Field Surveys Suggest Biennial Reproduction Cycle and Competition-triggered Dispersal of the Endangered Chinese Crocodile Lizard
2022-09-27ShuyiLUOYujieYANGChunshengYANGJunGUOXudongQINHaiyaoCENHongxinXIEandZhengjunWU
Shuyi LUO ,Yujie YANG ,Chunsheng YANG ,Jun GUO ,Xudong QIN ,Haiyao CEN ,Hongxin XIE,3* and Zhengjun WU
1 Daguishan National Nature Reserve for Crocodile Lizards,Hezhou 542800,Guangxi,China
2 Key Laboratory of Animal Ecology and Conservation Biology,Institute of Zoology,Chinese Academy of Sciences,Beijing 100101,China
3 University of Chinese Academy of Sciences,Beijing 100049,China
4 Guangxi Key Laboratory of Rare and Endangered Animal Ecology,College of Life Science,Guangxi Normal University,Guilin 541006,Guangxi,China
Abstract The endangered Chinese crocodile lizard(Shinisaurus crocodilurus) is a habitat specialist living in streams of mountain forests in southern China and northern Vietnam.Conservation efforts are increasing for recovering its wild populations.However,the growth,reproduction,and dispersal ability of crocodile lizards in the wild are largely unknown.We conducted field surveys of the crocodile lizard population in Daguishan National Nature Reserve,one of the largest extant wild populations of crocodile lizards,for three consecutive years in Guangxi,China.We found that crocodile lizards generally reach sexual maturity at the age of 2.5 years in the wild.Unlike most viviparous lizards,which reproduce annually,the crocodile lizard shows a biennial reproductive cycle.The number of observed juveniles and subadults fluctuated between years,whereas that of adults remained relatively stable.Non-adults had longer three-year dispersal distance than adults.Crocodile lizards showed preference for backwater pools in the stream.Competition for better resources may be the main trigger for dispersal.
Keywords age structure,demography,migration,population dynamic,sexual maturity
1.Introduction
The Chinese crocodile lizard (Shinisaurus crocodilurus) is a semiaquatic viviparous lizard,living in streams of evergreen mountain forests (Huang,2009;van Schingenet al.,2015),and is now restricted to several isolated areas in southern China and northern Vietnam.Its wild populations have been threatened by habitat destruction and poaching for pet trade (van Schingenet al.,2014).The population size ofS.crocodiluruswas estimated at 1,000 in the wild (Huanget al.,2008;van Schingenet al.,2016).Consequently,it has an endangered status on the Red List of the World Conservation Union (Nguyenet al.,2014) and listed on the Convention on International Trade in Endangered Species Appendix I in 2016 to prohibit international trade.In China,where most crocodile lizards live,nature reserves were built to protect extant wild populations,and breeding centers were set up to maintain captive populations for genetic rescue and reintroduction (Liet al.,2019).However,little is known about its growth,reproduction,and dispersal ability in the wild.Such knowledge provides important information for population dynamics and hence,conservation management (Ebrahimi and Bull,2014;Laneet al.,2021;Nicholsonet al.,2020).
Crocodile lizards were reported to reach sexual maturity at 3.5-4 years of age in the wild (Zhang,2002);however,captive female lizards can breed at the age of two years (Liet al.,2019).Empirical evidence suggests that captive lizards grow faster than wild lizards (Luoet al.,2018),which leads to the earlier sexual maturation of captive populations.The approximate age of crocodile lizards is often determined based on snoutvent length (SVL) (van Schingenet al.,2014;van Schingenet al.,2016),but the growth rate of wild lizards is unknown.Direct observation of the growth of wild individuals is thus needed to more accurately estimate the age of lizards in the wild.
Crocodile lizards mate from late March or early April (i.e.,after emergence from hibernation) to late May.Well-developed lizards stay in the maternal uterus through the winter until birth in next spring,leading to a gestation time of up to 10 months (Liet al.,2019).Most viviparous lizards have a short gestation time of 2-4 months and reproduce annually (Stewart and Blackburn,2014).Prolonged gestation,reported mostly in lizards living in harsh environments,such as high-altitude regions (Borettoet al.,2018;Cabezas-Carteset al.,2010;Castroet al.,2018;Olsson and Shine,1999) or high-latitude regions(Fernandezet al.,2015;Holmes and Cree,2006;Ibargüengoytía and Casalins,2007;Wilson and Cree,2003),often leads to biennial reproductive cycles.However,long gestations do not always prevent annual reproduction.For example,the BrazilianMabuyalizard,Mabuya heathi,reproduces annually despite a gestation period of about 12 months (Vitt and Blackburn,1983).Whether prolonged gestation results in the skipping of reproductive seasons in crocodile lizards is unknown.
