Effects of silver nanoparticle size,concentration and coating on soil quality as indicated by arylsulfatase and sulfite oxidase activities
2022-11-01YutongXUEPrashankMISHRAFriedaEIVAZIandZahraAFRASIABI
Yutong XUEPrashank MISHRAFrieda EIVAZI and Zahra AFRASIABI
1Environmental Studies Concentration,Soka Universityof America,Aliso Viejo CA 92656(USA)
2Nicholas School of the Environment,Duke University,Durham NC 27707(USA)
3Department of Agriculture and Environmental Sciences,Lincoln University,Jefferson CityMO 65101(USA)
ABSTRACT Recently,the nanotechnology industry has seen a growing interest in integrating silver nanoparticles(AgNPs)into agricultural products,which increases soil exposure to these particles.This demands an investigation into the effect of AgNPs on soil health.Changes in soil enzyme activities upon exposure to AgNPs can serve as early indicators of any adverse effects that these particles may have on soil quality.This study aimed to determine the effects of AgNP size,concentration,coating,and exposure time on the activities of two sulfur cycle enzymes,arylsulfatase and sulfite oxidase.To investigate the sensitivity of soil enzyme activity to AgNP contamination,silt loam soil samples were treated with 30,80,and 200 nm-sized AgNPs coated with citrate,lipoic acid,and polyvinylpyrrolidone at 1,10,and 100 mg Ag kg-1 soil,with the changes in enzyme activities monitored at 3 h,3 d,and 30 d after treatment.For comparison,the effects of silver(Ag)ions on the enzyme activities were studied under similar treatment conditions.For most of the concentrations tested,the inhibitory effects of AgNPs on different enzymes differed,with a much stronger effect on sulfite oxidase activity than on arylsulfatase activity.The AgNP concentration and exposure time played much important roles than coating type and particle size in the effects of AgNPs on soil enzyme activities.
KeyWords: citrate,lipoic acid,polyvinylpyrrolidone,soil health,sulfur cycle enzyme
INTRODUCTION
Over the past few decades, in the field of agriculture,silver nanoparticles (AgNPs) have been one of the most utilized metallic nanomaterials to suppress or eliminate a wide range of plant pathogens (Parket al., 2006). Silver nanoparticles provide a distinct advantage over traditional pesticides owing to their high stability in the field,extensive coverage, and indifference to environmental fluctuations(Mishra and Singh,2015).Plant pathogen management is shifting to the use of AgNPs because of their limited potency against nontarget organisms,especially humans,which is an advantage over traditional chemically produced pesticides(Joet al., 2009). Kimet al. (2012) found that AgNPs at 10–100 mg Ag kg-1could decrease the pathogenicity ofCladosporium cucumerinumby 90%in the production of such crops as strawberries,peppers,eggplants,and tomatoes. Silver nanoparticles were also found to be excellent suppressants ofEscherichia coliwhen applied at 15–500 mg Ag kg-1(Bryaskovaet al.,2011).
Another characteristic of AgNPs is their ability to enhance crop yields or resilience against natural stressors.Jhanzabet al.(2015)found significant yield improvement in wheat production upon the addition of 25 mg kg-1AgNPs to the experimental soil.Iqbalet al.(2019)found that AgNPs at 50 and 75 mg kg-1protected wheat plants from heat damage at the trifoliate stage through improvements in morphological growth.The wide application of AgNP-containing products,through direct spraying on the plants/soil or pretreating the seeds before sowing(Sharmaet al.,2018),inevitably introduces a large quantity of AgNPs into ecosystems,especially soil.While the precise interactions between AgNPs and soil biota are yet to be elucidated,they have been shown to be harmful and cannot be ignored(Mishra and Singh,2015).
Soil enzymes play key roles in regulating the cycles of carbon(C),nitrogen(N),sulfur(S)and other nutrients by mediating the biochemical reactions involved in the decomposition of organic matter.Soil enzyme activities are very sensitive to changes in soil properties caused by environmental or human factors (Sherene, 2017). Therefore, soil enzyme activities can be used as an indicator of changes in soil quality and health(Bowleset al.,2014;Acosta-Martínezet al.,2018).
The response of agricultural soils to engineered nanoparticles has been thoroughly reviewed by Gardea-Torresdeyet al. (2014). Silver nanoparticles have shown to significantly inhibit the enzyme activities of cellobiohydrolase,β-1,4-xylosidase, acid phosphatase,β-1,4-Nacetylglucosaminidase (Asadishadet al., 2018), urease,dehydrogenase,and phosphatase(Caoet al.,2017).Multiple factors contribute to the inhibitory/stimulatory effect of nanoparticles on soil enzyme activities,including nanoparticle size,concentration,coating,and exposure period.Few studies have investigated the influence of such factors of AgNPs on soil enzyme activities.Considering this,the main objective of this study was to assess the effects of different coatings, sizes, concentrations, and exposure periods of AgNPs on the activities of soil enzymes.The two enzymes targeted in this study,arylsulfatase and sulfite oxidase,are involved in soil biogeochemical S cycle and can provide a clearer understanding of how exposure to AgNPs can impact soil quality(Tabatabai and Bremner,1970;Bilen and Dick,2011).
Sulfur occurs in soils in both inorganic and organic forms(90%–98%organic)and is present in most surface soils of humid and semi-humid regions(Scherer,2001;Tabatabai,2005). There is continuous cycling between organic and inorganic S in soils (Kloseet al, 2011). All organisms participate in the biological S cycle in ecosystems.Plants absorb S,which is an essential element for their growth in the form of sulfate anions(Castellano and Dick,1991).Ester sulfates represent an important fraction of total organic S in soil (30%–75%). Arylsulfatase releases sulfate anions from ester sulfate through enzymatic hydrolysis(Fitzgerald,1978). Sulfite oxidases are another important class of S cycle enzymes in soil that oxidize inorganic or organic S compounds to sulfates(Bilen and Dick,2011).Therefore,studies of enzyme activities enhance our understanding of agricultural practices and could ensure the sustainable development of human activities in relation to natural soil conditions.
Additionally, metal-based nanoparticles can dissolve into the adjacent solution or environment because of their thermodynamic instability(Bormet al.,2006).The rate and extent of dissolution depends on the physical properties of the nanoparticles and the nature of the surface coating agents.In this study,the size,surface charge,and dissolution of AgNPs were monitored in the soil water extract to further interpret the enzymatic bioassays.Owing to the inherent toxicity of silver(Ag)ions(Kolesnikovet al.,2020),we incorporated ionic Ag(as AgNO3)into this study as a separate control group to factor in the effects of Ag ions in contrast with those of the AgNP treatments.
