Elevational Variation in Reproductive Strategy of a Widespread Lizard:High-Elevation Females Lay Fewer but Larger Eggs
2022-09-27GideonGywaDEMEXinHAOLiangMABaojunSUNandWeiguoDU
Gideon Gywa DEME ,Xin HAO ,Liang MA ,Baojun SUN and Weiguo DU*
1 Key Laboratory of Animal Ecology and Conservation Biology,Institute of Zoology,Chinese Academy of Sciences,Beijing 100101,China
2 University of Chinese Academy of Sciences,Beijing 100039,China
3 College of Forestry,Hainan University,Haikou 570228,Hainan,China
Abstract Identifying how reproductive strategies such as the trade-off between clutch size versus egg mass vary with elevational gradients is essential for our understanding of life-history evolution.We studied lacertid lizards (Eremias argus) in China,from six populations at different altitudes,to assess elevational variation in reproductive strategy.We found significant between-population variation in maternal body size and clutch mass,but these variations were not explained by elevational differences.However,high-elevation females tended to produce smaller clutches of larger eggs compared with their low-elevation counterparts,demonstrating an elevational change in the trade-off between egg size and number.The egg size-number trade-off is a reproductive strategy that may favor large offspring,better enabling them to survive severe and unpredictable environments found at high elevations.
Keywords between-population variation,clutch size,egg size,elevational gradient,Eremias argus, local adaptation,reproductive output
1.Introduction
The evolutionary diversity of life-history traits is a result of natural selection on reproductive strategies during adaptive radiation.Organisms that have radiated along latitudinal and elevational gradients show variation in life-history traits at embryonic and post-embryonic stages,often due to local adaptation (Fitch,1980;Zammuto,1986;Stearns,1992;Hille and Cooper,2015;Haoet al.,2021).A well-known life-history hypothesis is that body size varies with latitude or elevation(Blackburnet al.,1999;Ashton and Feldman,2003).In terms of reproductive strategies,the offspring size-number trade-off has also been found to vary along latitudinal and elevational gradients (Weatherset al.,2002;Meiri and Dayan,2003;Duet al.,2014).For example,ectothermic vertebrates’ females from highlatitude environments tend to produce larger eggs than those from low-latitude environments (Laugenet al.,2003;Ji and Wang,2005;Moritaet al.,2009;Duet al.,2014).This reproductive strategy along environmental gradients may be influenced by a combination of extrinsic (e.g.,climate variation) and intrinsic(e.g.,female body size) factors (Hille and Cooper,2015;Meiriet al.,2020).
Environmental factors such as temperature and oxygen also vary along elevational gradients,and therefore induce elevational variation in food availability and seasonal activity periods for animals (Fitch,1980).In response to these environmental changes,animals are predicted to show different life-history traits.Firstly,body size is predicted to vary along elevational gradients.According to Bergmann’s rule,organisms at higher latitudes should be larger.However,Bergmann’s rule cannot be universally applied to all animals.In reptiles,for example,turtles follow the rule,but squamates(lizards and snakes) generally do not (Ashton and Feldman,2003).Furthermore,oxygen and food availability decrease as elevation increases and,thus,may constrain the body size of ectotherms,leading to a reversal of Bergmann’s rule (Jinet al.,2006).Secondly,reproductive output may differ along elevational gradients.Reproductive output can be considerably affected by body size,with larger individuals having greater reproductive output than smaller individuals (e.g.,Ashton and Feldman,2003;Angillettaet al.,2004;Sears and Angilletta,2004),but,again,this pattern is not universal (e.g.,Stearns,1992;Castilla and Bauwens,2000;Shanbhaget al.,2000;Duet al.,2012;Suárez-Varónet al.,2019).Moreover,body size is not the only explanatory variable for reproductive output;other factors such as temperature and food availability can significantly affect reproduction in a diverse array of animals (Stearns,1992;Du,2006;Maet al.,2014).Thirdly,the offspring size-number trade-off may vary along elevational gradients.This trade-off may be influenced by female body size,food availability or climate change (Fitch,1980).A common reproductive tactic of avian taxa at high elevations is for females to decrease offspring number and increase offspring size,thereby increasing potential fitness because of local adaptation to their environments(Badyaev,1997;Weatherset al.,2002).
