Tuning the reactivity of Al-Ni by fine coating of halogen-containing energetic composites
2022-10-17SulnYngKejunMengWuxiXieHongqiNieQiLongYn
Su-ln Yng ,Ke-jun Meng ,Wu-xi Xie ,Hong-qi Nie ,Qi-Long Yn ,*
a Science and Technology on Combustion,Internal Flow and Thermo-Structure Laboratory,Northwestern Polytechnical University,Xi'an,710072,China
b Xi'an Modern Chemistry Research Institute,Xi'an,710065,China
Keywords:Arrested high energy ball milling Intermetallic alloy Combustion performance Energetic composites Reactivity control
ABSTRACT In this paper,various core-shell structured Al-Ni@ECs composites have been prepared by a spray-drying technique.The involved ECs refer to the energetic composites (ECs) of ammonium perchlorate/nitrocellulose(AP/NC,NA)and polyvinylidene fluoride/hexanitrohexaazaisowurtzitane(PVDF/CL-20,PC).Two Al-Ni mixtures were prepared at atomic ratios of 1:1 and 1:3 and named as Al/Ni and Al/3Ni,respectively.The thermal reactivity and combustion behaviors of Al-Ni@ECs composites have been comprehensively investigated.Results showed that the reactivity and combustion performance of Al-Ni could be enhanced by introducing both NA and PC energetic composites.Among which the Al/Ni@NA composite exhibited higher reactivity and improved combustion performance.The measured flame propagation rate (v =20.6 mm/s),average combustion wave temperature (Tmax =1567.0 °C) and maximum temperature rise rate (γt =1633.6 °C/s) of Al/Ni@NA are higher than that of the Al/Ni (v =15.8 mm/s,Tmax =858.0 °C,and γt =143.5 °C/s).The enhancement in combustion properties could be due to presence of the acidic gaseous products from ECs,which could etch the Al2O3 shell on the surface of Al particles,and make the inner active Al to be easier transported,so that an intimate and faster intermetallic reaction between Al and Ni would be realized.Furthermore,the morphologies and chemical compositions of the condensed combustion products (CCPs) of Al-Ni@ECs composites were found to be different depending on the types of ECs.The compositions of CCPs are dominated with the Al-Ni intermetallics,combining with a trace amount of Al5O6N and Al2O3.
1.Introduction
Al-Ni reactive materials,including composite particles and multilayered foils,are a class of energetic materials (EMs) with high-energy content [1-3].These materials can undergo intermetallic reaction with a significant amount of heat release and forming composites with high mechanical strength [4,5].Due to this promising feature,Al-Ni reactive materials have been widely used in various energetic applications such as reactive fragments for warhead[6],reactive shaped charge liner[7],insensitive penetrator based on nano-structured EMs[8,9],However,Al-Ni mixtures are not able to be readily ignited especially when their sizes are in micron scale.The relatively low exothermicity of reactions between metallic reactants may results in a high energy barrier for reliable ignition.In addition,the naturally formed oxide shell on the surface of Al particles is likely to lead to the lowered reactivity between the Al and Ni,thereby limiting its broader application[10-12].In order to improve the ignition and energy release efficiency of Al-Ni,the novel design and relevant advanced preparation of Al-Ni materials have shown to be promising according to recent literature summarized as follows.
Various methods used for the preparation of Al-Ni reactive materials with lower ignition threshold and higher combustion performance have been proposed [13-18].For instance,Hadjiafxenti et al.produced Al-Ni nanocomposite powders by low energy ball milling (LEBM),which exhibit a lower ignition temperature below 600 K [19-21].The increased reactivity of Al-Ni nanocomposites produced by ball milling is likely due to the fact that the nano-Ni was embedded in Al matrix without formation of oxide barriers or intermediate layers [1,21,22].Except for composite particles,Al-Ni foils with varied bilayer thicknesses could be fabricated at different atomic ratios by using sputtering method.The onset temperatures of those multilayered foils are below 800 K,higher than its nanoparticle style [23].Mukasyan et al.proposed that the combustion wave in the Ni/Al nano-foil appears to be a sequential two-stage process,which involves the chemical and physical exothermic transformations [24].Gunduz et al.reported another two-stage reaction in their experimental and modeling work,which includes the flame front propagates near the reverse peritectic transformation temperature of NiAlinto NiAl and melts at 1406 K.The reaction continues with the growth of NiAl until the melting temperature of 1911 K [25-27].The above-mentioned preparation methods may provide Al and Ni with more intimate and high surface area contacts,which are critical to the selfpropagation combustion of the solid-state reactions.In this way,reactions that normally require high heat input for initiation can be realized at lower temperatures.
In addition to the preparation methods that were used to reduce ignition threshold and improve the combustion performance of Al-Ni by increasing the intimate contact,the additives can also be used to enhance the reactivity of Al-Ni by accelerating of the reaction rate and reducing the agglomeration.Researchers have managed to use metallic additives to prepare Al-Ni/M (M: molybdenum,copper and magnesium).The initial temperatures of Al-Ni were found to be increased to various levels depending on the type of metals used.Among them,Cu shows the most significant effect on the combustion process of Al-Ni with a remarkable increase in the flame temperature from 2000 K to 3000 K[28].For fluoropolymers,the addition of PTFE to Al-Ni may decrease the critical shock pressure for initiation of shock-induced chemical reaction,due to a lowered the apparent activation energy and increased the chemical reaction efficiency of Al-Ni in the presence of fluorine as a highly oxidative element.In particular,with the addition of PTFE,the pre-ignition reaction (PIR) occurs between AlOand PTFE,so that the heat release from PIR reaction plays a positive role in the promotion of intermetallic reaction between Al and Ni[11].Besides,the agglomeration was greatly reduced due to generation of gaseous product (AlF) during PIR,so that the reaction efficiency may also be enhanced.In addition to PTFE,other types of fluorine-containing polymers could also react with AlOpassivation layer that facilitates the exposure of the active Al,thereby promoting the reactivity[29-31].