MATERIALS AND METHODS
Soil,reagents,and AgNP characterization
The experimental soil used in this study was from the top layer(0–15 cm)of an agricultural field(38°31′34.3′′N,92°08′27.9′′W) at the George Washington Carver Farm,Lincoln University of Missouri,USA.The soil is a Wrengart-Gatewood silt loam,and its properties are listed in Table I.Prior to enzyme activity assays,the soil samples were thoroughly mixed,air-dried,passed through a 2-mm sieve,and stored in sealed and shaded plastic bags.
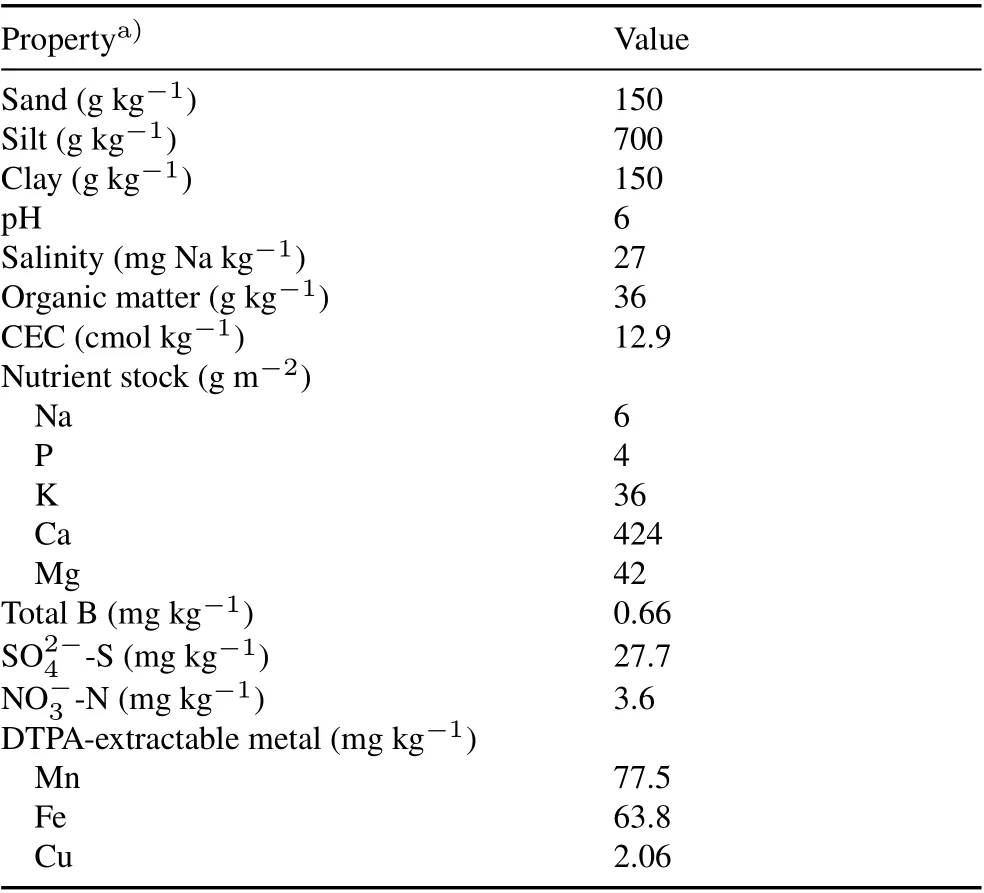
TABLE I Physicochemical properties of the experimental soil collected from the top layer(0–15 cm)of an agricultural field at the George Washington Carver Farm,Lincoln University of Missouri,USA
A total of nine samples of AgNPs, in three coatings of lipoic acid, citrate, and polyvinylpyrrolidone (PVP)and three sizes (i.e., 30, 80, and 200 nm) for each coating, were purchased from MilliporeSigma, USA at a stock concentration of 0.02 mg mL-1. Silver nitrate(AgNO3, analytical grade, 99.0%), sodium citrate dihydrate(HOC(COONa)(CH2COONa)2·2H2O,≥99.0%),tris-(hydroxymethyl)aminomethane(THAM)-hydrochloric acid(THAM-HCl,American Chemical Society reagent grade,≥99.8), potassium ferricyanide (K3Fe(CN)6, 99%), potassium sulfite (K2SO3, 90%), ethylenediaminetetraacetic acid disodium salt dihydrate (Na2EDTA·2H2O, ≥99%),calcium chloride (CaCl2,≥93%), sodium acetate trihydrate (CH3COONa·3H2O, ≥99%),p-nitrophenyl sulfate(C6H5NO6S, ≥99%),p-nitrophenol (O2NC6H4OH, ≥99%),and toluene(C6H5CH3,≥99%)were also purchased from MilliporeSigma. All reagents were used as received without any further modification. Ultrapure water with a resistivity of 18.2 MΩ cm (Millipore, USA) was used to prepare all solutions.
Ultraviolet-visible (UV-vis) spectra of the nanoparticles from 300 to 700 nm were obtained using a Lambda 365 UV-vis spectrophotometer(Perkin Elmer,USA).The hydrodynamic size and zeta potential of the nanospheres were measured with a ZetaSizer Nano instrument(Malvern Panalytical Ltd.,USA)after AgNPs were suspended in soil extract for 3 h and 30 d.The soil extract was prepared by mixing 2 g dry soil in 100 mL deionized (DI)water with constant shaking at 250 r min-1for 12 h.The thoroughly mixed soil slurry was centrifuged at 605×gfor 10 min,and the supernatant was filtered through a 0.1-μm filter(Millex,Millipore,USA).The filtered soil extract was mixed with AgNPs at a concentration of 10 mg Ag L-1.
The extent of the AgNP dissolution in the soil extract was measured with an inductively coupled plasma mass spectrometer(ICP-MS)(NexION 350Q,PerkinElmer,USA).To measure the total Ag concentrations(isotope107Ag),the suspensions were digested with 70%HNO3in a Milestone Ethos Up microwave system at 150°C for 10 min. The digested solutions were then diluted with 1%HNO3,and the dissolved Ag was separated from the AgNPs by ultrafiltration using Amicon®Ultra centrifugal filters with a nominal molecular weight limit of 3 kDa at 3 260×gfor 1 h.The concentration of Ag in the filtrate was compared with the Ag concentration prior to ultrafiltration.In the control experiments (i.e., Ag+in DI water), more than 95% of the Ag+passed through the 3 kDa filters.However,when dissolved Ag+ions were added to the soil extract at the same concentration,more than 90%of the ions were retained on the filter,likely due to the complexation of the Ag ions with the dissolved organic matter.