A wide range of studies have demonstrated latitudinal variation in the egg size-number trade-off in reptiles (Angillettaet al.,2004;Duet al.,2005,2014),however,we still have relatively little understanding of elevational patterns involved in this trade-off (but see Luet al.,2018b;Sunet al.,2013;Meiri,2018;Meiriet al.,2020).Here we investigate elevational variation in reproductive life-history traits using a widespread lizard species (Eremias argus),to identify how the egg size-number trade-off varies along elevational gradients.By measuring lifehistory traits such as female body size,clutch mass,clutch size,and egg mass in six populations ofE.argusranging in elevation from 300 m to 2800 m above sea level (asl),we can test the above-mentioned hypotheses on life-history variation along elevational gradients.Specifically,we predict that (1) in a reversal of Bergmann’s rule,lizards from high elevations have a smaller body size and,therefore,less reproductive output than lizards from low elevations,and (2) females at high elevations lay fewer but larger eggs compared with their counterparts at low elevations.
2.Materials and Methods
2.1.Study animal and collectionEremias argusis a small(~70 mm snout-vent length [SVL]) oviparous lacertid lizard that is widely distributed across China,from sea level to ca.2800 m asl (Zhaoet al.,1999),providing an excellent model system for the study of elevational differences in reproductive life-history traits of lizards.Females of this species can produce two clutches of 2-5 parchment-shelled eggs per clutch,during spring and summer across geographical gradients.The first clutch of eggs are laid from early April into early June,and second clutches from early June to mid-July (Zhaoet al.,1999;Maet al.,2019b).
We captured gravid female lizards by hand during the early reproductive season from six different locations in China over four years (Year: 2011;Shidu County,Beijing: 300 m asl,39°38' N,115°35' E;n=30♀;Year: 2012;Harbin,Heilongjiang: 500 m asl,45°48' N,126°32' E,n=16♀;Year: 2014;Xingtai County,Hebei:600 m asl,37°04' N,114°30' E,n=26♀;Year: 2014 and 2021;Erdos,Inner Mongolia: 1300 m asl,39°36' N,109°46' E,n=34♀;Year:2014;Jingtai County,Gansu: 2300 m asl,37°11' N,104°03' E,n=14♀;Year: 2014;Gonghe County,Qinghai: 2600 m asl,36°17' N,100°37' E;n=11♀).Once captured,all gravid females were transferred to nearby field laboratories where they laid their eggs.
2.2.Animal husbandry and reproductive life-history traitsGravid females were weighed (body mass [BM];±0.01 g)and measured (SVL;±0.05 mm) and individually housed in glass terraria (600×300×400,L×W×D mm) filled with 10 mm of moist sand and pieces of clay tile as shelters.Lizards were maintained in rooms at the field laboratories with a light cycle of 10L:14D (0800 h on and 1800 h off).Additionally,a 100 W light bulb was suspended above each terrarium to provide the lizards with additional heat and thermoregulatory opportunities from 0800 h to 1800 h.We fed the lizardsad libitumwith food and water daily (mealworms and crickets dusted with additional vitamins and minerals).Gravid lizards were housed in the laboratory for an average of 14 days before clutches were produced.
Terraria were checked four times a day for freshly laid eggs (0800 h,1100 h,1400 h and 1800 h).Once found,eggs were immediately collected and weighed (±0.001 g).We then calculated the average mass of all eggs in a single clutch (=mean egg mass) and the total mass of all the eggs in a single clutch (=clutch mass).Once the females had laid eggs,we measured their snout-vent length (SVL;±0.05 mm) and postpartum body mass(BM;±0.01 g).Afterwards,females were released at the site from where they had been captured.