Dispersal ability is an important factor influencing population dynamics (Shang,2010).Dispersal between populations allows gene flow,which is important for endangered species (Dale,2001;Kirchneret al.,2003) that are often confined to small fragmented populations,and therefore,threatened with inbreeding depression (Hedrick and Garcia-Dorado,2016).The crocodile lizard only lives along streams;its annual home range was reported to be 69.34 ± 71.11 m(n=21) using the radio telemetry method,showing limited dispersal ability (Qin,2019).However,juveniles were not tracked due to the limitation of methods (body size must be able to carry a radio transmitter).Natal dispersal is a critical force of population spread,recolonization,and gene flow(Sutherlandet al.,2000).Assessment of natal dispersal is needed for understanding the crocodile lizard’s dispersal ability.
In this study,we monitored a wild crocodile lizard population for three consecutive years.The individualbased data allowed us to quantify the growth,population demography,and movement of individuals in this three-year period.Here we focused on analyzing age structure fluctuations between years to study the reproductive cycle and exploring factors that influence long-term dispersal of the crocodile lizard.We aimed to gain insights into the cause and strength of dispersal in crocodile lizards,which could be useful for habitat conservation and assisted migration for genetic rescue or reintroduction of new populations.
2.Materials and Methods
2.1.Study site and field surveysWe collected long-term field monitoring data from the Daguishan National Nature Reserve(24°09′N,111°81′E),Guangxi Province,China.Crocodile lizards mainly inhabit three adjacent streams (Figure 1A),namely Dachaichong (DCC,1,750 m),Yusanchong (YSC,1,650 m),and Chishuichong (CSC,900 m).We used position signs (Figure 1B),which were set up along the stream every 10 m,to locate the position of the spotted lizard.Crocodile lizards are diurnal and usually reside on branches or leaves above water during night (Figure 1B) (Yuetal.,2006;Yanget al.,2020).This allowed us to capture the animals during field surveys at night and collect data with minimal disturbance to their daily activity.Each lizard has a unique tail stripe pattern,which can be used as an individual identifier (Figure 1C) (Wang,2011).Field monitoring has been performed by the reserve since 2009,but without consistency and detailed data recording.In this study,we conducted field surveys from 2018 to 2020 in the three streams,2-4 times each year,during the non-hibernation season of crocodile lizards (from May to October,Table 1).Each survey was performed by 3-4 people for safety reason and for in-depth observation.After 8 pm on sunny days,we began at position zero downstream and walked upwards along the stream until the end of the stream.When we could not walk through a stream in one night (because of time limitation or weather conditions),we continued from the end point of the previous day.Upon spotting a lizard asleep on a perch,we carefully captured it with latex-gloved hands,recorded data,and then released it immediately at the same place to minimize disturbance.We collected data for body mass,SVL,sex,position,and whether the lizard was within a 0.5-m range of a backwater pool (the part of a stream that flows slower than the main current,usually wider and deeper than the upper and lower parts of the stream and formed after a small waterfall)(Figure 1D).Body mass was measured with a portable electronic scale to the nearest 0.1 g.SVL was measured with a steel ruler to the nearest 1 mm.The position of the lizard was recorded by measuring its distance from the nearest position sign using a measuring tape to the nearest 0.5 m.Pictures of tail stripes were taken for each individual.

Table 1 Summary of field surveys conducted in three streams of the Daguishan National Nature Reserve from 2018 to 2020.

Figure 1 A: Distribution of the three streams where Chinese crocodile lizards inhabit in the Daguishan National Nature Reserve;Map approval number: GS(2020)3185.B: Position signs along the stream.C: The unique tail stripe pattern of Chinese crocodile lizards.D: A typical backwater pool in the stream;Backwater pools are usually formed after a small waterfall.