Soil treatment
To prepare for enzyme assays, soil samples were pretreated with DI water(1 mL DI water for every 1 g soil)to assist in the homogenization process upon the addition of AgNP solutions.Then,stock AgNP solution was added to obtain concentrations of 1,10,and 100 mg Ag kg-1soil.In addition,to determine the effects of Ag ions on soil enzyme activities,AgNO3was used at the same concentrations and following the same procedures as those of the AgNPs.The Ag-treated samples were shaken for 10 s for proper homogenization and incubated in a dark environment for either 3 h,3 d, or 30 d at 25±2°C to compare the time-dependent influences of the nanoparticles and Ag ion solutions on the enzyme activities.
At the end of each incubation time interval(i.e.,3 h,3 d,and 30 d),a number of samples were taken for enzyme assays.For each nanoparticle treatment, enzyme, and incubation period,27 soil samples in total were incubated,including one for each size,concentration,and coating.In addition,six soil samples were incubated for each AgNO3concentrations and six samples for each control.
Enzyme activityanalysis
The sulfite oxidase activity assay protocol established by Bilen and Dick(2011)was used.First,2 g soil was mixed with a AgNP suspension and then incubated at 25°C for 3 h,3 d,and 30 d.Sulfite oxidase exhibits optimal activity at pH 8(Wilson and Kappler,2009).Therefore,reagent pH was measured prior to treatment and,if needed,adjusted to 8 with 0.2 mol L-1NaOH or HCl.
Two milliliters of each of 8.0 mmol L-1THAM buffer,8.0 mmol L-1Na2EDTA,0.5 mol L-1CaCl2, 8.0 mmol L-1K2SO3, and 2.5 mmol L-1K3Fe(CN)6were added to the soil mixture and swirled gently to mix.Immediately afterward, 0.5 mL of the mixture was pipetted out and centrifuged at 4 350 r min-1for 5 min. The remaining mixture was then incubated at 37°C for 4 h. The color intensity of the supernatants of the 0.5 mL mixture was measured at 420 nm with a Synergy H1 Hybrid™Multimode Reader(BioTek Instruments Inc.,USA)to obtain the absorbance of K3Fe(CN)6prior to the 4-h incubation.The yellow color exhibited by K3Fe(CN)6would disappear when Fe3+is reduced by sulfite oxidase.After the 4-h incubation,the remaining mixture was centrifuged at 4 350 r min-1for 5 min, and the color intensity of the supernatants was measured at 420 nm to obtain the absorbance of K3Fe(CN)6.Both before and after incubation,the absorbance readings were compared with the calibration curve created using 0,0.2,0.4,0.8,and 1.0 μmol mL-1K3Fe(CN)6in the assay medium.The activity of sulfite oxidase in each sample was calculated as micromoles of K3Fe(CN)6reduced per gram of soil per 4 h.
For the control group, the same soil mixture with all reagents and AgNPs but no K3Fe(CN)6was incubated for 4 h.After incubation,2 mL K3Fe(CN)6was added immediately to the mixture and swirled briefly to mix.The resulting solution was then immediately centrifuged with other treatment groups.Next,300 μL supernatants of both the control and the treatment samples were pipetted onto a 48-well plate,which was inserted into a Synergy H1 Hybrid™Multi-mode Reader(BioTek Instruments Inc.,USA)for absorbance reading at 420 nm.
To measure arylsulfatase activity, a modified protocol proposed by Tabatabai and Bremner(1970)was used.The activity of arylsulfatase was determined based on the release ofp-nitrophenol when the soils were mixed withp-nitrophenyl sulfate and toluene at 37°C for 1 h to achieve an optimum enzyme activity reading. One gram of moist soil mixed with AgNPs and AgNO3suspensions was then incubated at 25°C for 3 h, 3 d, and 30 d. Prior to incubation, 0.25 mL toluene was added to the soil samples to stop further synthesis of the enzyme by living cells and to prevent the assimilation of any enzyme reaction products during incubation.Then,4 mL 0.5 mol L-1acetate buffer(pH 5.8)and 1 mL 0.05 mol L-1p-nitrophenyl sulfate were added to the mixture and incubated at 37°C for 1 h,followed by the addition of 1 mL 0.5 mol L-1CaCl2and 4 mL 0.5 mol L-1NaOH. The color intensity was measured at 420 nm and referenced against the standard working solution ofp-nitrophenol.
For the controls, the same soil mixture with all the reagents exceptp-nitrophenyl solution was incubated for 1 h at 37°C. After the incubation period, 1 mL 0.5 mol L-1CaCl2,4 mL 0.5 mol L-1NaOH,and 1 mL 0.05 mol L-1p-nitrophenyl solutions were added. The mixture was then immediately centrifuged, followed by color intensity measurement.
Statistical analysis
For consistent reporting, the enzyme activities of the treated soil samples were normalized to those of the control.Since the incubation period significantly affected the enzyme activities,the results of each incubation period were reported separately.The effect of the experimental variables was studied using generalized linear models. To establish the statistical significance of the main effects and the interaction effects of concentration,size,and coating on soil enzyme activities,an analysis of variance(ANOVA)test was employed.No individual main effects were reported if the interaction effects were significant.This study used Tukey’s honestly significant difference(HSD)to find significant differences between the soil treatments and the control for every incubation time period,at an alpha level of 0.05.Statistical analyses were performed using SAS software(Version 9.4,2013),and Python 3.7.4 was used to evaluate and plot the normalized enzyme activities along with the standard errors.
RESULTS
Physicochemical properties of the AgNPs
All AgNPs used in this study exhibited maximum absorption values in the 397–492 nm range, which were in accordance with the reference values reported by the vendor(Table II).In this study,zeta potential and hydrodynamic diameter were measured for all AgNPs in the soil extract at two time intervals:3 h(representing the initial condition of the nanoparticles in the soil water extract)and 30 d(Table III).
At the 3-h exposure period,the zeta potentials measured for all AgNPs confirmed stabilization by the surface coatings.Within 30 d of suspension in the soil water extract,the surface charges of the citrate-coated AgNPs did not change very much.However,the magnitude of the zeta potential increased(to a less negative value)for the PVP-and lipoic acid-coated particles.This is most likely due to the absorption of naturalorganic matter or other ions on the surface,aggregation with other colloids,and/or sulfidation(Asadishadet al.,2018).