Besides fluoropolymers,the same effect can be observed for the transition metals used as coating agents.A thin Ni coating layer on the surface of Al particle was shown that the agglomeration of the CCPs would be prevented.In this way,the ignition temperature of Al was reduced to 760-950 K and the front velocity was increased by a factor of 4 as compared to the unmodified ones [2,32].The ignition mechanism was found to be correlated directly with intermetallic exothermic reactions between Al and Ni [2,33,34].It was explained that the improvement in combustion performance is a result of the cracking AlOshell due to thermal stress that promotes ignition of Al.In order to further improve the combustion performances of Al-Ni,it would be desirable if the coating agents can react with the passivation layer of Al and forming gaseous products.Except for fluoropolymer,the coating of halogencontaining EMs on the surface of Al-Ni has the great potential to meet both requirements.
It has been shown that coating modification with halogencontaining oxidants may lead to a significant enhancement of ignition and combustion by improving the reactivity of Al[35-38].In addition,the coating technique has a certain positive effect on improvement of the mechanical properties of Al-based composites,which is,however,not in the scope of this study.Our group have conducted various investigations on the coating of Si with optimized ECs.As typical examples,the Si@PVDF/CL-20 (PVDF/CL-20 with the mass ratio of 1:6),Si@AP/NC,(AP/NC with the mass ratio of 2:1)composites with a core-shell structure have been successfully synthesized by using spray-drying technology [39,40].The results showed that Si@ECs can undergo a more complete reaction between Si and the decomposition products of ECs during the combustion process.The ECs have relatively lower ignition threshold,higher reactivity and better stability.Those ECs could be an appropriate candidate for enhancing the combustion performance of Al-Ni.Therefore,it is of great interest to investigate the effect of energetic composites AP/NC and PVDF/CL-20 on the combustion performance of Al-Ni.
In this work,the Al-Ni@ECs at various Al-Ni atomic ratios have been prepared by arrested high energy ball milling (AHEBM) followed with spray-drying technique.The morphologies and compositions of prepared composites and the CCPs were characterized by scanning electron microscopy (SEM) and X-ray diffraction technique,respectively.The thermal reactivity and combustion performances of Al-Ni@ECs composites were evaluated by thermal analyses and customized combustion diagnostic system.The thermodynamic calculation of the full-range chemical equilibrium of the Al-Ni@ECs was conducted by HSC software as a supporting information to elucidate the mechanisms of the enhanced combustion.
2.Experimental
2.1.Raw materials
The micro-spherical aluminum powder (μ-Al) with an average diameter of 1 μm,nano-spherical nickel particles (-Ni) with a mean diameter of 100 nm,acetone,and dimethylformamide(DMF)were purchased from Sigma-Aldrich company.NC with the nitrogen content of 12.6 wt%,AP (≥99.5%),CL-20 (≥99.5%),PVDF(≥99.9%) were supplied by Xi’an Modern Chemistry Research Institute.
2.2.Preparation methods
In order to obtain the maximized energy output,the ECs including AP/NC(NA)and PVDF/CL-20(PC)were optimized at mass ratio of 2:1 and 1:6,respectively.The maximum energy release of NA and PC were 5919 J/g and 3536 J/g,respectively[39,40].Coating of ECs on Al-Ni particles were prepared by spray-drying technique.For the practical applications,it is beneficial to use the least mass content of ECs in the reaction mixture,which is capable of yielding a sufficiently low ignition temperature and initiating the rapid selfpropagating combustion.To achieve a thermo-chemical activation mode,the smallest quantity of ECs used for the activation of Al-Ni in this system that was experimentally obtained is 10 wt%.The process for preparation of Al-Ni@ECs composites is illustrated in Fig.1.
Ball milling time is the major controlled factor variable in preparation of Al/Ni and Al/3Ni composites at molar ratios of 1:1 and 1:3,respectively.It has been shown that the heat release of Al/Ni starts to decrease when the milling time increases up to 2 h,whereas it is 6 h for Al/3Ni(more details are shown in Section 3.1).Therefore,the milling times used for preparation of Al/Ni and Al/3Ni are fixed to be 2 h and 6 h,respectively.The preparation of Al/3Ni reactive composite is briefly introduced as follows:taking Al/Ni as an example,3.15 g Al and 6.85 g Ni powders are milled in a 250 mL stainless steel jar by using a planetary ball milling facility(XQM-2-DW,China) for 2 h.The rotation speed was 300 rpm,and the diameter of stainless steel ball is 5 mm.The mass ratio of ball to powder was 10:1.In this process,20 mL mixed solution of DMF and acetone with the volume ratio of 4:1 was used as the processing media.It is also the case for preparation of Al/3Ni,but the only difference is that the milling time was increased to 6 h.

Fig.1.Schematic illustration for the preparation process of Al-Ni@ECs composites by ball-milling followed with spray-drying technique.
