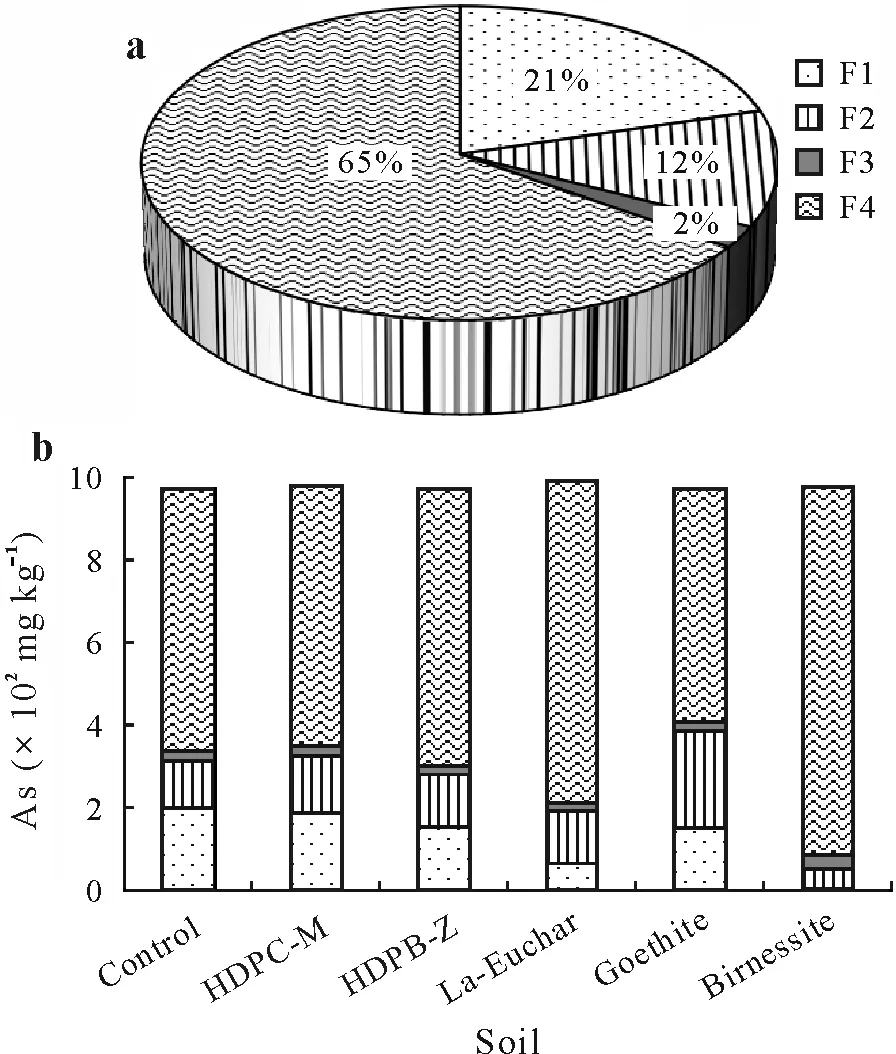
Fig.4 Percentages of different As fractions(F1–F4)in the total As in the unamended control soil (a) and contents of different As fractions in the unamended control soil and adsorbent-amended soils(b)after three months of incubation.The test soil was collected from a dryland in Armidale,New South Wales,Australia and spiked with 1 000 mg kg-1 As one month before the incubation.F1=exchangeable;F2=reducible;F3=oxidizable;F4=residual.The adsorbents include hexadecylpyridinium chloride-modified montmorillonite(HDPC-M),hexadecylpyridinium bromide-modified zeolite(HDPB-Z),La-doped magnetic biochar produced from eucalyptus bark(La-Euchar),goethite,and birnessite.