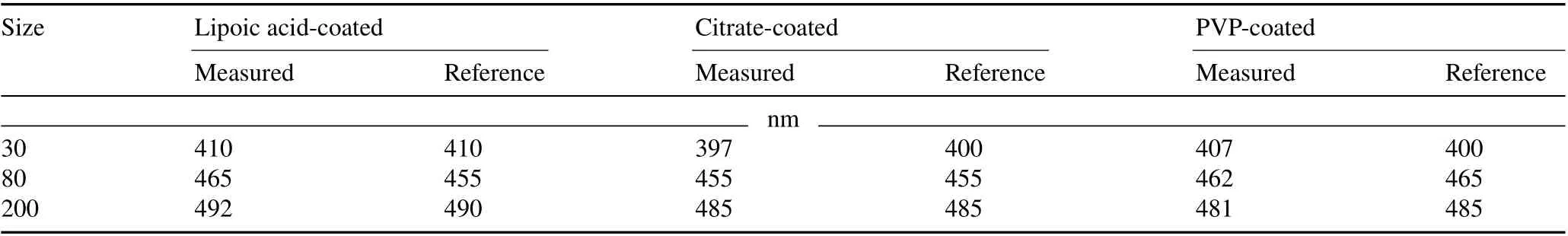
TABLE II Wavelengths where the silver nanoparticles(AgNPs)that were lipoic acid-,citrate-,or polyvinylpyrrolidone(PVP)-coated and in three sizes of 30,80,and 200 nm exhibited their maximum absorption values and the reference values reported by the vender
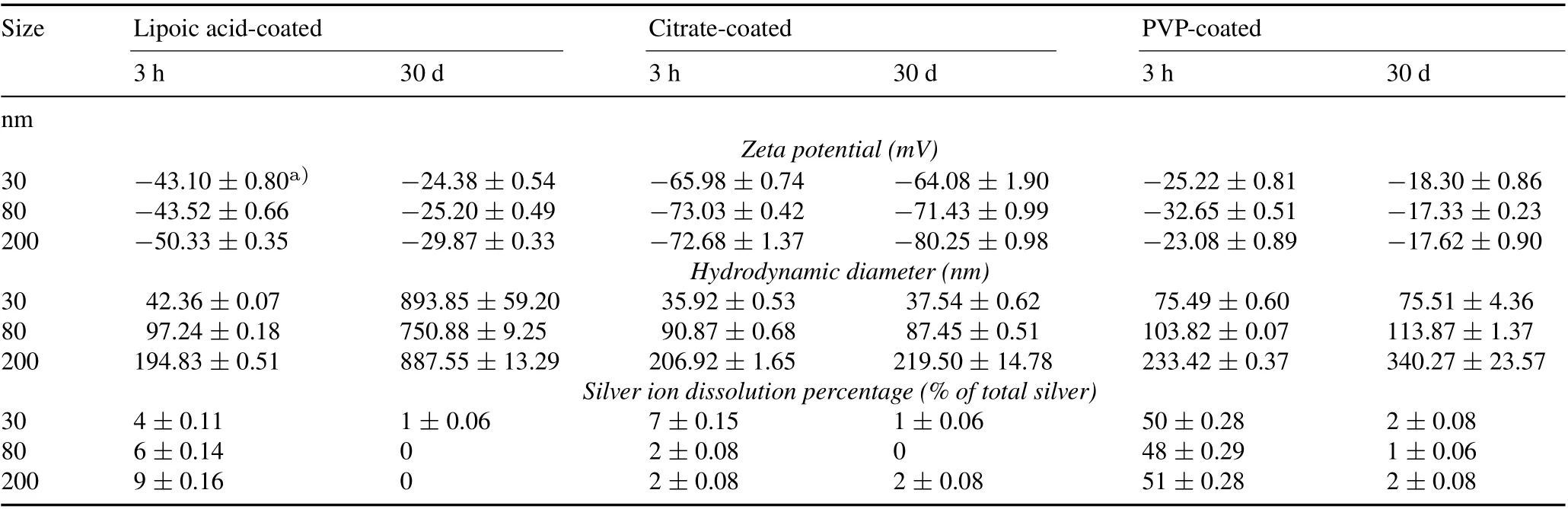
TABLE III Zeta potentials, hydrodynamic diameters, and silver ion dissolution percentages of the silver nanoparticles (AgNPs) that were lipoic acid-, citrate-, or polyvinylpyrrolidone(PVP)-coated and in three sizes of 30,80,and 200 nm after being suspended in soil water extract(soil:water=1:50)at 10 mg AgNPs L-1 for 3 h and 30 d
At the initial condition(3 h),measurements of the hydrodynamic diameters were all in good agreement with the transmission electron microscopy sizes reported by the vendor(Fig.S1,see Supplementary Material for Fig.S1).Over the 30-d period,the hydrodynamic size of the lipoic acidcoated AgNPs increased markedly,suggesting the occurrence of aggregation,which is consistent with the increased zeta potential values of these particles.The hydrodynamic sizes of the citrate-and PVP-coated particles remained the same over the 30-d period,indicating the high stability of these particles in soil systems.
Directly determining the dissolution pattern of AgNPs in a complex soil matrix is not a straightforward process.Therefore,to quantify the extent of the dissolution of AgNPs using ICP-MS,the Ag+content was measured as a percentage of the total metal content in the soil water extract over 3 h and 3 d (Table III). For the AgNPs with a same coating,the size of the nanoparticles did not affect their solubility,and a constant release of Ag+ions per unit surface area was not observed.A high dissolution of approximately 50%was only observed in the PVP-coated AgNPs over a 3-h time period.Comparatively,the other two coatings showed 1%–9%dissolution.In the longer exposure time(30 d),the Ag ion content in the soil water extract decreased dramatically to 0%–2%for all AgNPs.
Effects of AgNPs on soil rylsulfatase activity
In this study, AgNPs of three sizes (i.e., 30, 80, and 200 nm)and in three coatings(i.e.,citrate,PVP,and lipoic acid)were added to soil at three concentrations(i.e.,1,10,and 100 mg Ag kg-1)to measure the activities of arylsulfatase and sulfite oxidase after 3-h,3-d,and 30-d exposure,with each treatment normalized to its respective control(Table SI,see Supplementary Material for Table SI).Because it was found that the duration of the incubation significantly affected the activities (P≤0.05) of both arylsulfatase and sulfite oxidase,each enzyme’s results in relation to their incubation time are provided separately. For both arylsulfatase and sulfite oxidase, significant interaction effects (P≤0.05)were found for both time×concentration×size and time×concentration×coating.
