Improvement of phosphorus uptake,phosphorus use efficiency,and grain yield of upland rice(Oryza sativa L.)in response to phosphate-solubilizing bacteria blended with phosphorus fertilizer
2022-11-01PratibhaRAWATAnitaSHARMADeeptiSHANKHDHARandShaileshChandraSHANKHDHAR
Pratibha RAWATAnita SHARMADeepti SHANKHDHAR and Shailesh Chandra SHANKHDHAR
1Department of Plant Physiology, College of Basic Sciences&Humanities, Govind Ballabh Pant University of Agriculture&Technology, Pantnagar-263145(India)
2Department of Microbiology,College of Basic Sciences&Humanities,Govind Ballabh Pant Universityof Agriculture&Technology,Pantnagar-263145(India)
ABSTRACT Phosphorus(P)limitation in soil is a major concern for crop productivity.However,the use of chemical fertilizer is hazardous to the environment and costly.Therefore,the use of phosphate-solubilizing bacteria(PSB)is an eco-friendly approach for a sustainable agricultural system.In the present study,a field trial was conducted for two consecutive years to study the effects of three PSB strains isolated,Bacillus licheniformis,Pantoea dispersa,and Staphylococcus sp.,with different P fertilizer rates on P uptake,P use efficiency(PUE),and grain yield of rice.The activities of soil enzymes were also studied in relation to PSB treatments.Comparative analysis of the yield and biochemical parameters revealed that inoculation of PSB consortium could reduce almost 50%of the recommended P dose in rice cultivation.Three PSB strains in combination with 50%P dose was most effective and showed the highest increases in P uptake and PUE as compared to the uninoculated control.Moreover,the PSB consortium combined with 50%P dose contributed to 50.58%and 35.64%yield increases compared to the uninoculated control for 2018 and 2019,respectively.Significant increases in the activities of soil dehydrogenase,alkaline phosphatase,and acid phosphatase were also recorded under PSB treatment.
KeyWords: bacterial inoculation,enzyme activity,phosphate solubilization index,recommended P dose,soil dehydrogenase,soil phosphatase
Rice(Oryza sativaL.)is a global food grain consumed by half of the world’s population (Huanget al., 2014).The global increase of human population and the subsequent rising concern for rice production have shifted focus to sustainability in rice cropping systems.Phosphorus(P)deficiency is the foremost nutritional constraint in upland rice production systems(Ismailet al., 2007). Upland rice accounts for approximately 9%of the total rice area in Asia,and in rain-fed conditions, it is grown as a direct-seeded crop without irrigation(Caviteet al.,2020).Upland rice is typically deficient in P because of unforeseeable drought(Fageriaet al., 1982). Drought induces P transformation in soil and decreases its bioavailability by increasing the organic and inorganic P bound with secondary minerals such as iron and aluminum oxide(Zhanget al.,2020).Based on this premise,research on rice sustainable cropping systems has been focused on improving the current low productivity of rice.
As a pivotal nutrient for plants, P is indispensable for metabolic processes such as photosynthesis, respiration,adenosine triphosphate generation,cell division,signal transduction,nucleic acid formation,membrane integrity,maturation,and stress attenuation(Vanceet al.,2003).Although total P found in soil is generally 50–3 000 mg kg-1,only 0.1%is available P for plant uptake(Zhuet al.,2018)due to P adsorption,immobilization,and precipitation with cations in soil, as well as interconversions to organic P (Kishoreet al.,2015).Approximately 67%of the world’s cultivable land is deficient in P(Dhillonet al.,2017),thus demanding the recurrent application of chemical fertilizers to satisfy crop nutritional requirements.This approach of inorganic nutrient supply to plants is neither economically feasible nor ecofriendly, as chemical fertilizers can remain in soil for a long time. The unbalanced application of fertilizers has therefore prolonged effects on the environment, affecting soil fertility,crop health,and carbon footprint and causing eutrophication and heavy metal accumulation in soil,which is hazardous to both human and animal health(Huanget al.,2017).Moreover,only 10%–20%of the applied P is recovered by the crops(Roseet al.,2010a).Consequently,there is an increasing interest in sustainable and environmentfriendly strategies for enhancing P biofortification and yield while alleviating the outcome of chemical fertilizers for a balanced agricultural system.
In this regard,phosphate-solubilizing microorganisms(PSMs)are potential candidates for enhancing phosphorous use efficiency(PUE)in plants and ameliorating the growth and yield of crops in a cost-effective and environmentfriendly approach. These bioinoculants solubilize insoluble P (Fe-P, Ca-P) into plant absorbable forms (HPO2-4 ,H2PO-4) through diverse mechanisms such as secretion of organic acids (citric acid, oxalic acid, gluconic acid,and succinic acid) and production of siderophores, exopolysaccharides, and enzymes, such as phosphatases and dehydrogenases (Tomeret al., 2016). They also accelerate plant growth and yield by secreting phytohormones,such as auxins, gibberellins, and cytokinins, synthesizing 1-aminocyclopropane-1-carboxylic acid, increasing nutrient uptake, and controlling plant diseases (Rawatet al.,2021).Inoculation of PSMs has been shown to increase the grain yield (GY) of a variety of crops, namelyO. sativaL. (Stephenet al., 2015),Zea maysL. (Ibarra-Galeanaet al.,2017),Cicer arietinumL.(Rajwaret al.,2018),andSaccharum officinarumL. (Safirzadehet al., 2019). Recently,Emamiet al.(2020)assessed the potential of a novel phosphate-solubilizing strainPseudomonasR185 that elevated PUE by 29.5%and GY up to 58%in the wheat cultivar Marvdasht. It was also reported that the application of a phosphate-solubilizingBacillus subtilisstrain with a blend of 17.5 kg P2O5ha-1improved corn GY by 29.1% and PUE by 100.5%, and with a blend of 105 kg P2O5ha-1,Azospirillum brasilienseraised corn yield by 34.7% and PUE by 54.6%(Pereiraet al.,2020).
Owing to the propitious role of phosphate-solubilizing bioinoculants in agricultural systems and the significance of low-cost rice production by utilizing agroecological practices,the present study investigated the positive interaction between isolated phosphate-solubilizing bacterial strains,PUE,and rice GY at different P application rates.
MATERIALS AND METHODS
Soil sampling and isolation of bacterial strains
Rhizospheric soil samples of field-grown rice at the vegetative stage were collected from Pantnagar(29.0369°N,79.4472°E), Udham Singh Nagar District, Uttarakhand,India. Soil samples were collected in triplicate by uprooting the plant and carefully collecting the soil adhered to roots, followed by mixing the soil to make a composite sample. Soil samples serially diluted (10-4–10-5) were then inoculated on nutrient agar and Pikovskaya’s agar to isolate phosphate-solubilizing bacteria(PSB)(Pikovskaya,1948). Discrete bacterial colonies appeared on the plates after 24–36h of incubation at 30°C,and they were purified by repeated streaking on nutrient agar.Pure bacterial isolates were inoculated on glycerol stocks and nutrient agar slants,and maintained at-80°C and 4°C,respectively.
In situ phosphate solubilization assayand identification of PSB
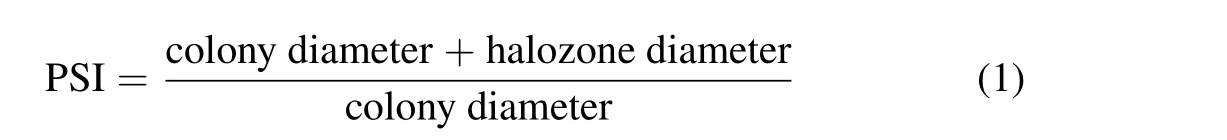
For qualitative estimation,isolated bacterial strains were point-inoculated on Pikovskaya’s agar plates and incubated at 28°C for 1 week.The phosphate solubilization index(PSI)was calculated based on the halozone and colony diameters(Singhet al.,2013)according to Eq.1:
The isolates were quantitatively screened on National Botanical Research Institute’s phosphate(NBRIP)growth medium at 28°C(Mehta and Nautiyal,2001).The active cultures of selected PSB isolates were incubated in 50 mL of NBRIP liquid medium for 6d on a rotary shaker(120 r min-1,28°C).Uninoculated medium served as the control.The culture broth medium was centrifuged at 8 000 r min-1at 4°C for 15 min,and 2 mL aliquots were then sampled aseptically to determine pH and soluble phosphate at different time intervals(0,2,4,and 6d)using the protocol of Fiske and Subbarow(1925).