The surprising point was the large proportion(21%)of non-specifically bound or exchangeable As,while only 12%of the total As was associated with Fe-Mn oxide phases(reducible).The formation of three different surface complexes on goethite for arsenate oxyanions has been reported,including a monodentate complex,a bidentate-binuclear complex,and a bidentate-mononuclear complex, depending on the oxyanion-Fe distance(Fendorfet al.,1997).Materaet al.(2003) investigated the As-bearing phases of contaminated soils using high-performance liquid chromatography(HPLC)-ICP-MS, SEM-EDX, XRD, and sequential chemical extraction,which led to the prediction of As sorption on Fe amorphous(hydr)oxides and formation of Fe arsenates.Zhanget al.(2018)studied the sequential extraction of As using the same fractionation method and reported that As mostly existed in the residual fraction (72.81%–96.65%),which is in agreement with the present study. However,the reducible,oxidizable,and exchangeable fractions were responsible for 0.49%–20.49%,0.13%–4.86%,and 0.12%–3.68%,respectively.The observed differences between our results and those of Zhanget al.(2018)might be attributed to factors such as the use of the spiking method and the low organic matter(<10 g kg-1)of the experimental soil in the present study.
The effects of the adsorbents on As fractionation in soil are depicted in Fig.4b.The amounts of As associated with the most labile fraction decreased dramatically,which was accompanied by a remarkable increase in the more recalcitrant fractions in the treated soils,revealing the As-immobilizing effects of all the tested adsorbents.However,the extent of these fluctuations varied depending on the immobilizing effects of adsorbents,and the changes in distribution pattern were more pronounced in soils amended with birnessite and La-Euchar.
The exchangeable fraction of As was significantly reduced (P <0.05) by 6%, 22%, 67%, 23%, and 99% in the soils amended with HDPC-M, HDPB-Z, La-Euchar,goethite,and birnessite,respectively(Fig.5).It is noticeable that the application of birnessite resulted in almost complete removal of As from the exchangeable fraction.Because Mn oxides possess high specific surface areas and polymorphic structures, they have been widely used for the removal of oxyanions from aqueous solutions (Lianget al.,2020).Furthermore,birnessite is a strong oxidant that can rapidly oxidize As(III) to As(V) (Lianget al., 2020).These structural and chemical features make birnessite one of the most effective adsorbents for As.The immobilizing effects of adsorbents on As decreased in the sequence of birnessite>La-Euchar>HDPB-Z>goethite>HDPC-M.There was a remarkable increase in the amount of As bound to the Fe and Mn oxide pools in the soils amended with HDPC-M and goethite,with more than 100%increase in the soil amended with goethite.The oxidizable fraction of As increased dramatically by approximately 57%.
The residual fraction of As increased significantly(P <0.05)in the soils amended with HDPB-Z,La-Euchar,and birnessite but decreased significantly (P <0.05) in the goethite-amended soil. The results of the sequential extractions demonstrated that As in the residual pool ranged between 63% and 89% of the total As. The highest percentage of As in the residual fraction was found for the birnessite-amended soils, indicating the immobilizing effects of synthetic adsorbents (Fig. 5). Lianget al. (2017)
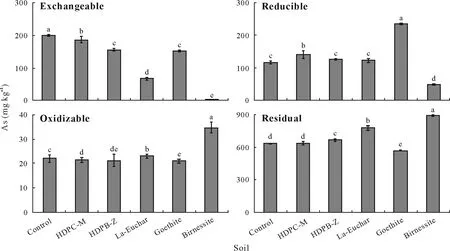
Fig.5 Contents of exchangeable,reducible,oxidizable,and residual As in the unamended control and adsorbent-amended soils after three months of incubation.The test soil was collected from a dryland in Armidale,New South Wales,Australia and spiked with 1 000 mg kg-1 As one month before the incubation.The adsorbents include hexadecylpyridinium chloride-modified montmorillonite(HDPC-M),hexadecylpyridinium bromide-modified zeolite(HDPB-Z),La-doped magnetic biochar produced from eucalyptus bark(La-Euchar),goethite,and birnessite.Error bars are standard errors of the means(n=3).Different letters indicate significant difference between soils according to Duncan’s multiple range test(P <0.05).
showed that modified ZVI co-ground with Mn dioxide could reduce the environmental availability of As by changing the labile and reducible As to residual As.Ettleret al.(2015)reported a significant decrease of As concentration in the labile fraction of the BCR method following the addition of Mn oxide.Although the effects of the synthetic adsorbents used in this study on the relative distribution of As in soils have not been studied, our results are consistent with the above studies,indicating distribution pattern changes of As after its successful immobilization in the soils.