Figure 1 shows the mean enzyme activities with AgNP treatment at three concentrations and three nanoparticle sizes(i.e.,30,80,and 200 nm).Stimulating effects were observed when soil samples were treated with the lowest concentration(i.e., 1 mg Ag kg-1soil) of 30-nm AgNPs over all three experimental time periods,with a significant stimulation of 47%at 30 d(P≤0.05).In addition,80-nm AgNPs showed more of a stimulating effect by the end of the 30-d incubation.In contrast,soils amended with larger particles(i.e.,200 nm AgNPs)initially showed reduced arylsulfatase activity with a significant inhibition of 39%at 3 d(P≤0.05).However,the results indicated that the enzyme activity recovered over time, with a significant stimulation of 45% at 30 d (P≤0.05).
The effect of exposure to an AgNP solution at 10 mg Ag kg-1soil within the initial 3 h was AgNP size-dependent(Fig. 1). A significant enhancement of 37% in enzyme activity was observed with the 30-nm AgNPs(P≤0.05),and as the size of the nanoparticles increased,the stimulating effect decreased to the extent that the 200-nm AgNPs resulted in a slight inhibition of enzyme activity.At this concentration,the inhibitory effect was observed at the 3-d exposure period in samples treated with all three sizes of nanoparticles(by 52%for 80 nm,and by 51%for 200 nm,P≤0.05).However,the activity increased after the 30-d period.
At the highest concentration of AgNP treatment (i.e.,100 mg Ag kg-1soil),the stimulation/inhibition effect on the arylsulfatase activity was size-dependent after 3-h and 30-d exposure,when larger particles had a greater inhibitory effect(Fig.1).After 3-d incubation,all three sizes of AgNPs inhibited the enzyme activity,with a significant inhibition of 54%(P≤0.05)by the 80-nm AgNPs,and this effect was not reversed even after a 30-d period.

Fig.1 Arylsulfatase activities in the soil samples treated with silver nanoparticles(AgNPs)of three sizes(i.e.,30,80,and 200 nm)and at three concentrations(i.e.,1,10,and 100 mg Ag kg-1 soil)for 3 h,3 d,and 30 d.The activities have been normalized to those in the control(no AgNP exposure).Error bars are standard errors of the means(n=3).An asterisk(*)indicates significant difference between the treatment and the control according to Tukey’s honestly significant difference at P ≤0.05.
Within the initial 3-h exposure to all three concentrations of citrate-coated AgNPs,the arylsulfatase activity increased(Fig. 2). After 3 d, the enzyme activity decreased, with significant inhibitions of 63%and 49%at 10 and 100 mg Ag kg-1soil,respectively(P≤0.05).However,after 30 d,the enzyme activity was fully restored and stimulated.
The PVP-coated AgNPs at 1 mg Ag kg-1soil had a stimulating effect on the arylsulfatase activity for all three incubation periods(Fig.2).When the concentration of PVPcoated AgNPs increased to 10 mg Ag kg-1, a significant stimulating effect was observed(by 44%,P≤0.05)at the initial time point of 3 h.The enzyme activity decreased when the exposure time was extended to 3 d,but after 30 d,full recovery of the activity was observed.When soil samples were amended with the highest concentration of PVP-coated AgNPs(i.e.,100 mg Ag kg-1soil),no significant effect was observed after 3 h, and the activity remained unchanged.However, after 3 d of exposure, the enzyme activity was inhibited significantly by 52%(P≤0.05),with no recovery after 30 d.
Overall,all samples treated with the three concentrations of lipoic acid-coated AgNPs showed lower enzyme activities compared with the untreated samples within the initial 3-h incubation(Fig.2).Inhibition continued over both the 3-d and 30-d time periods for all concentrations,except for the concentration of 1 mg Ag kg-1soil, which experienced enhanced enzyme activity after 30 d.
Effects of AgNPs on soil sulfite oxidase activity
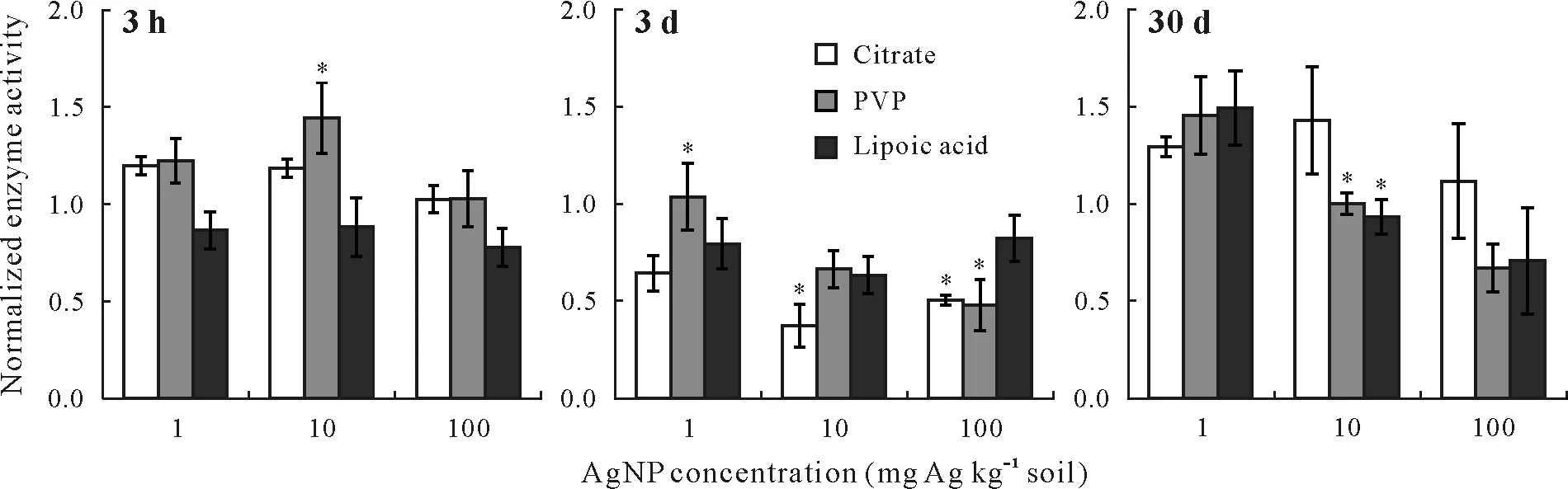
Fig.2 Arylsulfatase activities in the soil samples treated with silver nanoparticles(AgNPs)in three coatings(i.e.,citrate,polyvinylpyrrolidone(PVP),and lipoic acid)and at three concentrations(i.e.,1,10,and 100 mg Ag kg-1 soil)for 3 h,3 d,and 30 d.The activities have been normalized to those in the control(no AgNP exposure).Error bars are standard errors of the means(n = 3).An asterisk(*)indicates significant difference between the treatment and the control according to Tukey’s honestly significant difference at P ≤0.05.
