Sandwich structure for enhancing the interface reaction of hexanitrohexaazaisowurtzitane and nanoporous carbon scaffolds film to improve the thermal decomposition performance
2022-10-17ShuiZhuZichenHuYuqiCoXioxiLiYuqiFengXiongCoPengDeng
Shui-d Zhu ,Zi-chen Hu ,Yu-qi Co ,Xio-xi Li ,Yu-qi Feng ,Xiong Co ,* ,Peng Deng
a Shanxi Fire & Explosion-Proofing Safety Engineering and Technology Research Center,North University of China,Taiyuan,030051,PR China
b State Key Laboratory of Explosion Science and Technology,Beijing Institute of Technology,Beijing,100081,PR China
Keywords:CL-20 NCS Catalysis Thermal decomposition Sandwich structure
ABSTRACT Improving the thermal decomposition performance of hexanitrohexaazaisowurtzitane (CL-20) by appropriate methods is helpful to promote the combustion performance of CL-20-based solid propellants.In this study,we synthesized a sandwich structure of CL-20 and nanoporous carbon scaffolds film(NCS)and emphatically studied the thermal decomposition performance of the composite structure.Thermogravimetric analysis and differential scanning calorimetry were used to measure the thermal decomposition process of the composite structure.The kinetic parameters of thermal decomposition were calculated by the thermal dynamic analysis software AKTS.These results showed that the thermal decomposition performance of the sandwich structure of CL-20 and NCS was better than CL-20.Among the tested samples,NCS with a pore size of 15 nm had the best catalytic activity for the thermal decomposition of CL-20.Moreover,the thermal decomposition curve of the composite structure at the heating rate of 1 K/min was deconvoluted by mathematical method to study the thermal decomposition process.And a possible catalytic mechanism was proposed.The excellent thermal decomposition performance is due to the sandwich structure enhances the interface reaction of CL-20 and NCS.This work may promote the extensive use of CL-20 in the field of solid rocket propellant.
1.Introduction
With the rapid development of aerospace technology,chemical energy thrusters powered by solid fuel have attracted extensive attention due to their simple and reliable structure and low energy consumption[1,2].However,ammonium perchlorate(AP)is mostly used as an oxidant in the present solid rocket propellant,which has low heat release,high characteristic signal,and corrosive products during combustion[3-5].So,AP is more and more unable to meet the higher requirements of solid propellants.Therefore,it is urgent to find high-energy and environment-friendly oxidants for the further development of solid propellants.CL-20,as the fourthgeneration high-energy explosive,has the advantages of high density,high energy density,fast energy release rate,and low characteristic signal [6,7].It is expected to replace AP as a highenergy oxidant for solid propellant.However,the high burning rate and high-pressure index in the combustion process of CL-20 will affect its steady-state combustion,resulting in poor selfsustaining combustion performance [8].It can only be used sparingly as an additive in the solid propellant,which has limited to improve the performance of the propellant [9].According to reports,the thermal decomposition peak temperature of CL-20 can be reduced by adding metal oxides [10],graphene [11],carbon nanotubes,and other catalysts [12,13],thereby reducing the highpressure index of the CL-20 combustion process and enhancing its self-sustaining combustion performance [14].However,the larger particle size of CL-20 leads to a smaller contact area with the catalysts,which restricts the effect of the catalysts[15].Therefore,it is necessary to find a suitable material to increase the contact area with CL-20 and further enhance the thermal decomposition performance of CL-20.
NCS is a new type of carbon material.Its basic composition is 91-95%C,4-8%O and 1%H element[16].The surface structure of NCS is a uniform arrangement of nanopores,and the inner part is a three-dimensional connected pore structure.Each pore is connected with 12 surrounding holes [17].The pore size of NCS is adjustable and can be precisely controlled in the range of 2-1000 nm [18].The thickness of NCS can also be adjusted in the range of 0.5-1000 μm.Its compressive strength >3 MPa,shear strength >1 MPa,bending strength >1 MPa,conductivity >30000 s/m[18].NCS has the advantages of high strength,excellent heat and electric conduction,high temperature,and acid-alkali resistance,so it is widely used in the fields of nano hydrogen fuel cells [18],supercapacitor electrode materials[19],catalyst carrier[20],and so on.However,NCS has not been used in the field of energetic materials.