Three PSB isolates(PSB1,PSB2,and PSB3)with the highest PSI values were selected. Molecular characterization of these isolates was performed using 16S rRNA gene sequencing and phylogenetic analysis at Bioserve Biotechnologies,Hyderabad,India.The obtained gene sequences were submitted to the National Center for Biotechnology Information(NCBI)database after using the BLAST tool.Multiple sequence alignment was carried out using ClustalW,and phylogenetic analysis was conducted using the MEGA 5.0 software(https://www.megasoftware.net)as performed in Pankajet al.(2016).
Seed bacterization and field location
The seeds of the rice genotype Pant Dhan 26were obtained from the Seed Production Center,Govind Ballabh Pant University of Agriculture and Technology(GBPUAT),Pantnagar,Uttarakhand,India.The rice seeds were surfacesterilized with 0.1%sodium hypochlorite followed by washing with double-distilled water.The seeds were then soaked with an overnight grown liquid culture of the selected PSB strains at 1.9×108colony forming units per seed(containing 1%carboxymethyl cellulose as a sticking agent)and kept overnight at 28°C. Seeds were then shade-dried at room temperature for 2 h before being sown in the field. Seeds soaked with sterile distilled water served as the control.
The present investigation was conducted during the kharif season (June–November) in 2018 and 2019 at Dr.Norman E.Borlaug Crop Research Centre and Department of Plant Physiology,College of Basic Sciences and Humanities,GBPUAT. The experimental site lies in the Tarai plains,about 30 km southwards of the foothills of the Shivalik range of Himalayas(29oN,79.3oE,243.8 m above sea level),and experiences conditions of a subtropical and humid climate with hot dry summers and cool winters.The temperature and rainfall regimes of both seasons in the cropping years are presented in Fig.1.The soil at the sowing site during both years was Typic Hapludoll.The soil texture was silty clay loam with a pH of 7.5 and 7.8,and electrical conductivity of 0.268 and 0.272 dS m-1for 2018 and 2019,respectively.Soil available nitrogen stock was 175.61 and 189.43 kg ha-1(Subbiah and Asija,1956),available P stock was 17.5 and 18.0 kg ha-1(Olsonet al.,1954),and available potassium(K)stock was 122.86and 128.33 kg ha-1(Jackson,1973)for 2018 and 2019,respectively.
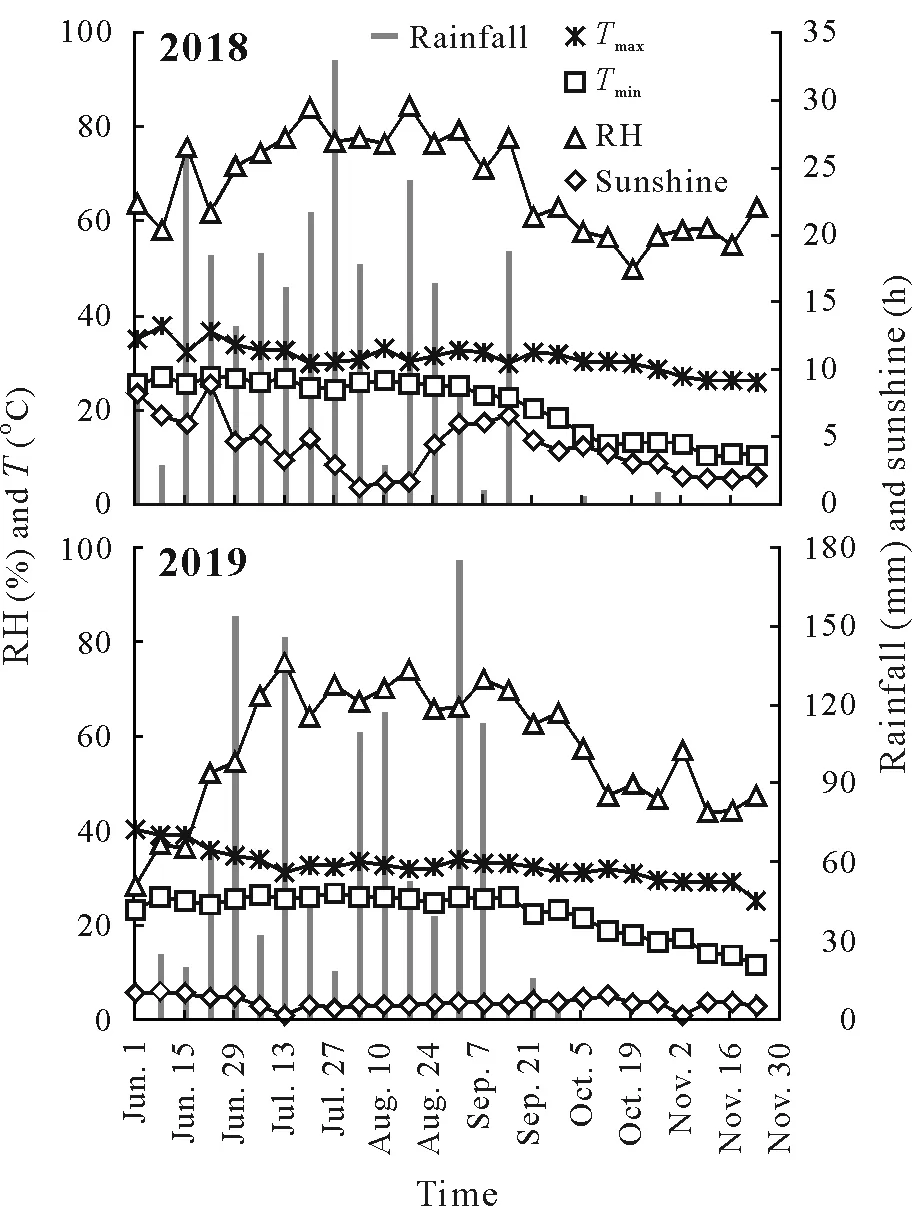
Fig.1 Weather conditions during the cropping seasons(June–November)of 2018 and 2019 at the experimental site in the Tarai plains,India.The data were obtained from the Meteorological Observatory at Norman E.Borlogue Crop Research Centre, Govind Ballabh Pant University of Agriculture and Technology,Pantnagar,India.T =temperature;Tmax =maximum temperature;Tmin =minimum temperature;RH=relative humidity.
Experimental design
In both years,rice seeds were directly sown in furrows opened 20 cm apart in the non-flooded field at a density of 110 kg seeds ha-1. The experiment was laid out in a split-plot design with three replications per treatment.The gross plot size was 30 m×24.5 m.Each of the 12 main plots was split into nine sub-plots(i.e.,number of total sub-plots was 108) measuring 1.6m× 1.6m. The sub-plots were separated from each other with proper bunds of 1-m width to avoid percolation between the sub-plots with different treatments.Randomization was performed at the main plot and sub-plot levels.The irrigation level was maintained at field capacity,i.e.,20.72%and 21.45%(measured through the gravimetric method) for the cropping years of 2018 and 2019, respectively, and the irrigation schedule was determined by the tensiometer installed in the field.There were four different levels of P fertilizer(0%,50%,75%,and 100%of the recommended dose of 45 kg P2O5ha-1).For each P level,there were nine PSB inoculation treatments:T1,uninoculated control;T2,PSB1;T3,PSB2;T4,PSB3;T5,PSB1 and PSB2;T6,PSB1 and PSB3;T7,PSB2 and PSB3;T8,PSB1,PSB2,and PSB3;and T9,standard strain(Pseudomonas putidaMTCC2476).The standard strain had already been studied as a PSB and it was obtained from the Microbial Type Culture Collection and Gene Bank,Institute of Microbial Technology, Chandigarh, India. Phosphorus and K were applied as basal fertilizers before sowing the seeds.Potassium was applied at 60 kg muriate of potash per hectare.Nitrogen(100 kg ha-1)was applied in four equal splits at tillering,maximum tillering,panicle initiation,and flowering stages.