Bioaccessibilityof As
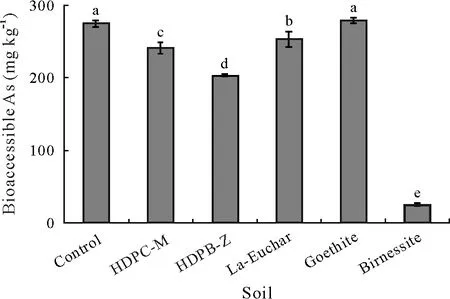
Fig. 6 Bioaccessible As (estimated by the simplified bioaccessibility extraction test)in the <250 μm fractions of unamended control soil and adsorbent-amended soils after three months of incubation. The test soil was collected from a dryland in Armidale, New South Wales, Australia and spiked with 1 000 mg kg-1 As one month before the incubation.The adsorbents include hexadecylpyridinium chloride-modified montmorillonite(HDPC-M), hexadecylpyridinium bromide-modified zeolite (HDPB-Z),La-doped magnetic biochar produced from eucalyptus bark(La-Euchar),goethite,and birnessite.Error bars are standard errors of the means(n=3).Different letters indicate significant difference between soils according to Duncan’s multiple range test(P <0.05).
Compared with the control, As bioaccessibility was reduced significantly (P <0.05) by 12% and 25% in the soils amended with HDPC-M and HDPB-Z,respectively,and it decreased dramatically(P <0.05),by about 90%,in the soil treated with birnessite(Fig.6).There are substantial variations in the bioaccessibility values of As reported by different authors, depending upon soil type andin-vitrobioaccessibility extraction test (Bagherifamet al., 2019).Xiaet al. (2016) investigated the bioaccessibility of As in spiked soils using the Unified BARGE Method(UBM)extraction and showed that As bioaccessibility was in the range of 40%–95%in gastric and 10%–96%in the intestinal phase, whereas Pascaudet al. (2014) obtained ranges of 2.3%–11.5% (gastric phase) and 3.1%–11.2% (intestinal phase) using the UBM test in a soil from a soccer field affected by mine tailings.Sarkar and Datta(2004)showed that only 33%–58% of the total As was bioaccessible in soils spiked with 45 mg kg-1As as sodium arsenate.Juhaszet al. (2007) investigated the bioaccessibility of As in 50 contaminated soils using the SBET method and reported As bioaccessibility values of 6%–89% for railway corridors,9%–89% for dip, 5%–36% for mine, and 15%–22% for gossan soils.Therefore,the proportions of bioaccessible As in our study agree well with the values reported by others.Sarkaret al. (2012) investigated the effects of bentonite modified by hexadecyltrimethyl ammonium(HDTMA)-Br and Arquad®2HT-75 and showed that organoclays can reduce the bioaccessible As by up to 58%.The observed differences between our results and those of Sarkaret al.(2012)could be related to the type and loading of surfactants,chemical properties of the soils, and the use of sodium arsenate to spike the soils.The results of our research agree with those of Hartleyet al.(2009)and Lee S Het al.(2011),who showed that amendments such as red mud,furnace slag,goethite,Fe grit,and lime can reduce the availability of As in polluted soils.