Afterwards,10 wt%of ECs as the coating agents are introduced to two typical Al-Ni composites as shown in Table 1.The uniformly ball milled Al-Ni reactive materials are firstly collected into a beaker,and then 10 wt% of ECs with 50 mL mixed solution are added.The resulted precursor solution was stirred for 2 h to ensure sufficient dispersion of the Al-Ni powders.Finally,the precursor solution was spray-dried to obtain the final composite coated products.The parameters for the spray-drying process are as follows:diameter of feed well is 1 mm,and fluid flow rate is 3 mL/min.The inlet and outlet temperature are kept at 170C and 110C,respectively.
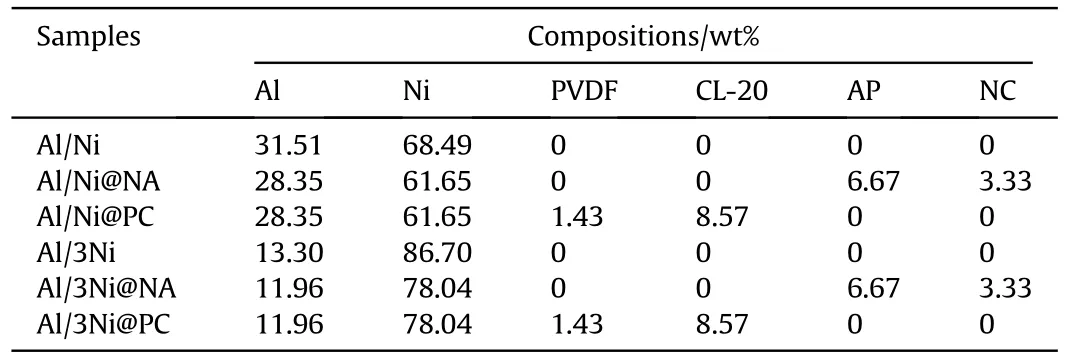
Table 1 The compositions of Al-Ni and Al-Ni@ECs composites.
The spray-dried powders are enclosed in a cylindrical mould with the internal dimension Ф 10×45 mmand uni-axially pressed at a pressure of 2 MPa for 5 min to make a dense sample.It was then placed in the sealed chamber with pressurized Ar for the combustion diagnosis.The CCPs were collected from DSC experiments and then characterized in terms of phase compositions and morphologies by using XRD and SEM techniques.The details of such characterizations are provided in the Supporting Information.
3.Results and discussion
3.1.The dependence of Al-Ni reactivity on milling time
The DSC experiments for all involved Al-Ni composites with milling time have been conducted,and the resulted heat flow curves as a function of temperature are shown in Fig.2.
As shown in Fig.2(a),there are two exothermic peaks at 579.9C and 618.1C for Al/Ni composite,when it was prepared by ballmilling of 0.5 h.However,the second exothermic peak disappeared when the ball milling time was in the range of 1-6 h.Interestingly,the second exothermic peak appears again for the composite after ball milling of 9 h.Moreover,the peak temperature of the exotherm for Al/Ni composite decreases from 579.9 to 562.6C as the ball milling time increases.However,when the milling time further increases (e.g.to 9 h),the exothermic peak shifts to a higher temperature,and it is also the case for Al/3Ni (shown in Fig.2(b)).
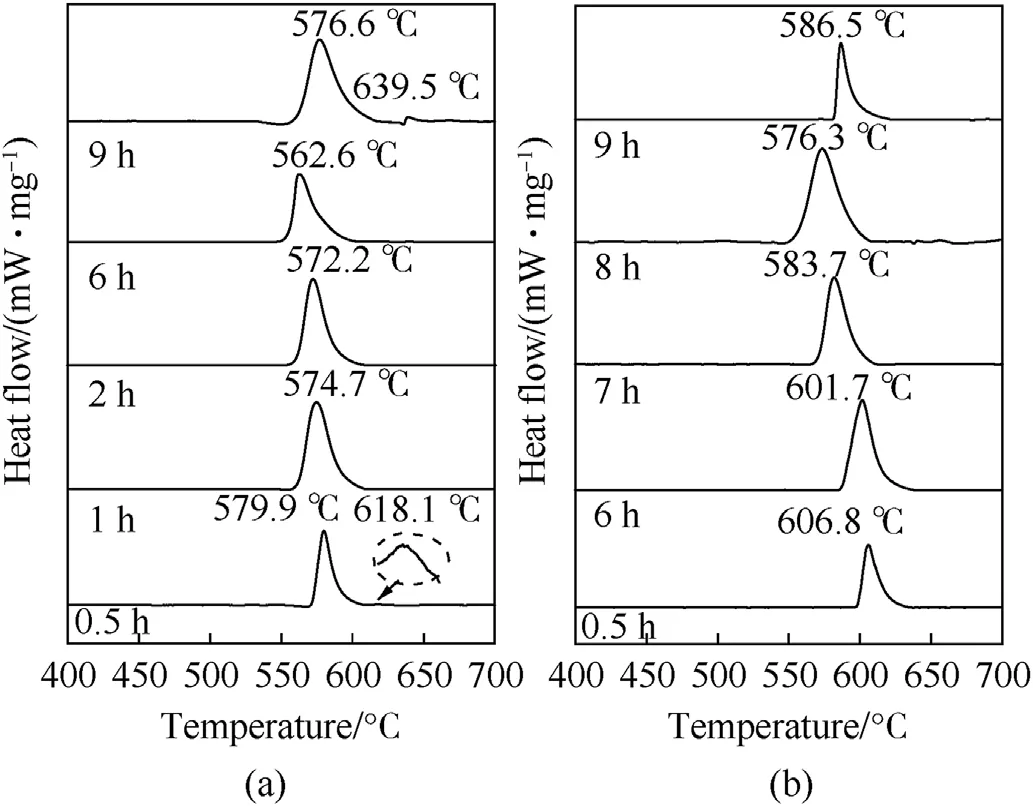
Fig.2.The DSC curves for Al/Ni and Al/3Ni samples obtained by change of ball-milling time: (a) Al/Ni;(b) Al/3Ni.