Sampling,analysis,and calculation
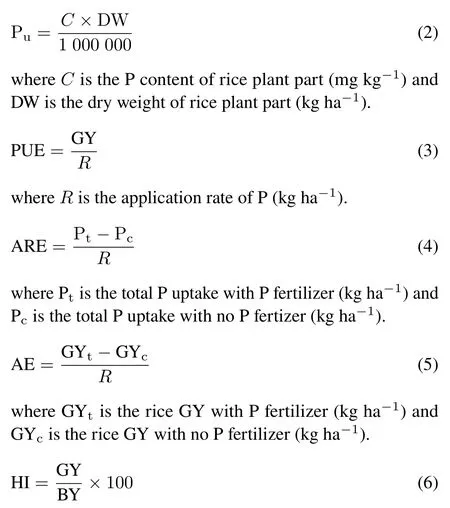
Soil samples (0–15 cm) were collected in triplicate from each plot before sowing,90 d after sowing,and after harvest in both years.The samples were stored at 4°C.Soil dehydrogenase(EC 1.1.1.1)activity was determined using the methods of Casida(1977).Soil alkaline phosphatase(EC 3.1.3.1)and acid phosphatase(EC 3.1.3.2)activities were measured using the methods described by Tabatabai and Bremner(1969).Soil available P content was estimated as described by Olsonet al.(1954).The microbial count of soil at the flowering stage of rice was estimated on nutrient agar using the serial dilution method (Wollum II, 1983). Rice plants from each plot were selected in triplicate at flowering and maturity and after harvest for P content, biological yield(BY),and GY estimation in both cropping years.The harvested crop plants were sun-dried for 5 d and weighed for total dry biomass(i.e.,BY).The grains were obtained by threshing the rice plants using a thresher. Sun-dried grains were weighed for GY(kg ha-1).The samples were oven-dried at 60°C to a constant weight, finely ground,and analyzed for P content in the grains,stems,and leaves according to the methods introduced by Jackson (1962).Phosphorus uptake(Pu,kg ha-1),PUE(kg kg-1),apparent recovery efficiency (ARE), agronomic efficiency (AE, kg kg-1),and harvest index(HI,%)were calculated using the following equations according to Baligaret al.(2001)and Hussein(2009).
Statistical analyses
Data for both years were subjected to analysis of variance(ANOVA)to test the effects of the different treatments.Duncan’s multiple range test was applied to determine significant differences between individual treatments atP <0.05.To test the relationship between different attributes,Pearson’s correlation coefficient was used.All tests were conducted using SPSS version 16.0.
RESULTS
Phosphorus-solubilizing efficiencyand identification of the isolated PSB
A total of 12 PSB strains were isolated from the soil on Pikovskaya agar and evaluated for halozone formation.Three different colonies with maximum PSI were selected(Table I,Fig.2)and subjected to quantitative P-solubilization on NBRIP liquid medium for 7 d.Maximum P-solubilization was observed on the fourth day of inoculation with a pH change in the medium(Table I).The 16S rRNA gene sequencing of the three strains PSB1, PSB2, and PSB3 showed 95.32%,95.09%,and 99.13%sequence similarity withBacillus licheniformis,Pantoea dispersa,andStaphylococcussp.,respectively.The 16S rRNA gene sequences of these strains were submitted to the NCBI database and allocated with NCBI accession numbers.
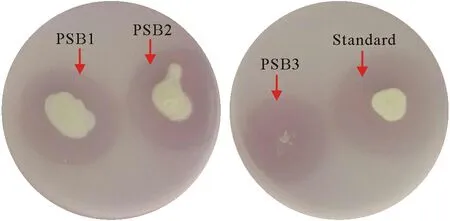
Fig.2 Halozone formation by the isolates on Pikovskaya’s agar containing tricalcium phosphate. The arrows show the halozones formed by the phosphate-solubilizing bacteria (PSB), indicating the solubilization of tricalcium phosphate. PSB1=Bacillus licheniformis; PSB2=Pantoea dispersa;PSB3=Staphylococcus sp.;Standard=Pseudomonas putida.
Phosphorus uptake in grains and vegetal tissues and yield attributes