As-immobilizing potential of the adsorbents
To determine the effects of various adsorbents on the amounts of strongly bound and loosely bound As, single extractions using 2 mol L-1HNO3and deionized water were performed, respectively. The amount of HNO3-extractable As was moderately increased in the soils amended with HDPC-M and La-Euchar,whereas HDPB-Z,goethite,and birnessite reduced the HNO3-extractable As by 30%,57%,and 92%,respectively,compared with the control soil(Fig.7).The proportions of HNO3-extractable As were found to be in the range of 0.05%–9%, with the soils amended with La-Euchar and birnessite showing the maximum and minimum amounts of extractable As,respectively.
The deionized water-extractable As,which represents the loosely bound or non-specifically bound exchangeable As,declined significantly(P <0.05)by 17%,47%,95%,45%,and 95% in the soils amended with HDPC-M, HDPB-Z,La-Euchar,goethite,and birnessite,respectively,compared with the control soil.The proportions of deionized waterextractable As varied between 4%and 0.02%,and the most notable decrease occurred in the soils amended with La-Euchar and birnessite, in which the amount of deionized water-extractable As decreased dramatically,by 95%,from 40 mg kg-1in the control to 2.3 mg kg-1.
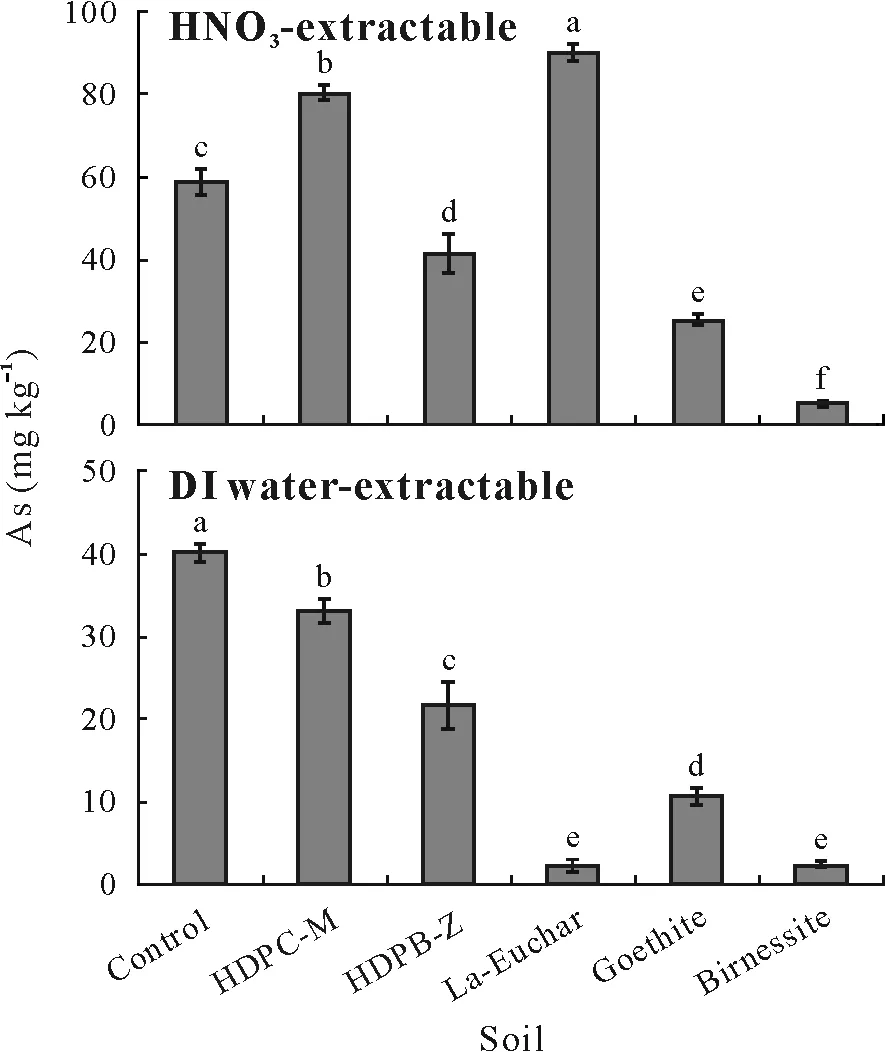
Fig.7 Contents of HNO3-and deionized(DI)water-extractable As in the unamended control soil and adsorbent-amended soils after three months of incubation. The test soil was collected from a dryland in Armidale,New South Wales, Australia and spiked with 1 000 mg kg-1 As one month before the incubation. The adsorbents include hexadecylpyridinium chloride-modified montmorillonite (HDPC-M), hexadecylpyridinium bromide-modified zeolite(HDPB-Z),La-doped magnetic biochar produced from eucalyptus bark(La-Euchar),goethite,and birnessite.Error bars are standard errors of the means (n = 3). Different letters indicate significant difference between soils according to Duncan’s multiple range test(P <0.05).