Fig.1.Preparation of CL-20/NCS sandwich structure.
Considering that the three-dimensional connected channel structure of NCS has more catalytic active sites,good thermal conductivity,and diffusion ability to gas products[21,22],the CL-20 was filled into NCS with pore sizes of 15 nm,50 nm,and 100 nm(NCS-15,NCS-50,NCS-100),and constructed a sandwich structure.The thermal decomposition property of the composite structure of CL-20/NCS was studied.NCS with different pore sizes both can improve the thermal decomposition performance of CL-20,reduced the decomposition peak temperature of CL-20 by 5.4-16.9C and the activation energy by 10.5-100.1 kJ/mol.Among them,the NCS with a pore size of 15 nm has the best effect.Besides,the possible mechanism of NCS to improve the thermal decomposition performance of CL-20 was proposed.This work provides the possibility for CL-20 to replace AP as a high-energy oxidizer for solid propellants as soon as possible.
2.Experimental section
2.1.Chemicals and materials
CL-20 was obtained from the Beijing Institute of Technology.NCS was purchased from Nanjing Momentum Materials Technologies Co.,Ltd.Acetone solution was purchased from Tianjin Xingyue Chemical Co.,Ltd.The solvent is analytically pure.
2.2.Preparation of CL-20/NCS

Fig.2.SEM images of NCS-15 (a),NCS-15 with a small amount of CL-20 (b),NCS-15 with 80 wt% CL-20 (c),and the cross-section of CL-20/NCS-15 (d).
CL-20/NCS was prepared by the method of template adsorption solution.The process is shown in Fig.1.1.7 g CL-20 was mixed with 5 mL acetone solution and stirred for 5 min at room temperature.Then the appropriate amount of the mixed solution was dropwise added to the NCS(1 cm × 1 cm) with different pore diameters.At last,the CL-20/NCS/acetone composite sample was heated in an oven at 50C until the acetone solution was completely volatilized.In this study,the mass ratio of CL-20 to NCS was 4:1.
2.3.Sample characterization
The surface morphology and microstructure of the samples were measured by scanning electron microscopy (SEM,BCPCAS4800).The element distribution of samples was measured by an energy dispersion X-ray spectrometer (EDS,BCPCAS4800).The crystal structure data of the samples were obtained by X-ray diffraction (XRD) using a DX-2700 diffractometer in the scanning angle range of 5-50.Nitrogen sorption isotherms were measured at 77.300 K with a TriStar II 3020 analyzer.The specific surface area(S) was estimated by Brunauer-Emmett-Teller (BET) method.
2.4.Catalytic activity measurement
The thermal decomposition process of the sandwich structure of CL-20/NCS was recorded by thermogravimetric analysis and differential scanning calorimetry(TG-DSC)using a Netzsch STA449F3 analyzer(Germany).The heating range was 40-500C.The heating rates were 1,5,10,15,and 20 K/min.The sweep gas and protective gas were high purity argon of 50 and 20 mL/min,respectively.
3.Results and discussion
3.1.Characterization of samples
The SEM images of NCS-15,NCS-15 with a small amount of CL-20,NCS-15 with 80 wt%CL-20,and the cross-section of CL-20/NCS-15 are shown in Fig.2.Fig.2a shows the NCS-15 is a porous structure.The diameters of these holes are large or small,but most of them are in the range of 10-20 nm.Fig.2b shows the NCS-15 with a small amount of CL-20 still have a large number of holes are not filled by CL-20.The micromorphology of the sandwich structure of CL-20/NCS-15 containing 80 wt% CL-20 is shown in Fig.2c.CL-20 crystallizes on the surface of NCS-15.Discontinuous holes with a diameter of 5-10 nm can still be seen on the surface of the composite structure.Fig.2d shows the micromorphology of the cross-section of CL-20/NCS-15,and the sandwich structure can be clearly seen.The results of SEM show that the CL-20/NCS sandwich structure was successfully prepared.

Fig.3.Elemental mappings (b-d) from the cross-section of CL-20/NCS-15 (a).

Fig.4.N2 adsorption-desorption isotherms (a) of NCS.XRD pattern (b) of NCS and CL-20/NCS.Average particle size (c) of CL-20 in composite samples.
Fig.3 shows the elemental composition and dispersion of the cross-section of CL-20/NCS-15.The energy filtering area of CL-20/NCS-15 is shown in Fig.3a.Fig.3b-d shows the elemental mappings of C,N,and O in CL-20/NCS-15.The EDS results once again prove that CL-20 and NCS form a sandwich structure.
The Nadsorption was carried out for the NCS-15,NCS-50,and NCS-100 in order to evaluate the permanent porosity.The results are shown in Fig.4a.NCS exhibits reversible type IV,which is one of the main characteristics of mesoporous materials [23,24].Table 1 shows that the surface area of NCS-15,NCS-50,and NCS-100 is calculated to be 692.2,217.0,and 70.2 m/g using the BET model,respectively.NCS-15 has the largest specific surface area.Fig.4b shows the crystal structure of raw CL-20,NCS,and CL-20/NCS composite structures.The results show that NCS is an amorphous structure.The raw CL-20 has the same diffraction peak as ε-CL-20(CCDC: 117779) [25,26].The CL-20/NCS has the same diffraction peak as α-CL-20 (CCDC: 117776) [27],which indicates that the crystal structure of CL-20 was changed in the process of recrystallization.And the transformation of crystal structure will lead to a slight decrease in the thermal decomposition performance and energy density of raw CL-20 [28].Fig.4c shows that the average particle size of CL-20 in NCS-15,NCS-50,and NCS-100 are 7.1,14.2,and 20.5 nm,respectively (calculated from the Scherrer equation)[29].The data shows that as the pore size of NCS increases,the particle size of CL-20 also increases.