The use of PSB in combination with different dosages of P fertilizer significantly augmented the P uptake in the stems,leaves,and grains as compared to the uninoculated controls at the flowering and maturity stages of rice in both years(Tables II and III).In addition,PSB application alone significantly improved the P uptake in vegetal tissues and grains without any P application, as compared to the uninoculated controls at the flowering and maturity stages in 2018 and 2019.Moreover,P uptake at maturity was higher than that at the flowering stage. The maximum percent increment in P uptake in the grains(98.15%and 76.28%),stem (224.02% and 203.62%), and leaves (204.22% and 183.52%) were observed with T8 blended with 50% P fertilizer at maturity in 2018 and 2019,respectively.
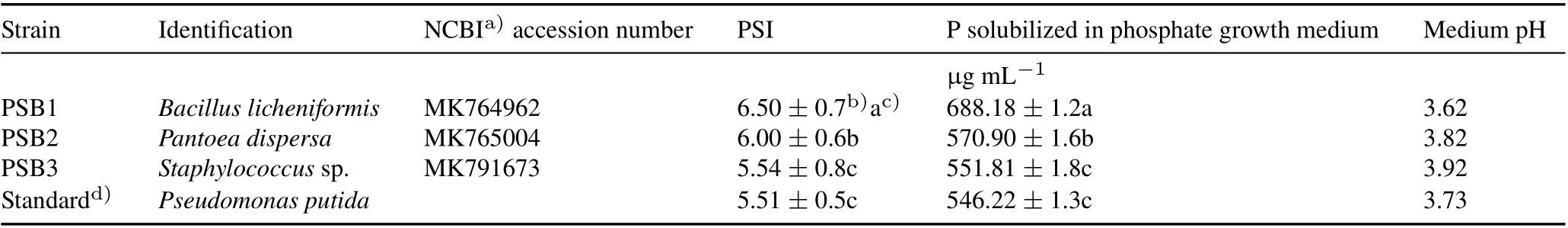
TABLE I Identification of the three selected bacterial strains with the highest phosphate solubilization index (PSI) values isolated from the rhizospheric soil of field-grown rice collected from Pantnagar,Udham Singh Nagar District,Uttarakhand,India
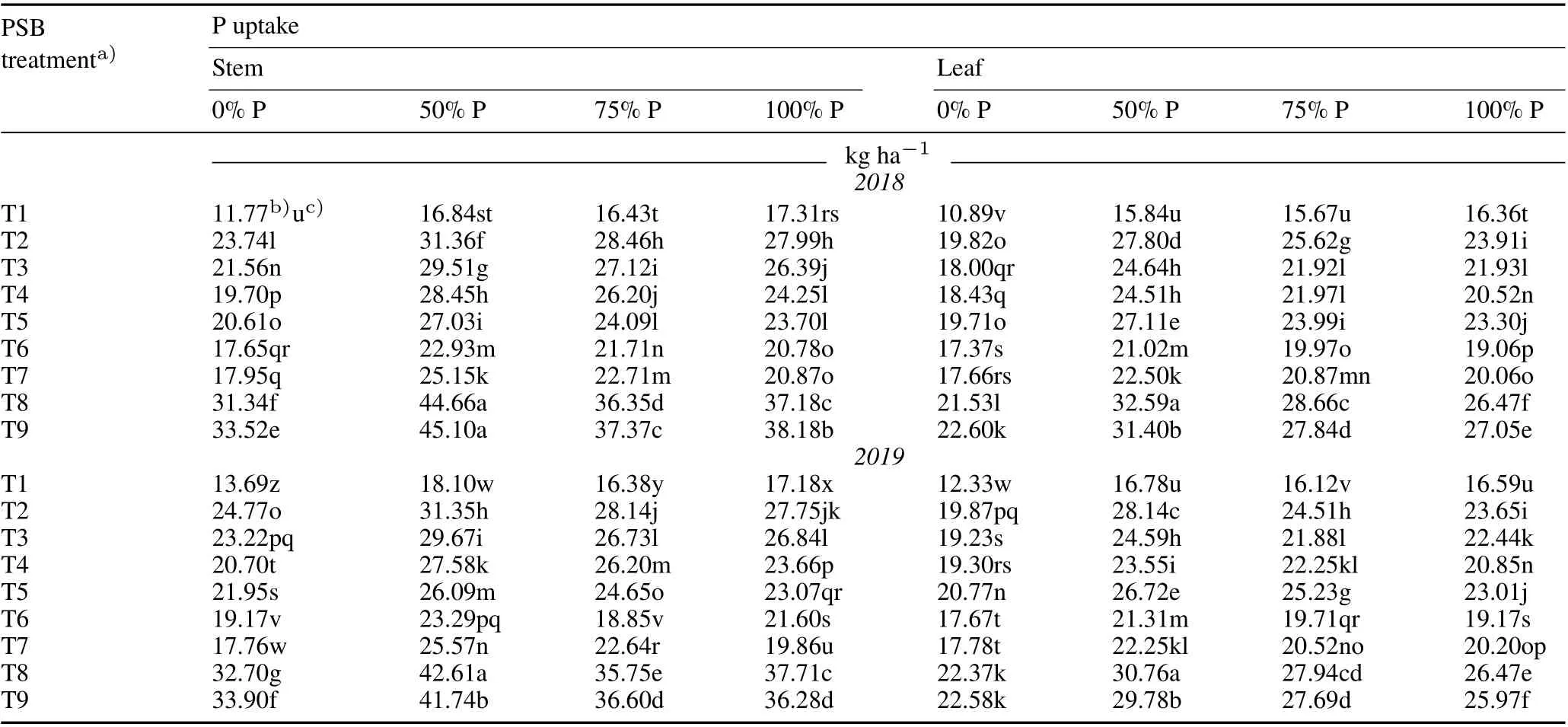
TABLE II Effect of phosphate-solubilizing bacteria(PSB)inoculation on P uptake in the stems and leaves of rice at the flowering stage under different P application rates(0%,50%,75%,and 100%of the recommended dose 45 kg P2O5 ha-1)during the kharif season(June–November)in 2018 and 2019 in the field experiment conducted in the Tarai plains,India
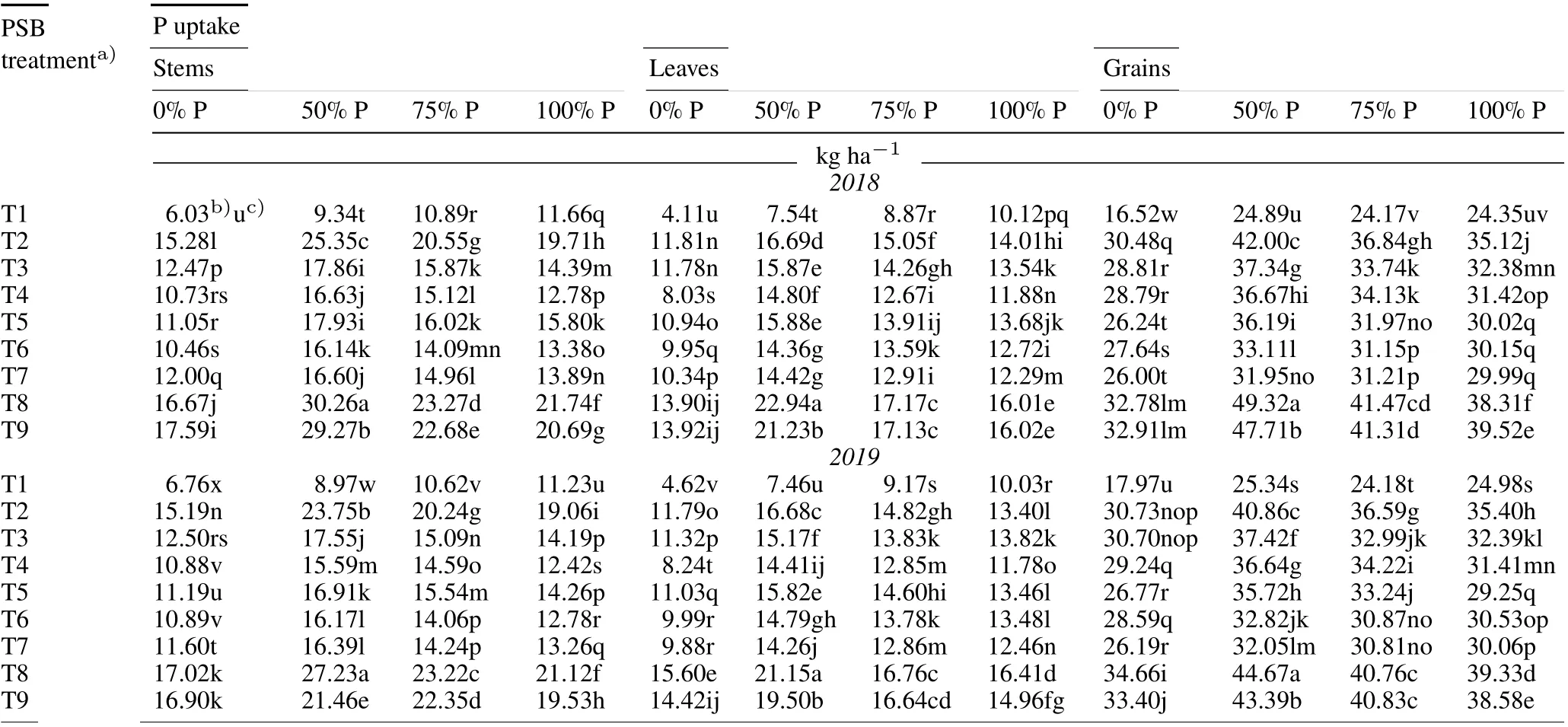
TABLE III Effect of phosphate-solubilizing bacteria (PSB) inoculation on P uptake in the stems, leaves, and grains of rice at the maturity stage under different P application rates(0%,50%,75%,and 100%of the recommended dose 45 kg P2O5 ha-1)during the kharif season(June–November)in 2018 and 2019 in the field experiment conducted in the Tarai plains,India
The GY of the uninoculated control with no P fertilizer was 4.13 and 4.73 t ha-1in 2018 and 2019, respectively,while the GY of the uninoculated control with 100%P fertilizer(5.85 and 5.88 t ha-1in 2018 and 2019,respectively)was much higher (Table IV). The highest GY (7.74 and 7.24 t ha-1in 2018 and 2019,respectively)was obtained in treatment T8 with 50%P fertilizer.The highest recorded GY was 50.58%and 35.64%in 2018 and 2019,respectively,in terms of percentage increase over the control.Promising GYs were also obtained in individual PSB treatments and consortium T8 without phosphate fertilizer over the absolute control, which were at par with the yield obtained with 100%of the recommended phosphate fertilizer dose for the cropping years.Biological yield was the highest(16.14 and 15.71 t ha-1)with T8 treatment in combination with 50%P fertilizer dose with an increase of 20.75%and 16.38%in 2018 and 2019,respectively,compared to the uninoculated control.
The highest HI was recorded in T8 with a 50%P application in 2018 and 2019(47.94%and 46.10%,respectively)(Table IV). In T8 alone, good HI values of 43.02% and 43.78%were observed in 2018 and 2019,respectively,which were higher than those achieved by the 100%recommended P fertilizer dose with no PSB.Phosphorus uptake in grains was significantly(P <0.01)and positively correlated with GY and HI in both 2018 and 2019 as illustrated in Tables V and VI.
PUE,ARE,and AE
In all PSB-treated plants,PUE increased significantly compared to the uninoculated control(Table VII).The most effective treatment in terms of P utilization was T8 in combination with 50% fertilizer with an increase of 50.59%and 35.64% in 2018 and 2019, respectively. Phosphorus use efficiency was significantly(P <0.01)and positively correlated with GY and HI in 2018 and 2019(Tables V and VI).