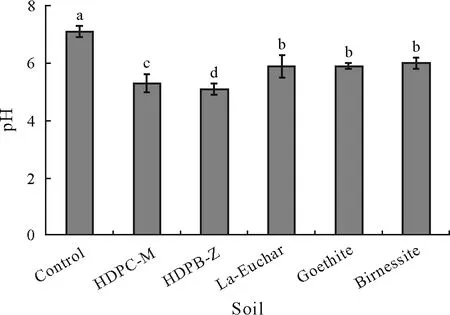
Fig.8 pH of the unamended control soil and adsorbent-amended soils after three months of incubation.The test soil was collected from a dryland in Armidale,New South Wales,Australia and spiked with 1 000 mg kg-1 As one month before the incubation.The adsorbents include hexadecylpyridinium chloride-modified montmorillonite(HDPC-M),hexadecylpyridinium bromide-modified zeolite(HDPB-Z),La-doped magnetic biochar produced from eucalyptus bark(La-Euchar),goethite,and birnessite.Error bars are standard errors of the means(n=3).Different letters indicate significant difference between soils according to Duncan’s multiple range test(P <0.05).
All the tested adsorbents significantly decreased(P <0.05)soil pH compared to the control soil(Fig.8).The most noticeable pH reductions were observed in the soils amended with organominerals,in which soil pH decreased from 7.1 in the control soil to 5.3 in the soil amended with HDPC-M and to 5.1 in the soil treated with HDPB-Z.Arsenic speciation in the environmental system is greatly affected by the redox potential (Eh) and pH, and H2ASO-4is the predominant As species at low pH(lower than approximately 6.9)under oxidizing environmental conditions.However,at higher pH,HAsO2-4 is mostly observed,and species such as H3AsO04and AsO3-4 may exist in strong acidic and basic conditions,respectively. The uncharged H3AsO04is mostly found as the main As species at pH lower than approximately 9.2 under reducing environments(Mohan and Pittman,2007).Because of the anionic properties of As,increasing the soil pH triggers pH-dependent As desorption and replacement of anionic As species with OH-ions,which consequently leads to increased As mobility (Moreno-Jiménezet al., 2012).While the maximum As(III)adsorption occurs at pH 8–9,the maximum arsenate adsorption onto clays and oxides is observed at low pH values,which drops quickly in the pH range of 5–9 for different soil constituents(Goldberg,2002;Gersztynet al.,2013).Therefore,the effects of amendments on the bioavailability of As in our experiments could be explained by the significant reduction in soil pH following the incorporation of synthetic adsorbents into the experimental soil.
The elemental mapping of the control soil revealed that C,O,Si,Mg,Al,phosphorus(P),K,Ca,Mn,and Fe might exist in As-bearing phases(Fig.9).However,the extents to which these elements were associated with As were different.Even though As was distributed homogeneously within the thin section of As-contaminated soil,the results of overlapping elemental maps showed that the As-rich areas were predominately co-located with Fe and Mn hotspots,which are known to be major As sinks due to their great affinity toward metalloids and related geochemical associations.