The detailed DSC parameters obtained for Al/Ni and Al/3Ni composites are summarized in Table 2.It can be noticed that the measured heat flows () increase first and then decrease with the increase of ball-milling time for both composites.There is an appropriate milling time,when the Al and Ni is homogeneously mixed and well contacted,so that the initial reaction temperature reach the lowest point.It also suggests that excessive ball milling leads to the partial intermetallic reaction between Al and Ni,so that the reactivity would be decreased and the measured heat of reaction becomes lower.According to the heat of reaction,the appropriate ball-milling time for Al/Ni should be about 2 h(e.g.832.0 J/g).In comparison,the best ball-milling time for Al/3Ni could be around6 h,where the maximum heat release was 598.8 J/g,lower than that of Al/Ni.

Table 2 The DSC parameters for Al/Ni and Al/3Ni prepared by ball-milling for different time.
3.2.Morphologies of optimized EC coated Al-Ni composites
The morphologies of Al,Ni,Al-Ni and Al/Ni@ECs are shown in Fig.3.For Al and Ni as the starting materials(Fig.3(a)and Fig.3(b)),their surfaces are neat and smooth.In case of the ball-milled Al-Ni composites,the surface of Al particle (Fig.3(c) and Fig.3(d)) becomes uneven,where the-Ni particles are randomly distributed.The surfaces of Al/Ni@NA and Al/Ni@PC composites show a relatively rough morphology(Fig.3(e)and Fig.3(f)).The initial particle size of Al is about 2 μm,and it increases a little once Ni is covered.Once ECs is included,the Al/3Ni or Al/Ni seem aggregated to about 3-5 μm(Fig.3(e)and Fig.3(f)).Meantime,the surface of these large particles is defected with lots of pores,showing increased specific surface areas.

Fig.3.The SEM micrographs of (a) Al,(b) Ni,(c) Al/Ni,(d) Al/3Ni,(e) Al/Ni@NA,(f) Al/Ni@PC;and the (g),(h) EDS element mapping obtained for (e) Al/Ni@NA,(f) Al/Ni@PC.
The obtained element mapping results for Al/Ni@NA and Al/Ni@PC are shown in Fig.3(g)and Fig.3(h),where the distributions of Cl/N/O and F/N/O illustrate the AP/NC and PVDF/CL-20 are uniformly coated on the surface of assembled aggregated Al/Ni particles.
3.3.Thermal reactivity of Al-Ni@ECs composites
The DSC experiments have been performed to investigate the thermal behaviors of Al-Ni@ECs composites and the corresponding heat flow curves are plotted in Fig.4.
An endothermic peak at 661.1C for pure Al can be clearly seen in Fig.4(b),which is corresponding to the melting of Al [43].Exothermic peaks displayed at 572.2C and 601.9C are observed for Al/Ni and Al/3Ni,respectively.It reveals that ECs decomposes much earlier than the intermetallic reaction.Those two exothermic peaks are attributed to formation of AlNi and AlNi,respectively[17,19].The detailed reaction mechanisms of Al-Ni are discussed in the following Section 3.3.2.
As the coating layer of Al/Ni,the first exothermic peak of AP/NC appears approximately at 205.4C,which is surely caused by the decomposition of NC[44]and it is 7.8C lower than that of pristine AP/NC.The following exothermic peak at 303.6C is due to decomposition of AP.In presence of Al/Ni,the two decomposition peaks of AP are merged into one,and the peak temperature is 9.3C lower than that of the secondof AP.The third exothermic step associated with a small peak at 506.7C may be caused by the reaction between the acidic condensed products of AP and the AlOpassivation layer,with heat release of 116.9 J/g[45].The final exothermic process should be attributed to the intermetallic reaction between Al and Ni,which has a peak at around 573.9C,the heat release is 771.0 J/g and the condensed alloy product was confirmed by XRD spectrum(see in Section 3.3.2).The total energy release of thermit and intermetalllic reactions of Al is 887.9 J/g,which is improved by 6.7% in comparison with pure Al/Ni.A very similar exothermic reaction process is shown for Al/3Ni@NA composite,the energy release of the Al-related reaction was 860.8 J/g,which was 28.8 J/g higher than Al/Ni.Obviously,the energy release of Al-Ni was increased when it is coated with ECs,the increased energy is due to the preheating and coupling effects of ECs thermolysis.In comparison,for Al/Ni@PC,the first exothermic peak at around 237.9C can be assigned to CL-20 thermolysis,which is only 0.3C lower than that of pristine PC.The second small exothermic peak at around 418.8C was due to the pre-ignition reaction (PIR)between PVDF and AlOpassivation layer[46].It is followed by the main intermetallic exothermic reaction with a peak at 596.5C.It is also the case for Al/3Ni@PC,where the first two exothermic peaks at 233.4C and 465.4C are due to the decomposition of CL-20 and the PIR reaction,respectively.The third and last reaction steps are partially overlapped with a final peak at 628.5C,respectively.Such a two-step exothermic pattern is covered with a heat release of 507.0 J/g,which is 353.8 J/g lower than that of Al/Ni@PC.The energy release of Al-related reaction of Al/3Ni@ECs is lower than that of Al/3Ni,which maybe due to the reduced reaction rate between Al/3Ni and the condensed products of ECs,so that part of the heat release is covered by the baseline and overlooked.The above results imply that the improved reactivity of Al-Ni@ECs could be due to a synergistic effect,where Al-Ni catalyzes the decomposition of ECs and the heat release of Al-Ni is promoted in presence of condensed thermolysis products of ECs,especially when the products are acidic and could easily react with the oxide layer of Al and Ni.