The ARE improved significantly in the PSB-treated plants with 50%P fertilizer rate,as compared to the uninoculated controls(Table VII).The highest increases in ARE were 41.94% and 35.00%, obtained in T8 with 50% P fertilizer dose,as compared to the respective controls,with recorded values of 0.88 and 0.81 in 2018 and 2019, respectively. With higher phosphate fertilization doses, the ARE was reduced significantly in all PSB-treated plants as compared to their the uninoculated control in 2018 and 2019.Fluctuation in AE was observed in the PSB treatments with different P doses.Agronomic efficiency was found to decline with an increase in fertilizer dosage as compared to the control.The 50%P fertilizer dose with T8 treatment was found to be optimum for high AE of plants(65.78 and 35.41 kg kg-1in 2018 and 2019,respectively).
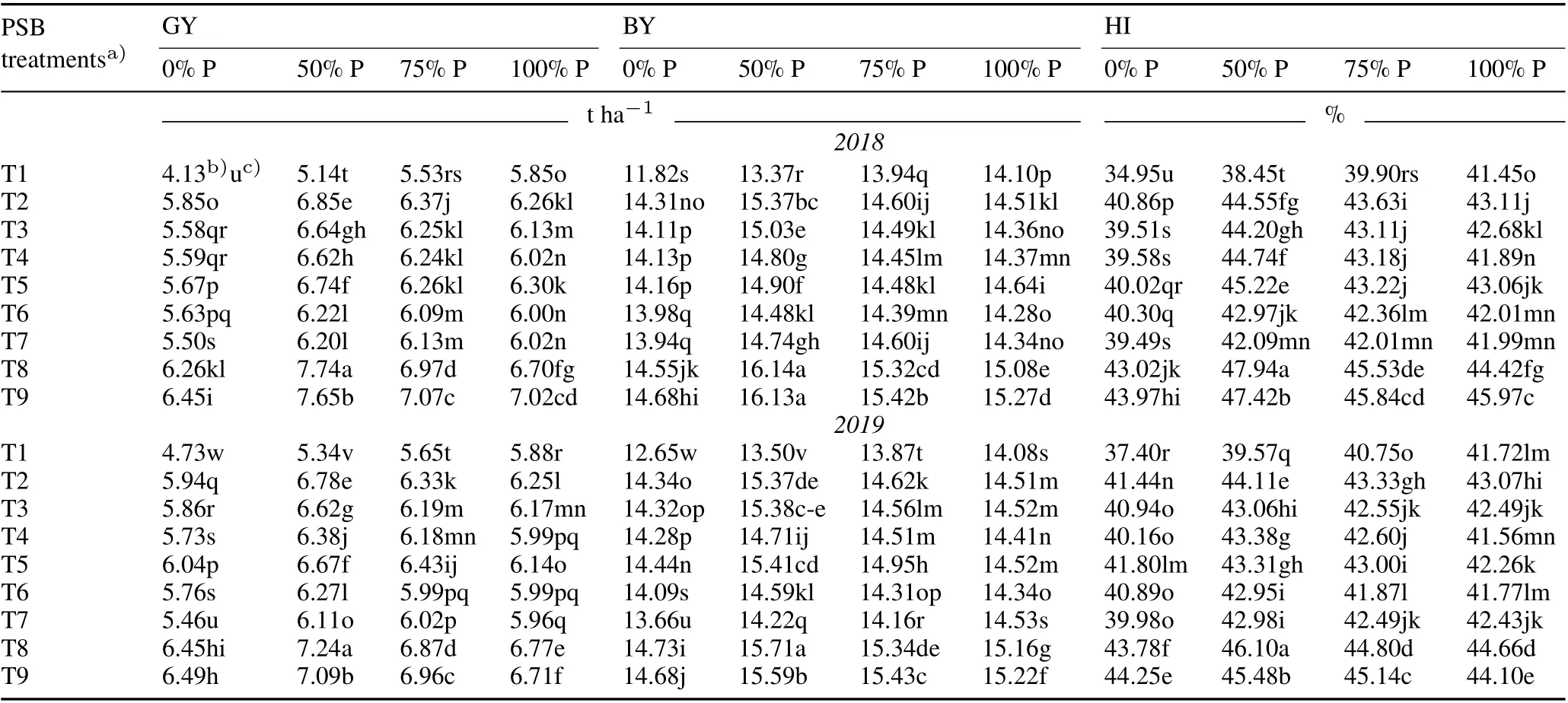
TABLE IV Effect of phosphate-solubilizing bacteria (PSB)inoculation on grain yield (GY), biological yield (BY), and harvest index (HI)of rice under various P application rates(0%,50%,75%,and 100%of the recommended dose 45 kg P2O5 ha-1)during the kharif season(June–November)in 2018 and 2019 in the field experiment conducted in the Tarai plains,India

TABLE V Pearson’s correlation coefficients between rice P uptake(Pu), P use efficiency(PUE),apparent recovery efficiency(ARE),agronomic efficiency(AE),biological yield(BY),grain yield(GY),and harvest index(HI)and soil available P content(AP),acid phosphatase activity(ACP),alkaline phosphatase activity(ALP),dehydrogenase activity(DHG),and microbial count(MC)in 2018 for the field experiment conducted in the Tarai plains,India
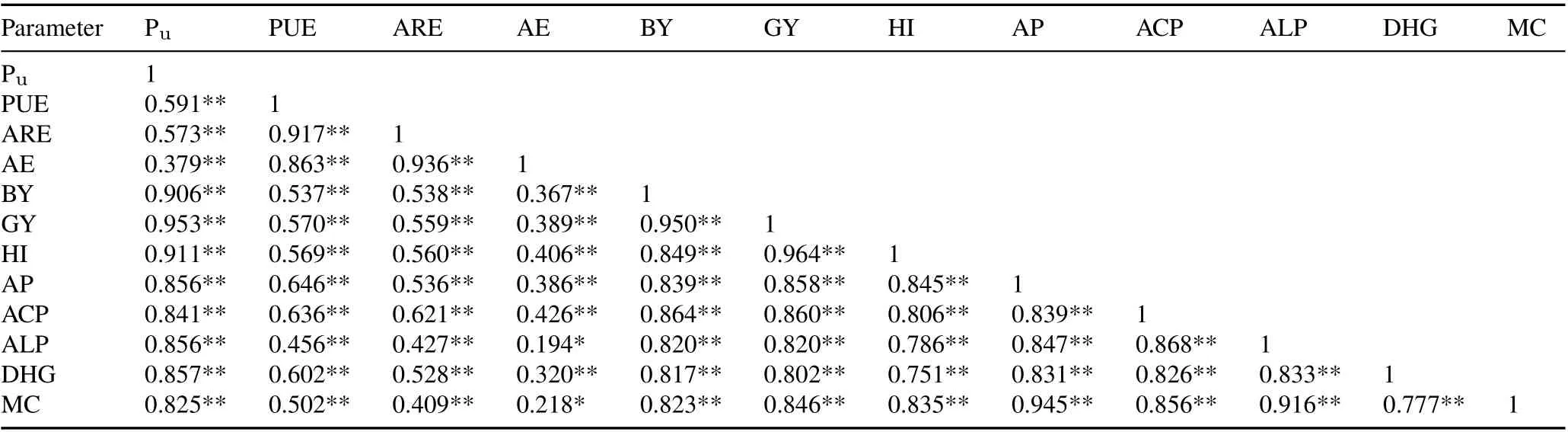
TABLE VI Pearson’s correlation coefficients between rice P uptake(Pu),P use efficiency(PUE),apparent recovery efficiency(ARE),agronomic efficiency(AE),rice biological yield(BY),grain yield(GY),and harvest index(HI)and soil available P content(AP),acid phosphatase activity(ACP),alkaline phosphatase activity(ALP),dehydrogenase activity(DHG),and microbial count(MC)in 2019 for the field experiment conducted in the Tarai plains,India
Soil enzyme activity,microbial count,and soil available P content
The PSB treatments significantly enhanced soil dehydrogenase activity at the flowering stage of rice as compared to the control soil under both P-fertilized and non-fertilized treatments and in both cropping years(Fig.3).The highest soil dehydrogenase activity was recorded for T8 with 50%P fertilizer dose,with an increment of 243.43%and 240.75%in 2018 and 2019,respectively.Maximum activities of soil acid and alkaline phosphatases at the flowering stage were also recorded for T8 with a 50%P fertilizer rate,with significant increases of 48.15%and 49.71%, respectively, in 2018 and 48.95%and 50.52%, respectively, in 2019 over 50%P fertilizer alone.