2.3.Statistical analysisWe performed all our analyses using R (version 4.0.5;R Development Core Team,2021).We first used the Shapiro-Wilk and Levene’s tests to assess the normality and homogeneity of variance of our data,respectively,and normalized the data using log-transformation when necessary.We compared statistical models using the likelihood ratio test and the Akaike-Information Criteria (AIC) for the model of best fit,which we then used in subsequent data analyses.We fitted all models with fixed variables (population origin),random effect (year) and correlated factors (tested by Pearson correlation analysis) as covariates (maternal body size).We used linear mixed effect models to test for between-population differences in maternal SVL and maternal body mass.We then used linear mixed effect models to assess the betweenpopulation difference in clutch mass and mean egg mass,with or without maternal body size as a covariate.To assess the between-population difference in clutch size with or without the effect of maternal body size,we used the generalized linear model with a Poisson distribution.Tukey’s post-hoc multiple comparisons tests were used to detect between-population variation in maternal body size and reproductive traits.We then used Pearson correlation analyses to assess elevational trends in maternal body size,clutch mass,clutch size,and mean egg mass.To remove the effect of maternal body size in these correlation analyses,we calculated the residuals of clutch mass,clutch size,and mean egg mass from regressions against maternal SVL.
3.Results
3.1.Elevational variation in maternal body sizeMaternal SVL and BM were significantly different among the six lizard populations we studied (Table 1).However,the differences in both maternal SVL and BM were not statistically associated with elevation (SVL:R=0.13,P=0.13;Figure 1A;BM:R=-0.16,P=0.07;Figure 1B).
3.2.Elevational variation in reproductive traitsClutch mass was significantly correlated with maternal SVL,with larger females having greater clutch masses than smaller females (F1,124=39.915,P<0.001).Clutch mass differed significantly between populations (F5,125=11.088,P<0.001),and this difference did not disappear even after the effect of maternal SVL had been removed (Table 1).Clutch mass residuals did not correlate with the elevational gradient (R=-0.14,P=0.11;Figure 2A).
Clutch size and mean egg mass were also significantly affected by maternal SVL,with larger females having larger clutch sizes (z=2.155,df=124,P=0.031) and heavier eggs (F1,124=16.924,P<0.001).Clutch size and mean egg mass differed significantly between populations (clutch size:F5,125=20.300,P=0.001;egg mass:F5,125=5.502,P<0.001).After the effect of maternal SVL was removed,the between-population variation in clutch size disappeared,but there was no change for mean egg mass (Tables 1-2).Clutch size was negatively correlated with increasing elevation,as shown by the decrease in residual clutch size as elevational gradient increased (R=-0.27,P=0.002;Figure 2B).Conversely,mean egg mass was positively correlated with increasing elevation,as shown by the increase in residual mean egg mass with increasing elevation (R=0.29,P=0.001;Figure 2C).Our results suggest that female lizards from low-altitude populations (e.g.,Harbin) may reduce investment in offspring quality (smaller eggs) to invest more in offspring number (larger clutch sizes),whereas the reverse is true for higher-elevation females,tending to produce fewer but larger eggs (Figure 2B-C).

Table 1 Summary of results on the effect of population origin on maternal body size and reproductive traits of Eremias argus.Values are of raw data measured and expressed as means ± s.e.Means with different superscripts differed significantly.Linear mixed effect models with maternal SVL as a covariate were used to remove the effect of maternal body size on reproductive traits.Bold font indicated a significant difference.