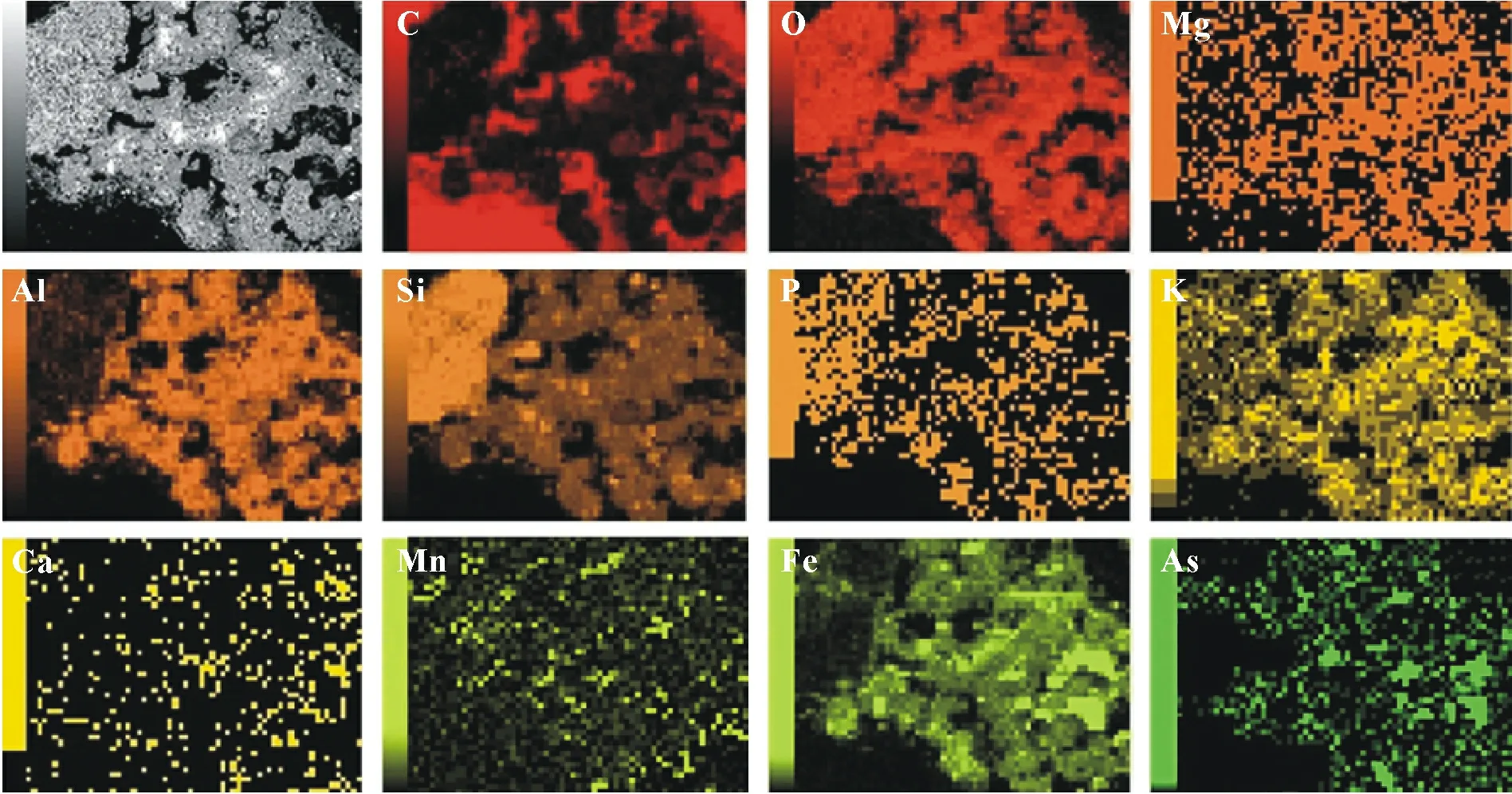
Fig.9 Scanning electron microscope(SEM)micrograph of a thin section of the unamended control soil after three months of incubation prepared in epoxy resin(the 1st picture)and related SEM-energy dispersive X-ray(EDX)maps of C,O,Mg,Al,Si,P,K,Ca,Mn,Fe,and As.Brighter points correspond to higher element contents.The test soil was collected from a dryland in Armidale,New South Wales,Australia and spiked with 1 000 mg kg-1 As one month before the incubation.