2.2.Individual identification and long-time trackingWe compared tail stripes of lizards observed from different surveys manually.On the first survey,we numbered each lizard we recorded in the order of natural numbers.On the following surveys,we compared newly observed lizards with the recorded individuals.Lizards with the same tail stripe pattern were assigned the same identification number,and newly recorded lizards were numbered continuously in sequence.Identification of individuals from different surveys at different times enabled us to track the growth and movement of lizards during the three-year period.In this study,we did not identify any inter-stream migrations,so we tracked individuals in each stream separately.
2.3.Data analysisPrecisely estimating the age of wild lizards is challenging.However,we could estimate their age group(juvenile,subadult,and adult) based on SVL (van Schingenet al.,2014;van Schingenet al.,2016).We identified juveniles based on the yellow area extending from their snout to the postorbital (Figure 2A),so that the exact age of the lizards is known during our surveys.Based on the observations with known age,we first tested factors influencing SVL using a general linear regression model (GLM).SVL was the dependent variable.Sex,sub-population (different streams),and growth time (defined as the main active season of crocodile lizards,i.e.,from May to September,153 d in total) were set as independent factors.We then built simple linear regressions of SVL against growth time.Regression coefficients were estimated and then used to calculate the growth time of our observations with unknown age,based on their SVL.Then age was estimated as the rounding of growth time divided by 153.Female sexual maturity of 121 mm in SVL was adopted from breeding records in the captive population (133.47 ± 8.73 mm for 19 lizards that reproduced at the age of two years) (Liet al.,2019).Male lizards were also able to mate at the age of two years in captivity.Male sexual maturity was set at 131 mm,according to recorded mating behavior (Yang,2019).Descriptive statistics are shown as mean ± SD.
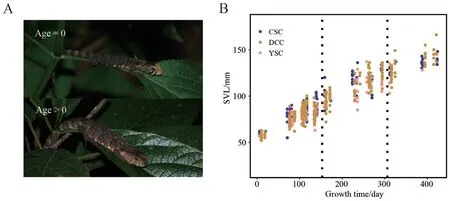
Figure 2 A: Identification of juvenile lizards (age=0) from the yellow spot on their heads.B: The linear correlation of snout-vent length (SVL) on the growth time of lizards with known age at each observation during our surveys;Vertical dot lines separate different growing seasons.
After estimating the age group for each observation,we counted the number of individuals in different age groups for assessing age structure and population fluctuation in the three streams separately.To make data comparable between years,observations from May 2020 and October in 2019 and observations in DCC in positions higher than 1,250 m in 2019 and 2020 were excluded in this analysis.We also used survey data of July from 2014 to 2019 to analyze the overall trend of population fluctuation in YSC,because surveys have been conducted in July every year.If surveys were conducted more than once a year,only data from the first survey was used.Interannual fluctuations in age structure were tested using Pearson’s chi-squared tests and changes in the proportions of juveniles,subadults,and adults between years were tested using pairwisez-tests for independent proportions with the Bonferroni-correctedP-values.Differences in the proportions of juveniles,subadults,and adults between even and odd years in YSC from 2014 to 2019 were tested using the Wilcoxon ranksum test.
The three-year dispersal distance was calculated as the longest distance between two observed positions of a lizard over three years,so only lizards observed both in 2018 and 2020 were retained for this analysis.Body condition of each lizard on an observation was calculated using the scaled mass index (SMI)(Peig and Green,2009).SMI values were calculated for each record using the mass and SVL,and the fixed body size was set as the mean of SVL of all records.We compared the dispersal distance of non-adults (age zero in 2018) and adults (age ≥ 2 in 2018) using GLM.Stream and sex were added as independent fixed variables.Two-way interactions between independent variables were also considered in the initial analyses,but then excluded due to their non-significant effects.Pearson’s chisquared test and pairwisez-tests for independent proportions were used to test the difference of frequencies of lizards in different age groups observed above a backwater pool.GLM was used to analyze body conditions with stream,sex,age group,and pool status (lizards living within a backwater pool or without) as independent variables.Two-way interactions were also not included because of non-significance.Data were analyzed using Excel 2016 and SPSS version 19.