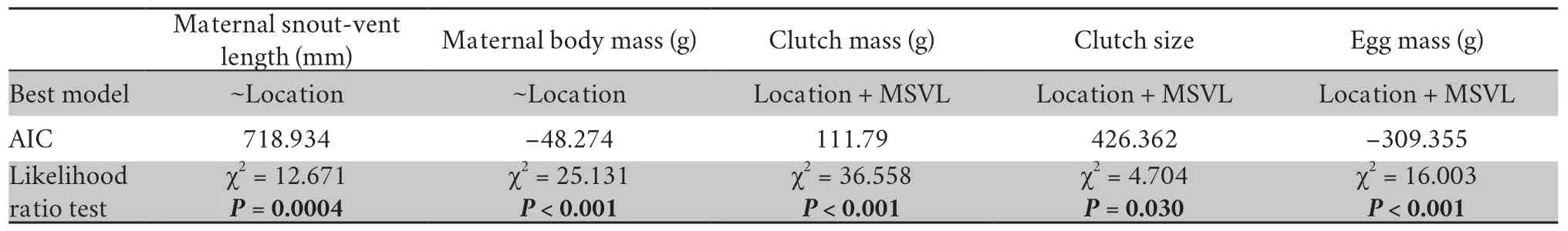
Table 2 Statistical significance (P<0.05) of different models for the effect of population origin on maternal body size and reproductive traits of Eremias argus.
4.Discussion
Our study revealed significant between-population differences in maternal body size,clutch mass,clutch size,and egg mass in the lizardE.argus,whose distribution spans a wide range of elevations.Inconsistent with our first prediction,betweenpopulation differences in maternal body size and clutch mass could not be explained by elevational gradients.However,our findings that high-elevation lizards produced smaller clutches of larger eggs (compared with low-elevation lizards whichproduced larger clutches of smaller eggs),is consistent with our second prediction (Figure 2B-C).These results suggest that the egg size-number trade-off in lizards shifts along elevational gradients,probably induced by environmental differences.

Figure 1 The relationships between (A) maternal snout-vent length and (B) maternal body mass and elevation (m) across six populations of lizard Eremias argus.Data are expressed as measured values depicted by closed circles (n=131).

Figure 2 The relationship between (A) residual clutch mass with no significant correlation,(B) residual clutch size with significant correlation,and (C) residual mean egg mass and elevation (m) with significant correlation across six populations of lizard Eremias argus.Residuals were calculated from regression against maternal snout-vent length.Data are expressed as measured values depicted by closed circles (n=131),and grey trendlines illustrate correlated relationships.
Maternal body size ofE.arguswas not related to elevation.This finding is inconsistent with previous studies showing that Bergmann’s Rule is reversed in lizards along latitudinal or elevational gradients (Ashton and Feldman,2003;Jinet al.,2006).Despite the non-relationship between body size and elevation in our study,the between-population variation in maternal body size suggests that local environmental conditions may underpin body size variation,at both proximate or ultimate levels (Chown and Klok,2003;Fosteret al.,2018).Geographic patterns in lizard body sizes are related to their local environment,suggesting that such patterns are largely driven by multiple environmental factors which are site-specific(Feldman and Meiri,2014;Guo,2016;Slavenkoet al.,2019;Meiriet al.,2020;Lianget al.,2021).Lizards with larger body sizes are often found in environments with warmer temperatures,or higher precipitation across major groups of Chinese lizards (e.g.,Lianget al.,2021).Additionally,seasonal changes in food availability and quality have been suggested to influence the evolution of body size in lizard species from different microhabitats (Tinkleet al.,1970;Martin,2002;Sunet al.,2014).For example,the between-population variation in maternal body size ofPhrynocephalus vlangaliiwas influenced mainly by seasonal limitations of food resources,which restricted offspring growth (Luet al.,2018b).Alternatively,between-population variation in maternal body size may have evolved during adaptive radiation and changing weather conditions in different localities (Angillettaet al.,2004;Seebacher and Shine,2004;Pincheira-Donosoet al.,2008;Luet al.,2018a;Norriset al.,2021).