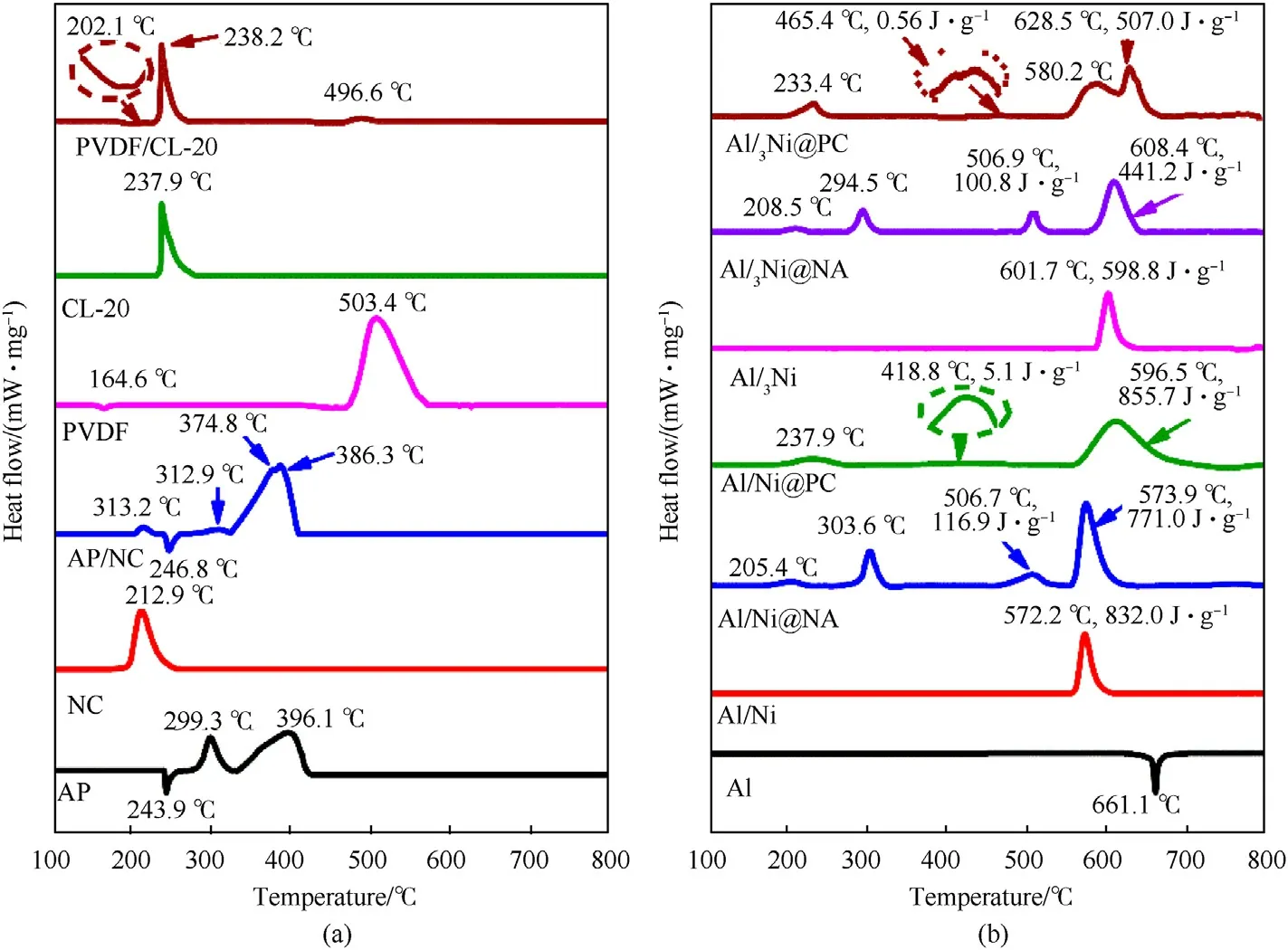
Fig.4.The DSC curves for (a) components of involved ECs and (b) the ECs coated Al-Ni composites.
In order to understand the condensed phase reaction mechanisms of Al-Ni@ECs composites,the XRD was implemented to identify all possible intermediate products quenched at different temperatures from DSC experiments.The obtained XRD spectra are shown in Fig.5.As expected,a strong diffraction peak of intermetallic compound is detected for the product collected at 800C,but the diffraction patterns are different depending on the types of ECs and atomic ratio of Al-Ni.
As shown in Fig.5(a),only the diffraction peaks of Al and Ni can be observed for the Al/Ni at room temperature.At 620C,the diffraction peaks of AlNi,AlNi,AlNi,and unreacted Al and Ni are shown together.At further elevated temperature of 800C,the diffraction peaks of unreacted Al and Ni disappear and the relative diffraction intensities of AlNi and AlNiare increased as well.Additionally,a new diffraction peak of AlNi is observed at this temperature.The results indicate that the intermetallic exothermic reactions between Al and Ni occurs in between 620C and 800C for Al/Ni.