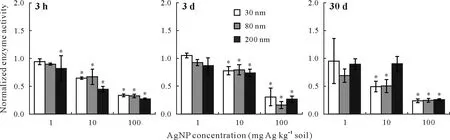
Fig. 3 Sulfite oxidase activities in the soil samples treated with silver nanoparticles (AgNPs) in three sizes (i.e., 30, 80, and 200 nm) and at three concentrations(i.e.,1,10,and 100 mg Ag kg-1 soil)for 3 h,3 d,and 30 d.The activities have been normalized to those in the control(no AgNP exposure).Error bars are standard errors of the means(n = 3).An asterisk(*)indicates significant difference between the treatment and the control according to Tukey’s honestly significant difference at P ≤0.05.
For all sizes of nanoparticles,the sulfite oxidase activity generally decreased across the 3-h,3-d,and 30-d periods of incubation at 1 mg Ag kg-1soil(Fig.3).At this concentration,a significant inhibition was observed in the sample amended with 200-nm AgNPs within the initial 3 h (by 18%,P≤0.05).Treating the samples with AgNPs of the three sizes at 10 mg Ag kg-1soil significantly(P≤0.05)reduced the enzyme activity by 33%–55%after the initial 3 h. After 3 d at this concentration, the enzyme activities were still significantly lower(P≤0.05)by 20%–26%compared with the untreated samples. Extending the time of exposure to 30 d,the 30-and 80-nm AgNPs further inhibited the enzyme activity by 51%and 50%,respectively.When the soil samples were incubated with the highest concentration of AgNPs at 100 mg Ag kg-1soil,a significant(P≤0.05)sulfite oxidase inhibition of 66%–73%was observed at the initial 3 h of incubation across almost all three sizes of AgNPs.This significant inhibition continued over 30 d.At the start of the experiment, the inhibition was AgNP size-dependent,with the 200-nm nanoparticles exhibiting a slightly higher inhibitory effect.After 30 d,no particular trend in size dependency was observed for the inhibitory effect of the nanoparticles,and the significant inhibition(P≤0.05)ranged from 74%to 76%.
After 3 h of exposure to the citrate-coated AgNPs,the sulfite oxidase activity was inhibited dose-dependently,where a significant inhibition of 32%and 67%was observed at 10 and 100 mg Ag kg-1soil,respectively(Fig.4).The 3-d and 30-d experimental results showed a continued inhibitory effect of the AgNPs on the enzyme activity,with a significant reduction(P≤0.05)of 70%–78%in the sample with the highest treatment concentration(i.e.,100 mg Ag kg-1soil).At all three experimental time points (i.e., 3 h, 3 d, and 30 d),the PVP-coated AgNPs inhibited the sulfite oxidase activity dose-dependently.At the concentrations of 10 and 100 mg Ag kg-1soil,a significant reduction(P≤0.05)in activity by 36%and 67%was observed,respectively,during the initial 3 h.The inhibitory effect continued over the 3-d and 30-d periods,and significant(P≤0.05)inhibition was observed at the highest treatment concentration of 100 mg Ag kg-1soil after 30 d(inhibition by 77%).The inhibitory effect of lipoic acid-coated AgNPs at all three tested exposure times was dose-dependent.At 1 mg Ag kg-1soil,the sulfite oxidase activity was significantly decreased(P≤0.05)by 25%for the initial 3 h. Although a slight stimulation was observed at the 3-d experimental interval,the activity once again decreased over the 30-d period.The lipoic acid-coated AgNPs at 10 mg Ag kg-1significantly(P≤0.05)decreased the sulfite oxidase activity by 55%during the 3-h exposure.Enzyme activity inhibition continued over the 3-d exposure period.Even after 30 d,the enzyme activity did not recover and was significantly inhibited by 57%(P≤0.05).When the soil samples were incubated with lipoic acid-coated AgNPs at the highest concentration of 100 mg Ag kg-1,the sulfite oxidase activity was reduced by 73%–89%across all three exposure times (i.e., 3 h, 3 d, and 30 d), and no enzyme recovery was observed.
Effects of Ag ions on soil rylsulfatase and sulfite oxidase activities
Both 1 and 10 mg Ag kg-1soil of AgNO3solution stimulated arylsulfatase activity during the first 3 h of incubation(Fig.5). When the time of exposure was extended to 3 d,AgNO3at these concentrations inhibited the enzyme activity.However,after 30 d,the enzyme activity fully recovered,and some stimulation in the activity was observed.At 100 mg Ag kg-1soil,the arylsulfatase activity decreased within the first 3 h of treatment.Prolonging the time of exposure to 3 d caused further inhibition of the enzyme activity.After 30 d,a slight recovery was observed,but the enzyme activity was still lower than that in the untreated sample.The sulfite oxidase activity of the soil samples treated with 1 and 10 mg Ag kg-1soil decreased within the initial hours of exposure,and the enzyme activity remained inhibited after the 30-d incubation. At 100 mg Ag kg-1soil, the enzyme activity significantly decreased(P≤0.05)by 63%within the initial hours of the experiment,and this inhibition continued at day 3,with no enzymatic activity recovery after 30 d.