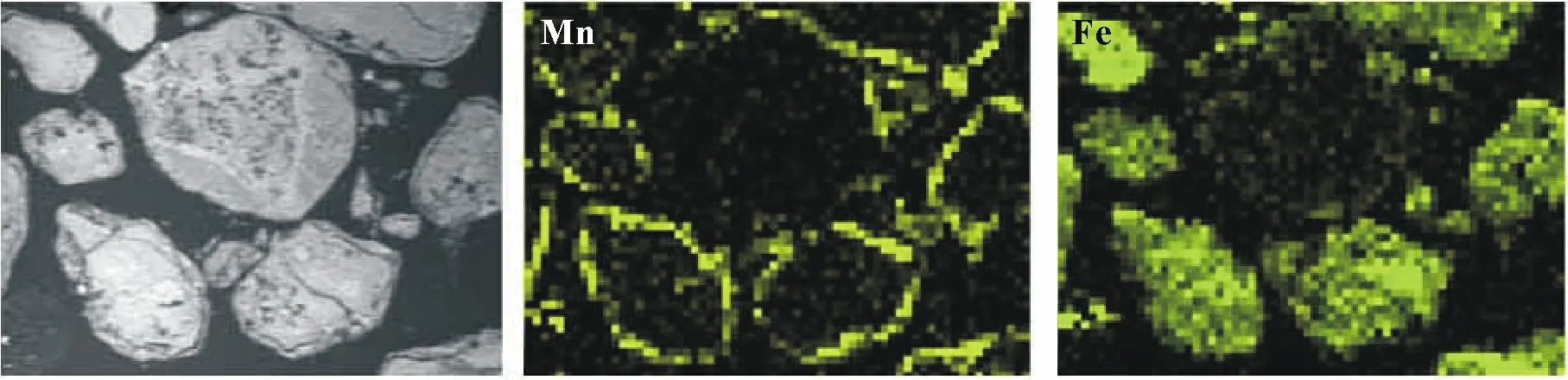
Fig.10 Scanning electron microscope(SEM)micrograph of a thin section of the birnessite-amended soil after three months of incubation prepared in epoxy resin(the 1st picture)and related SEM-energy dispersive X-ray(EDX)elemental maps of Mn and Fe.Brighter points correspond to higher element contents.The test soil was collected from a dryland in Armidale,New South Wales,Australia,spiked with 1 000 mg kg-1 As,and amened with birnessite(10%by weight)one month before the incubation.
The results of SEM-EDX Mn and Fe mapping of the birnessite-amended soil revealed that,unlike the homogeneous distribution of Mn in the control soil,the birnessiteamended soil grains exhibited well-defined Mn-rich contours in which Mn was predominantly concentrated along the borders of soil grains(Fig.10).Furthermore,it seems that incorporation of birnessite might affect the elemental distribution of Mn and As in the amended soil,as well as that of Fe,which was mainly distributed at higher concentrations in the inner part of soil grains,as evidenced by the brighter pixels of the Fe elemental map. Krishnamurti and Huang(1988)reported that Mn oxides can stimulate the formation of Fe oxides and the precipitation of amorphous Fe or Fe oxides/oxyhydroxides with different crystalline structures due to the oxidation of Fe(II)to Fe(III).
Manninget al.(2002)showed that MnO2can oxidize As(III) and facilitate the adsorption of As(V) on MnO2viathe formation of a bidentate binuclear corner-sharing complex of As(V)-MnO2.Moreover,these authors showed that the reductive dissolution process and surface alterations due to oxidation of arsenite resulted in an increase in arsenate adsorption through the creation of new reaction sites.It has been reported that both commercial hematite and goethite can adsorb more than 80% of arsenate, possibly because of the formation of an inner-sphere surface complex(Mamindy-Pajanyet al.,2009).Ettleret al.(2015)showed that amorphous Mn oxides could reduce the labile fraction of As and decrease As leaching by approximately 20%compared to the control soil.