Soil microbial count at the flowering stage of rice in both cropping years was significantly higher in the PSB treatments(Table VIII).The maximum microbial count was recorded in T8 with a 50%recommended P dose,which was 50.68%and 47.49%higher than the respective controls in 2018 and 2019,respectively.Before sowing,soil available P stock was 17.5 and 18.0 kg ha-1,whereas after harvest,soil available P content in the control was 14.12 and 14.96kg ha-1(23.93%and 20.32%lower,respectively)in 2018 and 2019,respectively.The highest soil available P(54 kg ha-1)was recorded in T8 with 50%recommended P.Significant correlations were observed between soil available P content,microbial count,and soil enzyme activities(Tables V and VI).
DISCUSSION
Bacterial strain identification and P-solubilizing potential of the isolates
Among the bacterial strains isolated,B.licheniformis,P.dispersa,andStaphylococcussp.were able to solubilize P in solid media,as indicated by the halozone formation and solubilization index.In quantitative estimation,the isolates solubilized more P than the standard strain on the fourth day of inoculation.This might be ascribed to the higher activity of P solubilization by the bacterial isolates,which may correlate with the nutritional status of native soil from which they wereisolated and higher organic acid production, as indicated by the pH changes in the media.In addition,pH decrease indicates acid production by PSB for the dissolution of P in the media(Vyas and Gulati,2009).Our results are similar to those of previous studies reporting the P solubilizing potential and growth-promoting effects ofBacillussp. in chickpea,wheat,and spinach(Dittaet al.,2018),Pantoeasp.in rice(Bhise and Dhandge,2019),andStaphylococcussp.in maize(Akramet al.,2016).
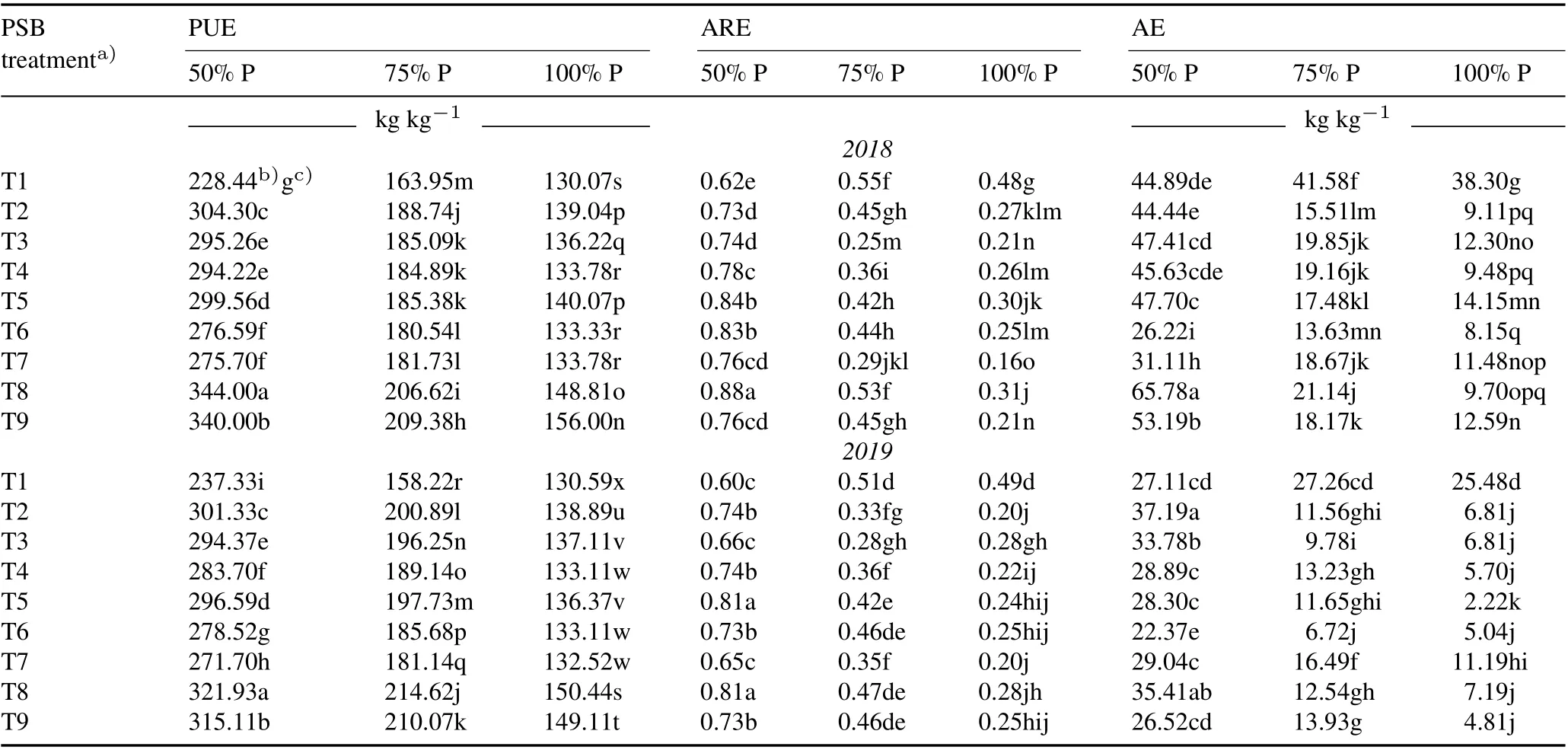
TABLE VII Effect of phosphate-solubilizing bacteria(PSB)inoculation on rice P use efficiency(PUE),apparent recovery efficiency(ARE),and agronomic efficiency(AE)under various P application rates(50%,75%,and 100%of the recommended dose 45 kg P2O5 ha-1)during the kharif season(June–November)in 2018 and 2019 in the field experiment conducted in the Tarai plains,India
Effects of PSB and P fertilizer on P uptake in grain and plant tissues and yield attributes
Our results demonstrate that the isolated PSB alone or their consortia in combination with the optimum level of P fertilizer (50% of the recommended dose) could be an effective strategy to increase P uptake in plants.The overall increase in P uptake in grain and vegetal tissues may be attributed to the more plant accessible P from supplemented P fertilizer and native P from soil by PSB inoculation.The solubilization of P was enhanced through organic acid and enzyme production,chelation,and exchange reactions(Sulemanet al., 2018), as well as higher biomass production,as reported by Paratey and Wani(2005).In all PSB-treated plants,P uptake in the stems and leaves was higher at the flowering stage than at the maturity stage of rice.Accumulation of high P in vegetative tissues is required in rice for the remobilization of P from aerial parts to the panicles for grain filling(Roseet al.,2010b).Therefore,high P is a prerequisite at the developing stage,which might result in high P content and uptake in the grains as compared to the stems and leaves at maturity. A significant increase in P uptake after PSB treatment and in combination with P fertilizer is an indicator of bioefficacy of the PSB,which increases the soil P content and makes P available for plant uptake(Hussainet al.,2019).
It is apparent from the yield data of 2018 and 2019 that PSB alone,or in combination with P fertilizer,significantly enhanced BY and GY compared to the control.The increase in GY might be due to enhanced P uptake by plants with increased plant growth due to the growth-promoting effect of PSB(Baiget al.,2012).Significant correlations between rice GY,P uptake,BY,and PUE and soil available P were found, supporting our results. However, few studies have been conducted to test the microbial efficacy for upland rice systems.In our study,a comparative yield was also obtained by PSB treatment alone as PSB improved P nutrition in the grains through the production of organic acids(data not shown)and phosphatase in the rhizosphere.The organic acids and phosphatase aid in the solubilization of soil unavailable P and enhance P uptake by plants for proper growth and grain development,thereby reducing the fertilizer input to the soil by 50%.The PSB inoculation can provide almost 50%of P required in upland rice systems,thereby lowering P fertilizer application rate.Nutrient imbalance,low P uptake,and lower PUE at higher P doses might be the reasons for the decline in PSB performance with 75%and 100%P fertilizer rates.Our results are in line with the findings of Bakhshandehet al. (2015), who reported a significant increase in GY and BY by 8.50%–26.9%and 12.4%–30.9%,respectively,in rice inoculated with diverse PSB strains alone, and a 67%decrease in fertilizer input when PSB inoculation was combined with P fertilizer application.