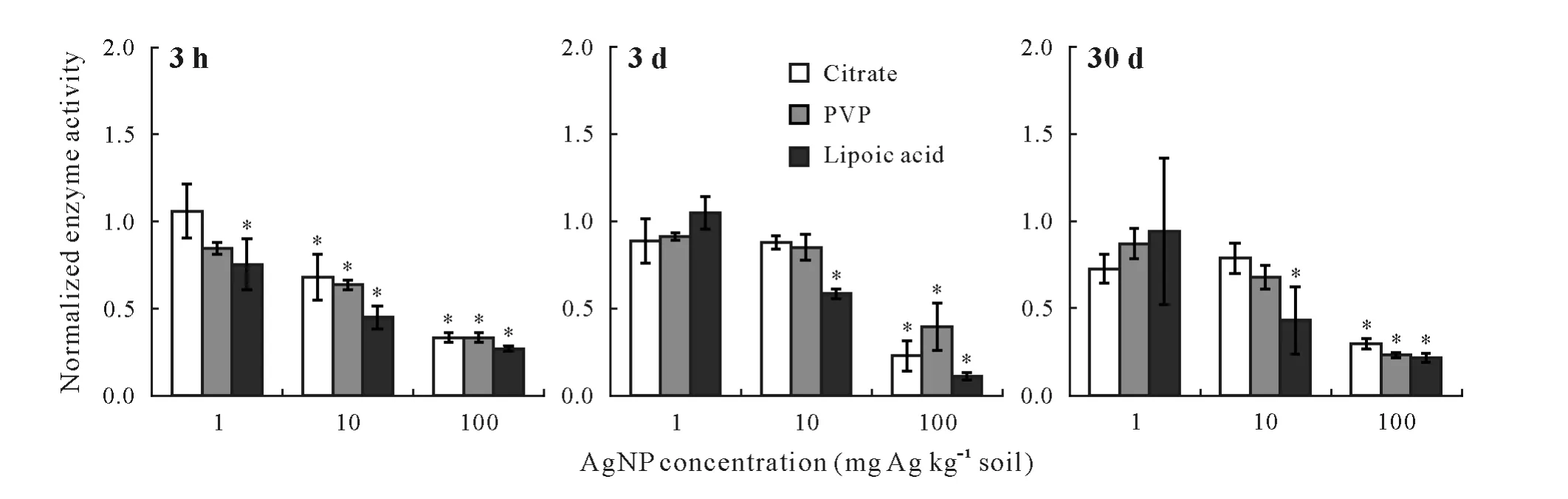
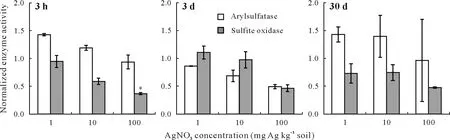
Fig.5 Arylsulfatase and sulfite oxidase activities in the soil samples treated with AgNO3 at three concentrations(i.e.,1,10,and 100 mg Ag kg-1 soil)for 3 h,3 d,and 30 d.The activities have been normalized to those in the control(no AgNO3 exposure).Error bars are standard errors of the means(n=3).An asterisk(*)indicates significant difference between the treatment and the control according to Tukey’s honestly significant difference at P ≤0.05.
DISCUSSION
Soil microorganisms regulate C,N,and S cycles through organic matter decomposition, immobilization, and mineralization.These biochemical reactions are mediated by enzymatic activity(Bandick and Dick,1999).Soil enzymes are assayed as indicators of microbial activity and are often used to assess soil quality.Hence,examining the enzymatic activities of soil exposed to nanoparticles can be a useful assessment tool to monitor the environmental risks associated with the presence of nanoparticles.
Arylsulfatase is the first sulfatase enzyme detected in nature (Fitzgerald, 1978) that catalyzes the hydrolysis of ester sulfates, in which the linkage with the sulfate is in the form of R-O-S(R represents a diverse group of organic moieties).Arylsulfatase has been detected in a wide range of agricultural and forest soils,marine and freshwater sediments,salt marshes,and arctic and wetland soils worldwide(Deng and Tabatabai,1997;Dineshet al.,2004;Wang and Lu,2006;Acosta-Martinezet al.,2008).
Microorganisms are believed to be the main source of arylsulfatase in soil(Fitzgerald,1978;Germidaet al.,1992).Arylsulfatase hydrolyzes ester-bonded S by fission of the O-S linkage in a pathway that can be summarized as follows:
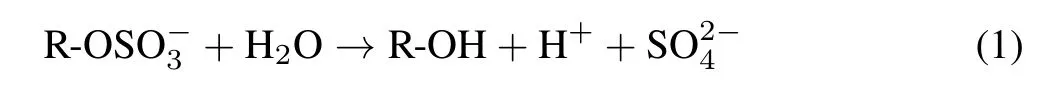
The other enzyme targeted in this study,sulfite oxidase,is a well-known metalloprotein redox soil enzyme that catalyzes a variety of transformations of C,S,and N,including the oxidation of sulfite to sulfate.Sulfite oxidase is involved in sulfite oxidation in soils treated with sulfites,or it naturally occurs in soils where S is cycled between the reduced(sulfite)and oxidized(sulfate)states,owing to flooding and drying(Bilen and Dick,2011).
The effect of any environmental contaminants on the activities of arylsulfatase and sulfite oxidase in soils deserves investigation because these two enzymes play key roles in the conversion of organic and reduced S to SO24-for plant uptake.Our results,similar to those of other studies on the effects of nanoparticles on soil microbial activities,reveal that the interaction with AgNPs differs with the type of enzyme (Jośkoet al., 2014; Rahmatpouret al., 2017;Asadishadet al., 2018). In this study, the sulfite oxidase activity was more sensitive to AgNPs and AgNO3exposure than the arylsulfatase activity.
The sulfite oxidase activity was inhibited with a clear dose-response relationship when soil samples were exposed to all three sizes of AgNPs.The enzyme activity decreased by approximately 75%at an AgNP treatment concentration of 100 mg Ag kg-1soil during the initial 3 h,and the enzyme activity did not recover after 30 d.At the same concentration,AgNO3showed a slightly less inhibitory effect on the sulfite oxidase activity (60% inhibition). In addition, similar to exposure to AgNPs,no enzymatic recovery was observed after 30 d.Compared with bulk heavy metals,engineered nanoparticles have large surface area-to-volume ratios,and therefore,greater reactivity,which could result in a higher inhibition on enzyme activity(Kimet al.,2013;Jośkoet al.,2014;Rahmatpouret al.2017).
Sulfite oxidase has a molybdenum cofactor as the essential component for catalyzing oxidation reactions, in which molybdenum is coordinated to a dithiolene group on the 6-alkyl side-chain of a pterin called molybdopterin(MPT)(Rajagopalan and Johnson,1992; Iobbi-Nivol and Leimkühler,2013).
Heavy metal ions other than molybdenum can bind tightly to the S-rich sites of molybdopterin cofactors,leading to enzyme inhibition. Metal ions, when present at high concentrations,are inserted nonspecifically into MPT,not requiring the molybdenum cofactor biosynthesis proteins MogA and MoeA(Neumann and Leimkühler,2008).
The high affinity of Ag as Ag+and AgNP to the thiol groups of enzymes has been well investigated (Moroneset al., 2005; Duránet al., 2016; Jianget al., 2019). In our study,the dose-dependent inhibition of sulfite oxidase activity in the AgNP and AgNO3treatments strongly suggests the interaction of Ag in nanoparticulate and ionic forms with the active site of the enzyme and a significant decrease in the number of free thiols available for enzyme function.