Table 1 The specific surface area of NCS.
3.2.Catalytic performance
The DSC curves of raw CL-20 and CL-20/NCS at the heating rate of 10 K/min are shown in Fig.5a.The DSC curves of CL-20 have only one peak.The peak represents the thermal decomposition process of CL-20.The peak temperature is 249.5C.Compared with raw CL-20,the exothermic peak temperature of the CL-20/NCS is reduced by 5.4-16.9C.As the pore size of NCS decreases from 100 nm to 15 nm,the thermal decomposition peak decreases from 244.1C to 232.6C.Also,the thermal decomposition peak of raw CL-20 is high and sharp,which indicates that the thermal decomposition process of raw CL-20 is rapid and intense.While,the decomposition peak of the CL-20/NCS is short and gentle,which indicates that the thermal decomposition process of CL-20/NCS is slow and stable.Fig.5b shows the TG curves of raw CL-20 and CL-20/NCS at the heating rate of 10 K/min.The mass-loss rate of raw CL-20 is 62%.The massloss rate of the CL-20/NCS is 38%-41%.This is due to the relatively small content of CL-20 in the sample of CL-20/NCS.Besides,the slope of the TG curve of CL-20/NCS is lower than that of raw CL-20.Among them,the slope of the TG curve of CL-20/NCS-15 is the smallest.This once again indicates that the thermal decomposition rate of raw CL-20 is higher than that of CL-20/NCS.And the temperature of CL-20/NCS-15 begins to lose mass is 144.6C,which is significantly lower than that of other samples.TG-DSC results show that NCS can catalyze the thermal decomposition of CL-20,and NCS-15 has the best catalytic effect.

Fig.5.DSC (a) and TG (b) curves of raw CL-20 and CL-20/NCS at the heating rate of 10 K/min.

Fig.6.Heat release (J/g) of raw CL-20 and CL-20/NCS.
Fig.6 shows the heat release of raw CL-20 and CL-20/NCS.The heat release of raw CL-20 is 2020 J/g.The heat release of CL-20/NCS-15,CL-20/NCS-50,CL-20/NCS-100 is 1189,965,and 1419 J/g,respectively.Compared with raw CL-20,the heat release of each of the composite structures of CL-20/NCS is decreased.Among them,the heat release of CL-20/NCS-50 is decreased the most,reaching 1055 J/g.
Fig.7a shows the thermal decomposition curve of raw CL-20 at different heating rates.Fig.7b-d shows the thermal decomposition curve of CL-20/NCS-15,CL-20/NCS-50,and CL-20/NCS-100 at different heating rates.Among them,the mass ratio of NCS in each composite structure is 20%.Recording the peak temperature of the exothermic peak of test samples during decomposition at different heating rates in Table 2.And several important kinetic parameters of the sample decomposition process can be calculated by the Kissinger Eq.(1),Friedman Eq.(2),and Ozawa Eq.(3).

Table 2 Tp and Ea of raw CL-20,CL-20/NCS-15,CL-20/NCS-50,and CL-20/NCS-100.

In these equations,is the peak temperature (C),β is the heating rate(K/min),is the apparent activation energy(J/mol),α is the conversion degree,is the frequency factor,is the reaction temperature(C),and R is the ideal gas constant(8.314 J/mol/K),αis the reaction mechanism function [30-32].