For Al/Ni@NA (Fig.5(a) and Fig.S1),the diffraction peaks of Al,Ni,and AP can be clearly seen in the XRD pattern at room temperature.At 520C,only the diffractions of Al and Ni are observed.It indicates that AP completely decomposed at this temperature,which is consistent with the DSC results.At 800C,the diffraction peaks are dominated by the intermetallic phase of AlNi,suggesting the intermetallic reaction between Al and Ni dominates at this temperature.For Al/Ni@PC,the XRD patterns are almost identical to those of Al/Ni@NA at each attempted temperature.
For Al/3Ni(Fig.5(b)),when the temperature elevates to 800C,the phase compositions of the final condensed product are dominated by AlNiand which is contaminated with small amounts of AlNiand AlNi.With the addition of ECs,a new phase of AlN is formed at 800C for Al/3Ni@ECs implying that the reaction takes place between N element from ECs and Al.This reaction partially consumes the Al,which is supposed to participate in the intermetallic reaction later,and thereby resulting in decreased practical reacting ratio between Al and Ni.
3.4.Combustion behavior of Al-Ni@ECs composites
The maximum energy releases of Al-Ni@ECs during the combustion process have been measured by using a bomb calorimeter.The heats of reaction are shown in Fig.6 and summarized in Table S1.
The variation in the measured heat of reaction for Al-Ni@ECs composites is shown in Fig.6.The highest energy generation is achieved by Al/Ni@NA,indicating that a higher combustion efficiency of Al/Ni could be realized by a minor use of NA.Compared with Al/Ni,the reaction heat of Al/Ni@NA and Al/Ni@PC are increased by 108%and 53%,respectively.It is strange that pure Al/3Ni cannot be easily ignited under 3 MPa of Ar.However,with the introducing of ECs,the ignition of Al/3Ni@ECs composites can be easily achieved.Herein,the theoretical energy release value of Al/3Ni(1230 J/g)is used as reported by Fischer et al.[47].to assess the effect of coating ECs on Al/3Ni.Compared with the theoretical energy release value of pure Al/3Ni,the reaction heat of Al/3Ni@NA was increased by 42%,whereas that of Al/3Ni@PC was increased by 22%.Obviously,the energy release of Al-Ni was greatly improved with the inclusion of ECs.

Fig.5.The XRD spectra of the residues of Al-Ni and Al-Ni@ECs after heat treatment at the different temperatures:(a)Al/Ni,Al/Ni@NA and Al/Ni@PC;(b)Al/3Ni,Al/3Ni@NA and Al/3Ni@PC.

Fig.6.Heat of reaction of Al-Ni@ECs composites measured by bomb calorimeter.
Meantime,the reaction heat of Al/Ni@ECs is higher than that of Al/3Ni@ECs,suggesting that Al-Ni with the atomic ratio of 1:1 has higher energy content than that of 1:3.The results indicate that both the atomic ratio of Al-Ni and the type of ECs have significant effects on the energy content and release rate of Al-Ni.The Al-Ni with the inclusion of PC seem to be less reactive as compared to NA.The possible reason could be related to the relative low exothermicity from the interfacial reaction between PC and Al-Ni.In fact,the reactivity of Al/CuO nanothermite composites with fluoropolymers has been measured with similar findings[48].They showed that hydrogen fluoride(HF)released from PVDF may react with Al/CuO,resulting in less energy due to higher heat of formation of fluorides than oxides.This less exothermic reaction may be responsible for the lower combustion rate and less heat release.Therefore,it can be concluded that the exothermicity of the reactions between ECs and Al-Ni plays a critical role in tunning energy contents and heat release rates of Al-Ni the involved composites.
fl
The combustion behaviors of Al-Ni@ECs composites have been studied using our customized combustion diagnostic system.The sequential snapshots of all samples burning in Ar have been done using a high-speed camera through a transparent window (Fig.7 and Fig.S2).All the samples except Al/3Ni were successfully ignited and proceeded to a self-sustainable combustion.The low ignitability of Al/3Ni is likely due to high chemical stability of Ni,especially when the fraction of Ni in the Al/3Ni composite is surpassing a certain threshold.The difficulty in ignition suggests that this composite at atomic ratio of 1:3 is insensitive to heat.Thus,the reactivity of the Al/3Ni@ECs may also be relatively lower compared to that of Al/Ni@ECs samples.
For Al/Ni,the light emission of the burned sample lasts for~2 s,which mainly involves the intermetallic reaction between Al and Ni.When the intermetallic reaction was completed,the brightness of the burned Al/Ni gradually reduces during cooling process.When it was coated with NA,the flame propagation process of Al-Ni@NA presents significant visible spots as well as irregular cracks on the surface of burned samples as shown in Fig.7(b)and d.At the same time,an axial elongation of Al-Ni@NA increases with the increase of burn time.The same phenomenon was shown in the case of Al/3Ni@NA.Such a behavior could be attributed to the effect of a large number of gaseous products (e.g.,HF,NO and CH)released from ECs,which were ejected from the sample surface and generating porous structures in the residues.Furthermore,the violent combustion reactions were observed for Al-Ni@NA,indicating that the reactivity of intermetallic reaction between Al and Ni has been greatly enhanced with the inclusion of NA.The flame front of Al/Ni@NA takes about~450 ms to reach the bottom of charge,since the flame propagation rate of NA is much faster than PVDF/CL-20.Moreover,the self-sustained combustion rate of Al/Ni@PC is smaller than that of Al/3Ni@PC,which is probably caused by the less exothermic reaction between the condensed phase products decomposed from PC and Al-Ni.