In this study,a low rate of dissolution was observed for most of the AgNP samples.Therefore,the authors postulate that the toxic properties of the AgNPs themselves,and not merely the dissolved Ag+ions, were responsible for the inhibition of soil enzyme activities. Similar conclusions were drawn when Shinet al.(2012)and Rahmatpouret al.(2017)studied the inhibitory effects of AgNPs and AgNO3on soil urease and alkaline phosphatase activities.The toxic effect of the heavy metals in nanoparticles can inactivate the protein groups and block the binding sites on the enzymes(Kızılkaya and Bayraklı,2005;Gaoet al.,2010).
The highest percentage of inhibition corresponded to the highest concentration of nanoparticle treatment,irrespective of the AgNP size.Although several enzyme activity datasets showed large standard deviations,these variations commonly exist in soil enzyme assays(Tonget al.,2007;Kimet al.,2011;Shinet al.,2012).
In contrast,AgNPs and AgNO3differentially affected the arylsulfatase activity.In general,the arylsulfatase activity was less affected by exposure to Ag solutions compared with sulfite oxidase activity.Peyrotet al.(2014)and Eivaziet al.(2018)also showed that arylsulfatase activity decrease could be expressed as a function of the Ag concentration in the soil.
Unlike sulfite oxidase,there is no evidence supporting the presence of thiol groups in the active site of arylsulfatase.In addition, it has been shown that the oxidation reaction catalyzed by arylsulfatase is irreversible,and no metal ion is required for its catalytic function(Germidaet al.,1992;Kloseet al., 2011). Hence, the presence of other heavy metals may not adversely affect arylsulfatase activity to the extent that they inhibit sulfite oxidase activity.
Earlier studies have shown that the length of time that soil enzymes are exposed to AgNPs may change the strength of the effect observed (Jośkoet al., 2014; Eivaziet al.,2018; Grünet al., 2019). In this study, the extension of the exposure time resulted in a reduced negative effect of the nanoparticles on arylsulfatase activity.The same effect was not observed for the sulfite oxidase. Over time, soil microorganisms can tolerate and metabolize nanoparticles as well as adapt to stress factors through regeneration and horizontal gene transfer (Bruinset al., 2000; Allison and Martini, 2008; Dineshet al., 2012; Liet al., 2017). In this study, another reason for the reduced inhibition and even stimulating effects of AgNPs on the selected enzyme activities could be the lower bioavailability of nanoparticles due to their immobilization through various kinds of sorption.
For both enzymes in this study, exposure to the low concentration AgNPs (i.e., 1 mg Ag kg-1soil) did not significantly reduce their activities. Similar findings were reported by de Oca-Vásquezet al.(2020)and Rahmatpouret al.(2017)whenβ-glucosidase,phosphatase,and urease in soil were exposed to low concentrations of AgNPs. In contrast,several studies reported the inhibitory effects of Ag-NPs at high concentrations on the activities of soil enzymes,including dehydrogenase,urease,phosphatase,cellobiohydrolase,β-1,4-xylosidase,β-1,4-N-acetylglucosaminidase,arylsulfatase,andβ-glucosidase(Shinet al.,2012;Caoet al.,2017;Asadishadet al.,2018).
In this study,we also examined the effects of different coatings on the activities of the two target enzymes.Surface coatings were used to stabilize AgNPs in solution by preventing their agglomeration,oxidation,and ion release.It has been determined that the coatings of nanoparticles greatly influence their biocompatibility, stability, and toxicity in aqueous solutions,biological systems,and the environment(Gebaueret al.,2012;Sharmaet al.,2014;Butleret al.,2015;Rahmatpouret al.,2017;Makamaet al.,2018).However,it is still unclear whether different surface coatings affect the interaction of AgNPs with biological environments(Reidy
et al.,2013;Martin et al.,2014).
In this study, soil samples were treated with AgNPs coated with citrate,PVP,or lipoic acid at three concentrations.The AgNPs in different coatings had different effects on the arylsulfatase and sulfite oxidase activities depending upon the AgNP concentration, with no specific trend observed. Asadishadet al. (2018) compared the inhibitory effects of citrate- and PVP-coated gold nanoparticles on five soil nutrient cycling enzymes and did not find any specific correlation between the coatings and enzyme activities.However, Arnaout and Gunsch (2012) reported that gum arabic- and citrate-coated AgNPs had a more inhibitory effect on the ammonia-oxidizing bacteriumNitrosomonaseuropaeathan PVP-coated AgNPs.In the present work,the nanoparticle concentration and exposure duration exerted a stronger effect on the enzyme activities than the nanoparticle size and surface coating.
CONCLUSIONS
This study confirmed that AgNPs and Ag ions differed in their effects on the activities of arylsulfatase and sulfite oxidase and that the enzymatic activities were mainly affected by the concentration of AgNPs and duration of exposure.Sulfite oxidase activity was inhibited upon exposure to all concentrations of AgNPs (i.e., 1, 10, and 100 mg Ag kg-1soil) in a dose-dependent manner, with no recovery of the enzyme activity after a 30-d period. Extending the AgNPs-soil contact time considerably altered the effect on the arylsulfatase activity.The effects of Ag ions dissolved from AgNPs were not significant,indicating that the adverse effects on enzyme functioning were caused by the toxic effect of the AgNPs themselves.This study establishes the importance of conducting multifactor studies to verify the threat to the biological quality of soils contaminated by AgNPs.
ACKNOWLEDGEMENTS
We convey our appreciation to Dr.Lisa Crummett for providing the microplate reader required to perform the enzyme assays. This study was funded by the National Institute of Food and Agriculture,United States Department of Agriculture(No.1007450).
SUPPLEMENTARY MATERIAL
Supplementary material for this article can be found in the online version.
杂志排行
Pedosphere的其它文章
- Comparing the Soil Conservation Service model with new machine learning algorithms for predicting cumulative infiltration in semi-arid regions
- Time effects of rice straw and engineered bacteria on reduction of exogenous Cu mobility in three typical Chinese soils
- Carbon nanomaterial addition changes soil nematode community in a tall fescue mesocosm
- In situ stabilization of arsenic in soil with organoclay,organozeolite,birnessite,goethite and lanthanum-doped magnetic biochar
- Improvement of phosphorus uptake,phosphorus use efficiency,and grain yield of upland rice(Oryza sativa L.)in response to phosphate-solubilizing bacteria blended with phosphorus fertilizer
- Elevated atmospheric CO2 reduces CH4 and N2O emissions under two contrasting rice cultivars from a subtropical paddy field in China