Fig.7.DSC curves of CL-20 (a),CL-20/NCS-15 (20 wt%) (b),CL-20/NCS-50 (20 wt%) (c),and CL-20/NCS-100 (20 wt%) (d) at different heating rates.
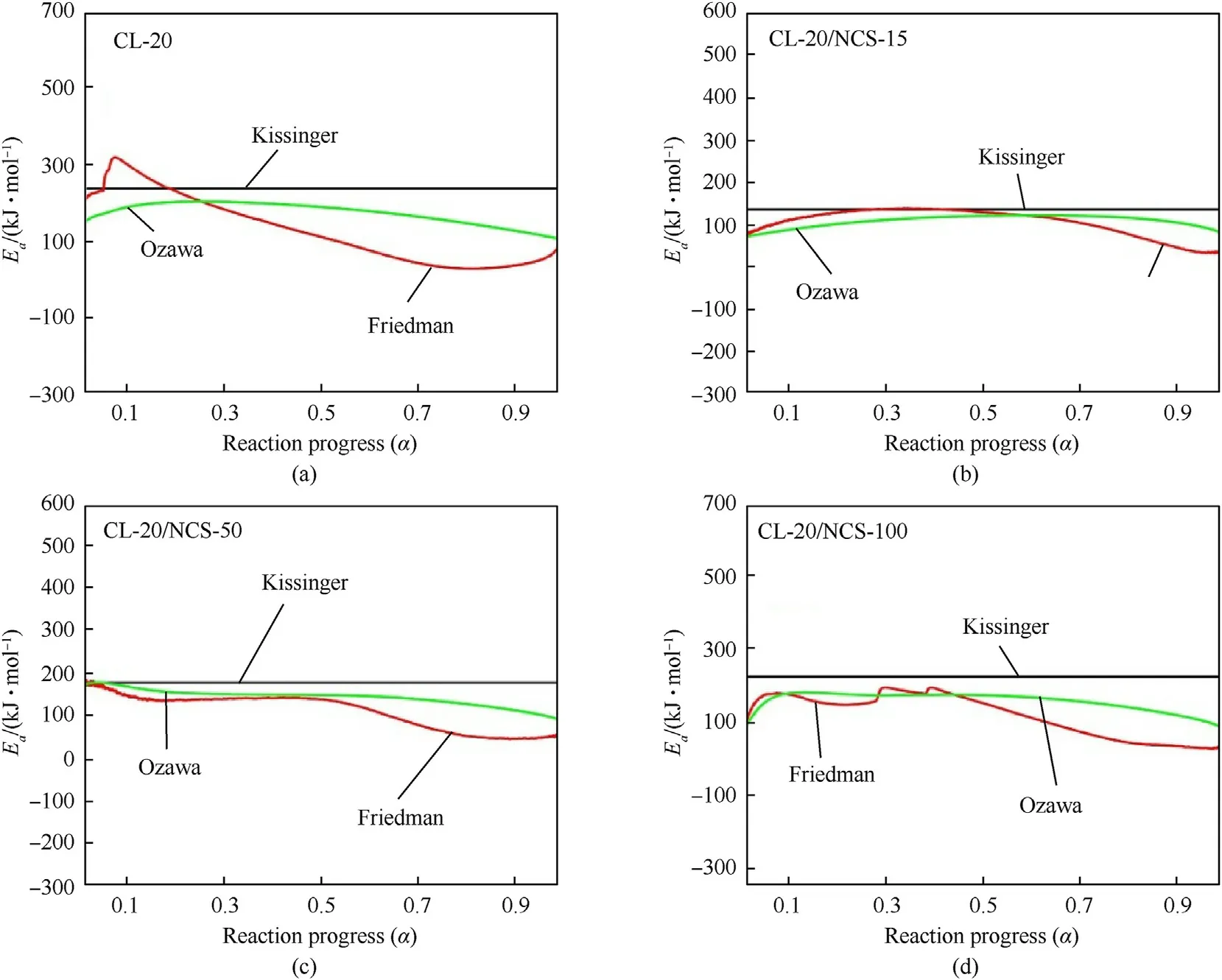
Fig.8. Ea of raw CL-20 (a),CL-20/NCS-15 (b),CL-20/NCS-50 (c),and CL-20/NCS-100 (d) calculated by three kinetic methods.
Fig.8 shows thecurve of the test samples obtained by the thermal dynamic analysis software AKTS using the above three methods.Theof the test samples calculated by the Kissinger method is recorded in Table 2.Theof raw CL-20 decomposition is 235.5 kJ/mol.The Eof the CL-20/NCS-15,CL-20/NCS-50,and CL-20/NCS-100 were reduced to 135.4,177.4,and 225.0 kJ/mol,respectively.These results indicate that NCS with three pore sizes both can promote the thermal decomposition of CL-20.In particular,NCS-15 greatly enhanced the decomposition performance of CL-20.In addition,thecurves obtained using the Friedman and Ozawa methods shown in the picture have the same trend as those calculated by the Kissinger method.This shows that thecalculated by these three methods is highly reliable.Table 3 shows the effect of different catalysts on the thermal decomposition performance of CL-20.It can be seen that the sample NCS-15 used in this study has more excellent catalytic performance than other catalysts in the previous report.