To further investigate the combustion behaviors of the prepared composites,the flame propagation rates have been calculated based on the recorded images by using high-speed camera in Fig.S2,and the results are summarized in Table 3.
From Table 3,it can be seen that the flame propagation rate of Al-Ni was increased with the inclusion of ECs.The flame propagation velocity of Al/Ni is 15.8 mm/s.In case of Al/Ni@NA,the flame propagation rate was increased by 30.0% from 15.82 mm/s to 20.6 mm/s under the effect of the NA coating.The same positive effect of PC coating on Al/Ni is obtained,where the flame propagation rate of Al/Ni@PC was 11.6%higher than pristine Al/Ni.For Al/3Ni@ECs composites,the propagation rate is nevertheless reduced with the increase of Ni.It suggests that despite Ni contributes to catalytic effect on ECs,excessive Ni would reduce the overall energy content in comparison to Al,thereby reduce the flame propagation rate.

Table 3 The flame propagation rate of Al-Ni-based reactive materials.
fi
Combustion wave temperature usually demonstrate the efficiency of the heat generation and the heat capacity of the combustion products.The temperature distribution and its dependence on burn time have been obtained by using the high-speed infrared camera.The recorded thermal images are displayed in Fig.S3,that allows one to preliminarily judge the relative difference in surface temperature distribution for the involved samples,the actual temperature could be much different,since the radiation parameter is very difficult to be accurately obtained.Anyway,these values are meaningful for a systematically comparing the effect of ECs on the improving the reactivity of intermetallic reaction of Al-Ni system.
As shown in Fig.S3,the maximum flame temperature of Al/Ni@ECs is about 1800 K,which is~500 K higher than that of Al/3Ni@ECs with less energy content.The bright red and blue vapors indicate that large amounts of gases are produced by ECs during their combustion processes.It is clear that Al/Ni@ECs composites release more gaseous products than Al/3Ni@ECs.The fast heat generation by gaseous production of ECs could be absorbed by Al-Ni for preheating,which has positive affect on the combustion efficiency/rate of Al-Ni.Furthermore,the secondary reaction of Al/Ni@ECs as depicted in Fig.S3(b) and Fig.S3(c) during the combustion process is featured with a secondary temperature rise of the burned sample,which was not observed for Al/3Ni@ECs.The secondary heat release process of Al/Ni@ECs is mainly dominated by the intermetallic reaction,which is greatly enhanced by the presence of the decomposed gaseous products by ECs as the catalysts or reactive sites,where porous structure has been formed.The absence of this phenomenon of Al/3Ni@ECs might be responsible for the relatively low reaction temperature change.It further confirms that an enhanced reactivity and higher combustion efficiency can be obtained for Al/Ni with an atomic ratio of 1:1.

Fig.7.The sequential snapshots taken for the samples of (a) Al/Ni,(b) Al/Ni@NA,(c) Al/Ni@PC,(d) Al/3Ni@NA and (e) Al/3Ni@PC burning in Ar.
For a better comparative analysis,the average combustion wave temperature of Al-Ni@ECs have been evaluated.The corresponding temperaturetime profiles and its derivatives are shown in Fig.8(a) and Fig.S3(b),respectively.All the curves are shifted manually along the-axis in order to avoid overlapping.In general,the average temperatures of the composites with ECs coating are several hundred Celsius higher than that of the reference sample of Al/Ni.Moreover,an average maximum combustion temperature()obtained for both Al/Ni@ECs composites are higher than that of Al/3Ni counterparts.
The temperature rise rate (γ),serves as the parameter indicating the intensity and efficiency of self-sustained combustion.It is affected by the gases production rate,thermite reaction rate and intermetallic reaction rate during the combustion process.Fig.8(b) shows the temperature rise rate derived for Al-Ni@ECs composites,where γis basically increased with the addition of ECs regardless of their types.For the best scenario observed,it is over 11 times higher than that of the reference sample.Furthermore,the temperature rise rate of Al/3Ni@ECs is relatively lower than that of Al/Ni@ECs,which can also be supported by the fact of the lower exothermicity of Al/3Ni.

Fig.8.(a) The combustion wave temperature profiles and (b) its derivatives as a function of time.
3.5.The structure of CCPs and proposed combustion mechanisms
The morphology and composition of the CCPs were characterized aiming to reveal the combustion reaction mechanisms.The CCPs of Al-Ni@ECs composites were collected for SEM,EDS and XRD analyses and the results are shown in Fig.9,Fig.S4 and Fig.10.Compared with Al/Ni shown in Fig.S4(a),the holes of the CCPs from Al-Ni@ECs(Fig.9,Fig.S4(b)and Fig.S4(c))are increased.It can be obtained that the gaseous products of Al-Ni@ECs are increased in comparison to that of Al/Ni without the ECs coating.The gaseous products on the interface layer can exclude the sintering and form lots of pores as literature reported [37].Those pores also provide new channels for the further reaction between Al-Ni and the condensed products of ECs.
The surface of the CCPs from Al/Ni@PC contains many hollow spheres with varied diameters ranging from 5 to 200 μm as shown in Fig.9(a).According to the previous studies,the hollow structure provides the penetrating channels for better heat and mass transfer,which leads to an enhanced intermetallic reaction rate of Al/Ni.The CCPs of Al/Ni@PC were examined by powder XRD techniques,where AlNi and a small amount of AlON(Fig.10)were discovered,indicating that the reaction between the gaseous products of ECs and Al occurred before intermetallic reaction of Al and Ni.