The effectiveness of C-rich biochars and modified biochars in the removal of heavy metals has been extensively researched because of their characteristics such as high surface area and charges,high porosity,plentiful supply of oxygen-containing functional groups,and high ion exchange capacity(Ahmadet al.,2014; Wuet al.,2020).Wuet al.(2020) showed that a novel Ca-based magnetic biochar is a useful amendment for As remediation owing to the formation of bidentate chelate and ternary surface complexes on the surface of Fe oxide,which consequently increased the adsorption capacity and changed the relative distribution of As.Other studies have confirmed the remediation potential of goethite-modified biochar (Zhuet al., 2020) and organoclays prepared from HDTMA-Br and Arquad®2HT-75(Sarkaret al.,2012)to reduce bioavailable As.The results of single extractions were in line with those of the sequential extractions and bioaccessibility tests,demonstrating that the tested synthetic adsorbents are promising candidates for As stabilization in terrestrial environments.
CONCLUSIONS
Contamination withAs is a major environmental problem because of its adverse health effect and toxicity to humans and ecological receptors.Therefore,there is an immediate need to design remediation methods for the rehabilitation of As-impacted lands.In this study,the effectiveness of various synthetic adsorbents forin situAs stabilization in soil was investigated after three months of incubation.The results revealed that residual As was the predominant fraction,which accounted for 65%of the total As,while the acid-soluble As,which is the most labile fraction,was responsible for 21%of the total As in the control soil.However,the distribution pattern of As was shifted from exchangeable to reducible and residual fractions of the amended soils following the incorporation of adsorbents.The results revealed that application of the synthetic adsorbents successfully reduced up to 90%,92%,and 95%of SBET-,HNO3-,and DI-extractable As,respectively,compared with the control.Goethite,HDPC-M,HDPB-Z,and La-Euchar dramatically reduced the bioavailability of As,and birnessite exhibited a great affinity for As and reduced the exchangeable As by more than 90%.Owing to their suitability,effectiveness,and simplicity,single and sequential chemical extractions in conjunction with bioaccessibility test could be considered useful tools for evaluating the performance of adsorbents used in remediation studies.The results of single and sequential extraction revealed that goethite, birnessite, HDPC-M, HDPB-Z, and La-Euchar dramatically decreased the bioavailability,bioaccessibility,and extractable amounts of soil As and therefore can be used as promising immobilizing agents for the stabilization of As in contaminated soils.
ACKNOWLEDGEMENTS
The author Saeed Bagherifam gratefully thanks the University of New England and the Australian government for providing scholarships for his Ph.D. program. We thank the Environmental Analysis Laboratory of the Southern Cross University,Australia for assisting in characterization of soil samples and analysis of soil extracts and Mr.Malcolm Lambert for technical assistance in SEM analysis.
SUPPLEMENTARY MATERIAL
Supplementary material for this article can be found in the online version.
杂志排行
Pedosphere的其它文章
- Effect of lignite amendment on carbon and nitrogen mineralization from raw and composted manure during incubation with soil
- Magnesium-fortified phosphate fertilizers improve nutrient uptake and plant growth without reducing phosphorus availability
- A highly effective polycyclic aromatic hydrocarbon-degrading bacterium,Paracoccus sp.HPD-2,shows opposite remediation potential in two soil types
- Recycling of isabgol(Plantago ovata Forsk.)straw biomass and mineral powder with bio-inoculants as an effective soil amendment for isabgol cultivation
- Enduring legacy of coal mining on the fungal community in a High Arctic soil after five decades
- Elevated atmospheric CO2 reduces CH4 and N2O emissions under two contrasting rice cultivars from a subtropical paddy field in China