Table 3 Effect of different catalysts on thermal decomposition performance of CL-20.
To further understand the thermal decomposition process of the CL-20/NCS composites structure,the DSC curve of these samples was measured at the heating rate of 1 K/min.Fig.9a shows that the raw CL-20 begins to decompose at 201.0C,and the heat flow reaches the maximum at 222.5C.There is only one exothermic stage.This is because that the thermal decomposition of CL-20 is a self-catalytic process.Fig.9b shows that the original DSC curve of CL-20/NCS-15 has two exothermic peaks that overlap each other.After deconvolution by mathematical methods,it can be turned into two independent exothermic processes.This indicates that thethermal decomposition of the CL-20/NCS-15 composite structure consists of two stages.The exothermic peak of the first stage is due to the interface reaction between CL-20 and NCS.Under heat excitation,The N-NObond of CL-20 is broken.The generated NOreacts with NCS at the interface to produce COand N.And releases a lot of heat.The exothermic peak of the second stage is due to the autocatalytic reaction of CL-20.Fig.9c shows that the thermal decomposition process of CL-20/NCS-50 is similar to that of CL-20/NCS-15 at the heating rate is 1 K/min,but the temperature of each decomposition stage is increased.Fig.9d shows that there is only one exothermic stage in the thermal decomposition process of CL-20/NCS-100,which is the same as the raw CL-20.This may be due to the large pore size of NCS-100,which can transfer NOout quickly and prevent the interface reaction.

Fig.9.DSC deconvolution curve of raw CL-20 (a),CL-20/NCS-15 (b),CL-20/NCS-50 (c),and CL-20/NCS-100 (d) at the heating rate of 1 K/min.

Fig.10.Schematic of the thermal decomposition mechanism of CL-20 catalyzed by NCS.
3.3.Catalytic mechanism
Fig.10 shows the thermal decomposition mechanism of CL-20 catalyzed by NCS.As shown in the figure,under thermal stimulation,CL-20 is decomposed.The main process of the decomposition reaction of CL-20 is the homolysis of the N-NObond[33,34].And after the N-NObond breaks,NOwill be produced.Due to a large number of pores in NCS,NOis transferred and diffused rapidly,which further promotes the homolysis of N-NO.At the same time,NCS with the larger specific surface area has more active sites.NOis absorbed to prevent its escape,and the interface reaction between NOand NCS with high carbon content is promoted.The heat generated by the reaction makes the residual CL-20 continue to decompose.Therefore,the activation energy and the decomposition peak temperature of the CL-20/NCS sandwich structure are decreased.However,NCS introduces a competition mechanism to promote the reaction between C and NO,and inhibits the catalytic effect of NOon CL-20,which reduces the reaction heat generated in the decomposition process.
4.Conclusions
In summary,the thermal decomposition performance of the sandwich structure of CL-20 and NCS was studied.The result showed that with 20 wt% NCS-15,the peak temperature of CL-20 was decreased by 16.9C,from 249.5 to 232.6C,which is lower than CL-20/NCS-50(235.6C)and CL-20/NCS-100(244.1C).Thewas decreased by 100.1 kJ/mol,from 235.5 to 135.4 kJ/mol,which is lower than CL-20/NCS-50 (177.4 kJ/mol) and CL-20/NCS-100(225.0 kJ/mol).These suggest that NCS-15 has the best catalytic activity for the thermal decomposition of CL-20.Furthermore,a possible catalytic mechanism for the thermal decomposition of CL-20 with NCS has been proposed.This is due to that NCS possesses a large specific surface area,more active sites,and excellent gas diffusion ability,which promotes its interface reaction with CL-20.Ultimately,the thermal decomposition performance of the CL-20/NCS composite structure is improved.This work may provide a new way to improve the performance of solid propellants.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
This work was supported by the National Natural Science Foundation of China (No: 21975227) and the Shanxi Province Graduate Student Innovation Project (No: 2020SY403).
杂志排行
Defence Technology的其它文章
- Establishment,simulation and verification of firepower safety control model
- Burning characteristics of high density foamed GAP/CL-20 propellants
- Cell-type continuous electromagnetic radiation system generating millimeter waves for active denial system applications
- Ablation characteristics of insulator under high-temperature gas dualpulse erosion
- Influence of shaped charge structure parameters on the formation of linear explosively formed projectiles
- Novel aluminum-based fuel: Facile preparation to improve thermal reactions