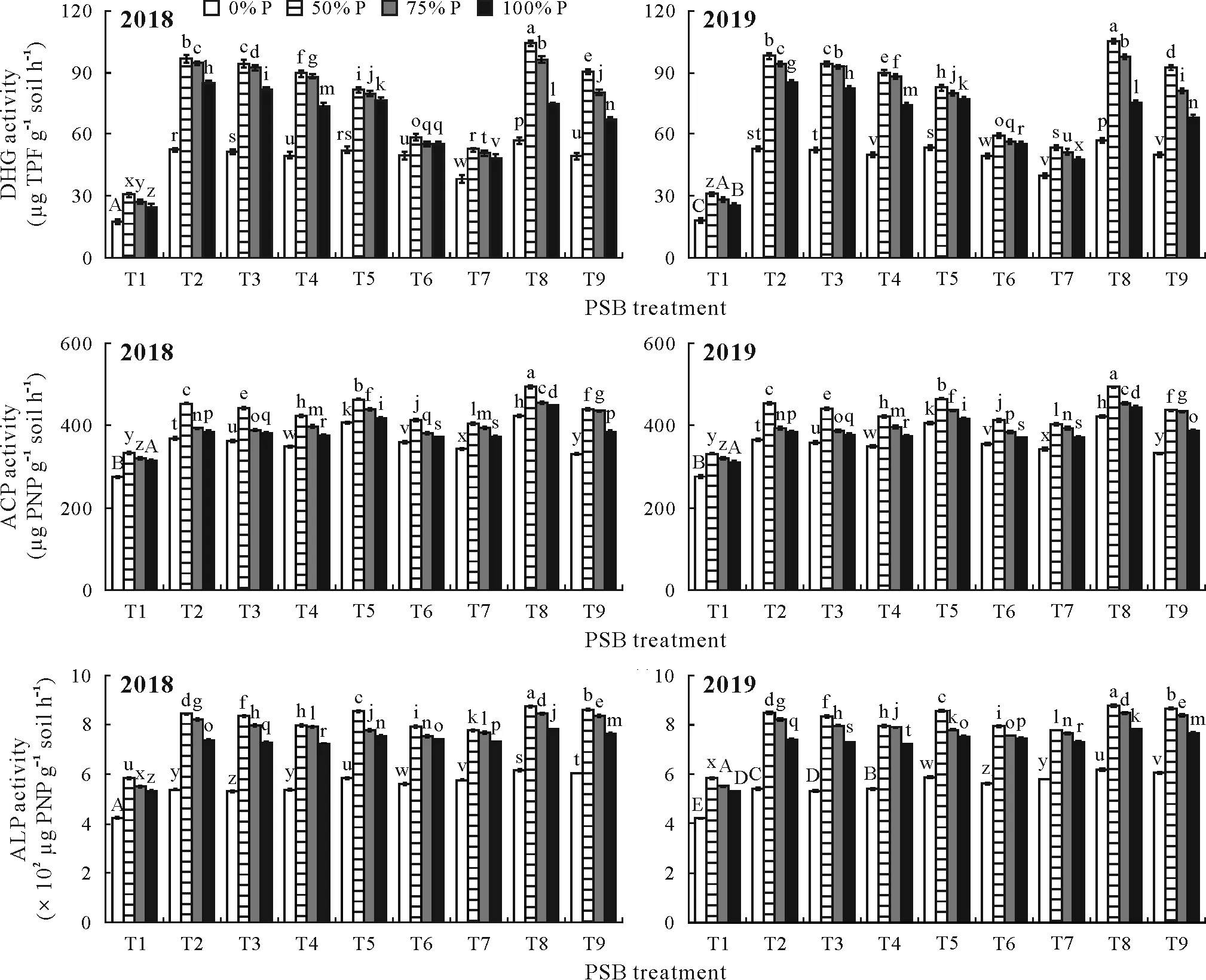
Fig. 3 Effect of phosphate-solubilizing bacteria (PSB) inoculation on the activities of dehydrogenase (DHG), acid phosphatase (ACP), and alkaline phosphatase(ALP)in soil at the flowering stage of rice under different P application rates(0%,50%,75%,and 100%of the recommended dose 45 kg P2O5 ha-1)during the kharif season(June–November)in 2018 and 2019 in the field experiment conducted in the Tarai plains,India.Bars are standard errors of the means(n=3).Different letters above the error bars indicate significant differences between the means based on Duncan’s multiple range test(P <0.05).T1=uninoculated control;T2=Bacillus licheniformis(PSB1);T3=Pantoea dispersa(PSB2);T4=Staphylococcus sp.(PSB3);T5=PSB1+PSB2;T6=PSB1+PSB3;T7=PSB2+PSB3;T8=PSB1+PSB2+PSB3;T9=standard PSB strain Pseudomonas putida.TPF=triphenylformazon;PNP=p-nitrophenol.
Total dry matter showed a similar trend to that of GY.Inoculation of PSB combined with chemical fertilizer application improved BY, possibly due to the production of growth-promoting hormones such as auxins and improvement of PUE by PSB(Sharonet al.,2016).Harvest index significantly correlated with BY and GY and showed a similar trend to that of GY.
Effects of PSB and P fertilization rates on P-related efficiencies
In both years, PUE was the highest for low fertilizer dose treatments along with PSB.Compared to the control,PSB treatment significantly improved PUE irrespective of fertilizer application rate.Our results reveal that PSB supplemented with a low rate of P fertilizer is better for high P efficiency.A decline in PUE with high fertilization rates can be attributed to the low uptake of applied P by the crop,as reported by Zhuet al. (2018). Moreover, a significant correlation between P uptake and GY confirms that GY also declines with a higher P dose due to low P uptake and nutritional imbalance(Zhuet al.,2012),which ultimately decreases PUE.Moreover,a higher dose of P fertilizer results in salt toxicity in the soil, which might account forthe lower nutrient uptake (Abd El Aziz, 2007). However,a significant improvement in PUE was obtained with PSB treatment supplemented with chemical fertilizer, possibly due to higher P uptake by the PSB, which was positively correlated with PUE(Nosratabadet al.,2017).