Maternal body size is one of the primary traits that directly or indirectly influences life-history traits in organisms,leading to covariation of fitness-related traits (Peters,1986;Roy,2008;Sibly and Brown,2007).Consistent with previous studies (Maet al.,2019a,b),femaleE.argusshowed a positive relationship between body size and clutch mass.This positive relationship makes female reproductive output susceptible to age because lizards show indeterminate growth (Tinkleet al.,1970).In this species,however,maternal body size is not the only determinant of reproductive output (clutch mass),because among-population differences in clutch mass still exist even after the effect of maternal SVL was removed from our analyses.Previous studies on different lizard species showed that variation in reproductive output was site-specific,depending on the size of the maternal body cavity or energy storage capacity for reproductive investment (Vitt and Congdon,1978;Parker and Begon,1986;Maet al.,2019a).Therefore,maternal reproductive output among populations ofE.argusmay be driven by the interplay between underlying intrinsic factors such as body size and age (Vitt and Congdon,1978;Duet al.,2005;Maet al.,2019b) and their adaptive radiation in response to their local microhabitat conditions (e.g.,Sunet al.,2014;Haoet al.,2021).
As we predicted,femaleE.argusproduced smaller clutches of larger eggs at high elevations (Figure 2B-C).Similarly,highelevation birds produce large offspring by compromising offspring number (Badyaev,1997;Weatherset al.,2002).This trade-off between egg number and size predominantly favors offspring quality,enabling offspring to survive the cold and unpredictable environmental conditions at high elevations(Yampolsky and Scheiner,1996;Fischeret al.,2003).Larger offspring have advantages related to competition,locomotor performance (i.e.,for escape and predation),and therefore survival rate (Duet al.,2014;Duet al.,2010;Pincheira-Donoso and Tregenza,2011).Reduced food availability has been suggested as a major proximate factor influencing the tradeoff between clutch size versus egg mass (Vitt and Congdon,1978;Hille and Cooper,2015).Whether reproductive strategies associated with the egg size-number trade-off are underpinned by environmental or genetic mechanisms is beyond the scope of our study,but merits further research.Future studies aimed at assessing the environmental and/or genetic mechanisms underlying the evolution of this reproductive strategy at population levels will shed new light on the different proximate and ultimate mechanisms that enable species to maximize reproduction and survival.
It is noteworthy that the populations we studied span a wide geographical distance (~2400 km) from Harbin to Gonghe.It is possible that the elevational pattern of life-history traits found in our study may also be due to other biotic and abiotic factors associated with widely-separated sites,in addition to temperature and oxygen that are closely related to elevation.Unfortunately,we currently do not have those detailed biotic factors like food availability,and predation pressure for each population.Future studies are needed to gain a comprehensive understanding of the correlations between biotic and abiotic factors and the life-history traits of lizards.
In conclusion,our results show that reproductive output varies among populations ofE.arguslizards,in part due to differences in maternal body size.Interestingly,our study also demonstrates elevational changes in the reproductive strategy associated with an egg size-number trade-off.High-elevation environments may induce female lizards to decrease offspring number (smaller clutch sizes) in favor of increasing offspring size (bigger eggs) to maximize ultimate fitness.However,understanding whether shifts in this trade-off along elevational gradients are simply due to environmental factors,or genetically determined as a result of local adaptation,requires further investigation.
AcknowledgementsWe thank Wang YANG for assisting in the data collection during the field work.This work was supported by grants from the Second Tibetan Plateau Scientific Expedition and Research Program (2019QZKK0501)and China’s Biodiversity Observation Network (Sino-BON).
杂志排行
Asian Herpetological Research的其它文章
- A New Brown Frog of the Genus Rana (Anura,Ranidae) from North China,with a Taxonomic Revision of the R.chensinensis Species Group
- Effect of Morphology and Age on the Closure Ability of Asian Box Turtles(Cuora)
- Partial Masquerading and Background Matching in Two Asian Box Turtle Species (Cuora spp.)
- Morphology and Histochemistry of Infralabial Glands of Two Species in the Slug-Eating Family Pareidae (Reptilia: Serpentes)
- Field Surveys Suggest Biennial Reproduction Cycle and Competition-triggered Dispersal of the Endangered Chinese Crocodile Lizard