Fig.9.(a)The SEM images of the CCPs from Al/Ni@PC;(b)The amplified SEM image of CCPs from Al/Ni@PC;(c)The SEM images of the CCPs from Al/3Ni@PC;(d)The amplified SEM image of one gradient for CCPs of Al/3Ni@PC;(e) the EDS mapping images for CCPs of (d) Al/3Ni@PC.

Fig.10.The XRD patterns of the CCPs.
For Al/3Ni@PC,a plenty of cubic crystals with smooth surfaces can be easily observed in their CCPs as shown in Fig.9.The EDS results show that the element of fluorine is dominated in the crystals,suggesting that they are F-containing compounds.As mentioned above,the reactivity of Al/3Ni@PC is relatively lower,which is responsible for the low reaction temperature as well as low burning rate of Al/3Ni@PC.Thus,the lowered reaction temperature and reduced burning rate may collectively be in favor of the growth of this F-containing crystal.However,such a crystal is unknown in the chemical database,and the specific formation mechanism needs to be further studied.Moreover,the CCPs of Al/3Ni@PC display a less porous structure in comparison to that of Al/Ni@PC.Since the formation of F-containing crystals would reduce the availability of F,so that the gaseous products are consequently reduced.The reduced gaseous products have a detrimental effect on the formation of porous structure,and thereby it significantly decreases the reaction rate of Al/3Ni.The CCPs of Al/3Ni@PC mainly contain AlNi,a small amount of AlN and AlON (Fig.10).The presence of some other unknown diffraction peaks in the CCPs of Al/3Ni@PC may be associated with the F-containing cubic crystals.
In order to further understand the reaction process,the equilibrium compositions of Al/Ni@NA at different temperatures were calculated by using HSC software.Fig.11 shows the equilibrated species of Al/Ni@NAtemperature.The quantities of HCl and AlNi decrease with the increase of temperature due to the formation of AlCl,suggesting that the reaction between HCl released from AP and shell of Al has occurred,which is beneficial to improve the combustion performance of the composite[49].With the increase of AlCl content,the AlO appears at higher temperature.The AlO is a primary combustion intermediate of Al,indicating that the reaction between ECs and Al occurs after the etching of AlOshell.Furthermore,the formation of AlON determined by XRD analysis further confirms that the reaction is most likely to occur between gaseous products of ECs and Al.In addition to the CCPs of AlNi,the calculation also shows that gaseous species such as NH,Ni,and AlCl are generated during the combustion process.The formation of gaseous substances would greatly change the reaction pathways,by shifting it from a solid-solid to solid-gas or even liquid-gas modes.The combustion performance was significantly improved under the combined combustion mode [50].

Fig.11.The dependence of equilibrated compositions on temperature calculated by HSC software for the Al/Ni@NA composite.
According to the above results,the overall reaction processes of Al-Ni@ECs are proposed as follows.First,ECs decompose and ignited with combustion products uniformly cover the particle of Al-Ni,and then the products or intermediates of ECs would react with preheated Al.The fast heating by ECs combustion can promote the flame propagation rate of Al-Ni.Additionally,the acidic products such as HCl and HF generated from ECs may etch the AlOshell,so that the inner active Al to be exposed and easily react with Ni.Therefore,more efficient energetic systems can be obtained by introducing these halogen containing ECs.
4.Conclusions
In this work,two types of ECs (NA and PC) were used to coat Al-Ni reactive materials by ball milling followed with spray-drying technique.The effects of ECs on the heat release,combustion characteristics of Al-Ni reactive materials,morphologies and compositions of the corresponding combustion products have been comprehensively studied.It has been shown that the combustion performances of those composites are greatly affected by introducing different types of halogen-containing ECs as coating agents.Particularly,the combustion performance of Al-Ni can be significantly improved by coating of NA.The flame propagation rate was increased from 15.8 mm/s to 20.6 mm/s,which was 30.0% higher than that of the reference.In addition,the combustion wave temperature of the corresponding surface modified composites was~500 K higher than that of the reference without surface modifications.The acidic gaseous products decomposed from halogencontaining energetic composites can react with AlOpassivation layer,which make the inner active Al to be exposed and easily react with Ni.Therefore,the intermetallic reaction between Al and Ni was greatly enhanced.
These results presented in this paper demonstrate that the halogen-containing energetic composites are the promising candidate for tuning the reactivity and combustion characteristics of the reactive intermetallic materials.Further efforts can be made on the clarification of the detailed combustion mechanisms of such composites with advanced techniques such as Time of Flight/Mass Spectroscopy (TOFMS).
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
This work was supported by the Nation Natural Science Foundation of China (Grant No.51776176) and the Fundamental Research Funds for the Central Universities,China (Grant No.G2017KY0301).This paper was also partially funded by NSAF project (Grant No.2030202) and sponsored by Innovation Foundation for Doctor Dissertation of Northwestern Polytechnical University(Grant No.CX2021048).
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dt.2022.01.007.
杂志排行
Defence Technology的其它文章
- Establishment,simulation and verification of firepower safety control model
- Burning characteristics of high density foamed GAP/CL-20 propellants
- Cell-type continuous electromagnetic radiation system generating millimeter waves for active denial system applications
- Sandwich structure for enhancing the interface reaction of hexanitrohexaazaisowurtzitane and nanoporous carbon scaffolds film to improve the thermal decomposition performance
- Ablation characteristics of insulator under high-temperature gas dualpulse erosion
- Influence of shaped charge structure parameters on the formation of linear explosively formed projectiles