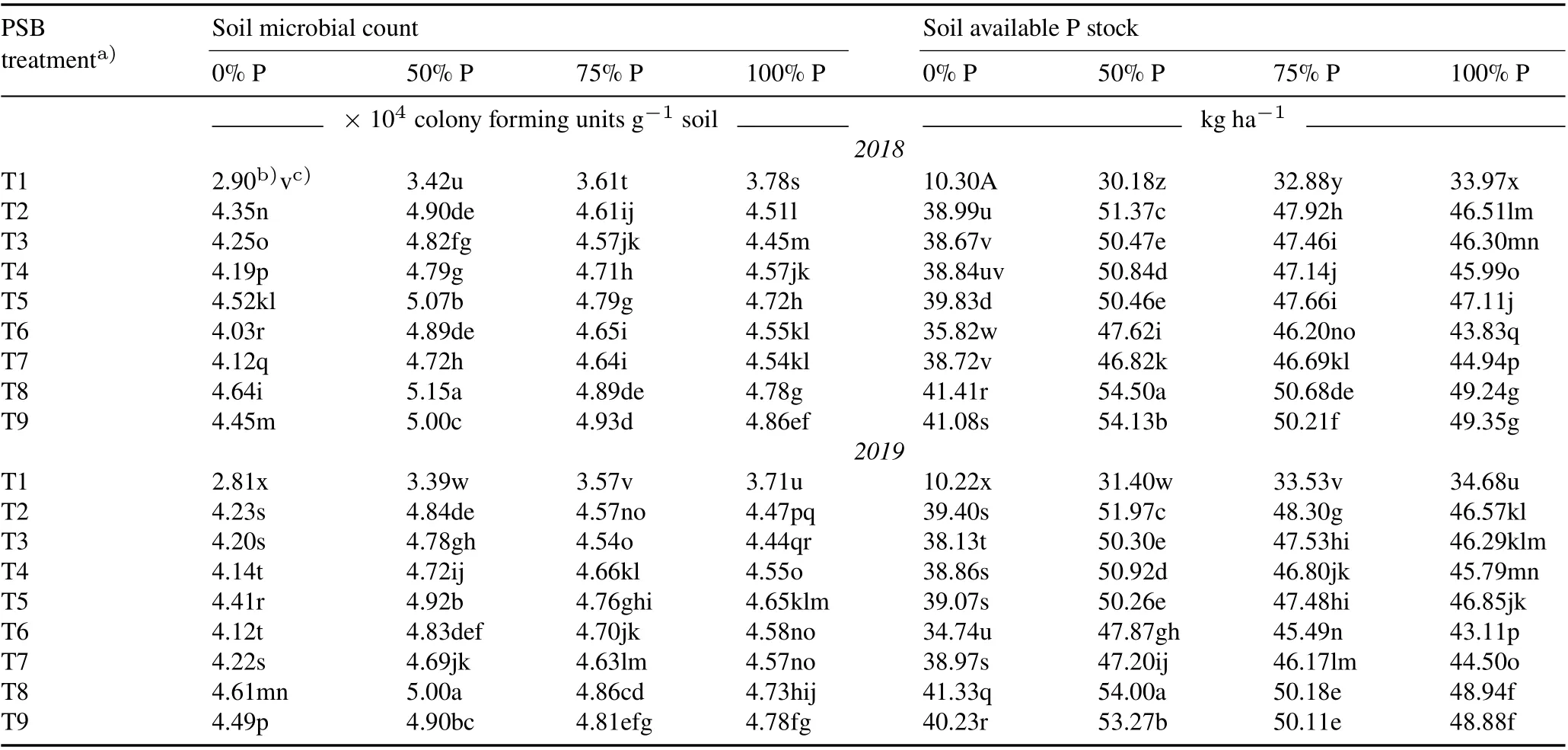
TABLE VIII Effect of phosphate-solubilizing bacteria (PSB) inoculation on soil microbial count and soil available P at the flowering stage of rice under various P application rates(0%,50%,75%,and 100%of the recommended dose 45 kg P2O5 ha-1)during the kharif season(June–November)in 2018 and 2019 in the field experiment conducted in the Tarai plains,India
A similar trend was seen in agronomic and apparent recovery efficiencies. Maximum values for both of these indices were obtained with PSB treatment plus a low P dose(50%recommended P)as compared to the respective controls. With high P fertilizer doses (75% and 100% of the recommended P), a steady decline in both agronomic and recovery efficiencies was observed as compared to lower fertilizer doses,irrespective of PSB treatment.Similar results were obtained by Venkateshet al.(2019)who reported a decline in agronomic and recovery efficiencies with increasing P fertilizer rate.This may be due to the inefficient utilization of P at high fertilizer doses during grain production. The higher capacity of plants to utilize the inherent P for grain and biomass production under P deficit conditions could also be the reason for high P indices at low fertilizer dosages.
Soil enzyme activities and available P content
Soil phosphatases and dehydrogenases are biological markers that represent microbial activity in the soil.Phosphatases are the key enzymes for the solubilization of organic P in soil(Sharmaet al.,2013).Both acid and alkaline phosphatases in soil increased significantly with PSB and P fertilizer treatment as compared to the absolute control at the flowering stage in both cropping seasons.This can be related to the high PSB population in inoculated treatments(Renellaet al., 2007). Inoculation of PSB combined with low rate of P fertilizer application(50%of the recommended dose)might have stimulated the synthesis of more acid and alkaline phosphatases to meet the P demand of plant and soil microbial populations(Roldanet al.,1997).Moreover,more P demand for plants at the flowering stage might be one of the factors for high phosphatase activity in soil.
Dehydrogenases are present in all microbial cells and oxidize soil organic matter,indicating soil metabolic activity. An increase in soil dehydrogenase activity through the application of biofertilizers indicates a higher microbial population in soil compared to chemical fertilizer application(Kauret al.,2017).In our study,using PSB plus fertilizer enhanced the soil dehydrogenase activity in the rice system.Improvement in soil enzymatic activity contributed to the efficient utilization of nutrients already present in soil,from the applied fertilizer,and from the organic matter present in soil by the soil microbes,which resulted in the proliferation of soil microbes.Furthermore,a significant correlation between soil microbial population and soil enzyme activity in this study is in accordance with the findings of Stephenet al.(2015) who reported that the application of biofertilizers improved soil microbial activity.
Soil microbial count was found to be maximum in the PSB treatment with 50%P fertilizer. Soil available P and biofertilizer application in seeds,soil,and roots are factors that affect microbial abundance in soil (Kumar and Rai,2020).Thus,P fertilizer is an active metabolic substrate for soil microorganisms,and its addition to soil activates soil microbial activity and growth(Bhattet al.,2016).
Soil P content in the absolute control soil was lower after rice harvest than before sowing.This can be ascribed to the absorption of P by plants to satisfy their metabolic needs during the developmental stages(Yanget al.,2016).Moreover,the high soil P content in PSB-treated plots with P fertilizer application might be due to the high PSB counts and enzymatic activities in soil, which resulted in soil P mineralization,as indicated by the significant correlations between microbial population,available P,and soil enzyme activities in our study(Shiet al.,2017).
To the best of our knowledge,our study is the first attempt to explore the P solubilizing and plant growth-promoting potential of bacteria belonging to the generaPantoea,Bacillus,andStaphylococcus,bothin vitroand under field conditions,for two consecutive years in an upland rice system.We reported significant increases in P uptake,PUE,GY,biomass production, soil P content, and soil microbial population from PSB inoculation,either individually or in consortium,in comparison to both the absolute and respective controls of the treatment.It was observed in this study that a much lower P fertilizer application(50%of recommended rate)combined with PSB treatment did not compromise the grain productivity in the rice system.The decline in GY and its attributes at 75%and 100%of the recommended P fertilizer dose might be due to salt toxicity and nutrient imbalance in soil,which ultimately led to a decline in P content,growth,and development of the crop.
CONCLUSIONS
This study highlighted the application of PSB strains isolated,B.licheniformis,P.dispersa,andStaphylococcussp.,as biofertilizers for increasing P uptake,biomass development,and P-related efficiencies in upland rice.Our results demonstrated the field implementation of individual PSB and their consortia that could substantially curtailed 50%of the recommended P fertilizer dose and improved rice GY,as compared to the uninoculated control.The GY obtained with PSB treatment without any fertilizer application was also comparable to the yield obtained with the combined use of PSB and 50%P dose.Furthermore,PSB with 50%P fertilizer significantly enhanced the soil available P status and soil metabolic activity compared to uninoculated control soil.In the present global scenario,where sustainable crop production and food security are major priorities,this agroecological practice could be a step towards a sustainable agricultural system that cuts food production costs and environmental hazards.
ACKNOWLEDGEMENTS
The authors are thankful to the Department of Plant Physiology,Govind Ballabh Pant University of Agriculture and Technology,Pantnagar,India for the materials and equipments used in this research,and for the Ph.D.scholarship provided to the first author.The authors also acknowledge the support provided by the All India Co-ordinated Research Project(AICRP),Indian Council of Agricultural Research(ICAR),New Delhi,India during the field study.
杂志排行
Pedosphere的其它文章
- Effect of lignite amendment on carbon and nitrogen mineralization from raw and composted manure during incubation with soil
- Magnesium-fortified phosphate fertilizers improve nutrient uptake and plant growth without reducing phosphorus availability
- A highly effective polycyclic aromatic hydrocarbon-degrading bacterium,Paracoccus sp.HPD-2,shows opposite remediation potential in two soil types
- Recycling of isabgol(Plantago ovata Forsk.)straw biomass and mineral powder with bio-inoculants as an effective soil amendment for isabgol cultivation
- Enduring legacy of coal mining on the fungal community in a High Arctic soil after five decades
- Elevated atmospheric CO2 reduces CH4 and N2O emissions under two contrasting rice cultivars from a subtropical paddy field in China