3.Results
Our field surveys from 2018 to 2020 resulted in 846 valid observations of lizards.After grouping of the same individual from different observations,366 lizards were recorded (DCC:183,YSC: 95,CSC: 88).Of the 86 lizards observed in the first survey in July 2018,64 (74.4%) were recaptured once or more.Forty-five lizards recorded both in 2018 and 2020 (DCC:22,YSC: 8,CSC: 15) were used for the analysis of three-year dispersal.
The SVL of lizards showed a linear correlation with their growth time (Figure 2B).Stream (F2,527=28.90,P<0.001) and growth time (F1,527=3,853,P<0.001) significantly influenced SVL.We therefore built simple linear regressions for SVL against growth time for each stream:
SVL (mm)=0.21 × growth time (day)+55.96
for YSC (R2=0.84,F(1,108)=557,P<0.001).The coefficient of growth time and the intercept was (0.22,56.98) and (0.20,63.94)for DCC (R2=0.90,F(1,270)=2,389,P<0.001) and CSC (R2=0.88,F(1,148)=1,043,P<0.001),respectively.We used the equations to estimate the age of lizard individuals with their SVL.We found that 93.1% of observed individuals in the wild reached sexual maturity (SVL ≥121 mm for females and ≥131 mm for males)at the age of 2.5 years (from July to September in the third growing season),and the average SVL was 140.17 ± 8.12 mm (n=29),which is comparable to that of adult lizards in captivity(He,2011;Liet al.,2019).In the following analysis,individuals with age ≥2 years were assigned as adults,and individuals with age zero and one as juveniles and subadults,respectively.
The age structure in the three streams showed similar patterns.Interannual fluctuations of age structure were significant in CSC (χ²(4)=15.711,P=0.003),DCC (χ²(4)=52.193,P<0.001),and YSC (χ²(4)=21.906,P<0.001).The proportion of juveniles and subadults fluctuated in a biennial pattern.Especially,the proportion of subadults varied significantly between adjacent years in all three streams from 2018 to 2020.However,the proportion of adults stayed relatively stable in all streams,except DCC,which showed an increasing trend (Figure 3A).The seven years of survey data from YSC confirmed the interannual fluctuations of age structure (χ²(12)=53.288,P<0.001).The proportion of juveniles and subadults varied between adjacent years,but statistical power decreased after Bonferroni’s correction (Figure 3B).The proportion of observed juveniles in even years was larger than that in odd years(Wilcoxon rank-sum test,P=0.034).The pattern was opposite for subadults (Wilcoxon rank-sum test,P=0.034),whereas it was absent in adults (Wilcoxon rank-sum test,P=1),supporting the biennial fluctuation cycle of juveniles and subadults.
Sex (F(1,40)=1.452,P=0.235) and stream (F(2,40)=0.761,P=0.474) did not significantly influence three-year dispersal distance.Non-adults had a significantly longer three-year dispersal distance than adults (F(1,40)=4.874,P=0.033) (Figure 4A).We counted the observed times of lizards in backwater pools according to age groups.We found that the frequencies of lizards found in backwater pools were different for different age groups (χ²(2)=66.425,P<0.001).Older lizards were found more frequently in backwater pools (Figure 4B).Furthermore,all 10 lizards with extremely low three-year dispersal distance(<10 m) were found in backwater pools in 2020.Thus,crocodile lizards,especially the adults,showed a preference for backwater pools.Moreover,lizards living in backwater pools had significantly better body condition than those not (F(1,836)=5.901,P=0.015).Sex (F(1,836)=10.372,P=0.001) and stream(F(1,836)=14.482,P<0.001) also significantly influenced the body condition of lizards.
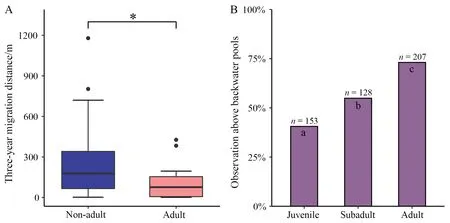
Figure 4 A: Comparison of three-year dispersal distance between adults and non-adults (age zero in 2018). The asterisk indicates P<0.05.B: Observed frequencies of Chinese crocodile lizards perching above backwater pools (i.e.,within a 0.5-meter range of a backwater pool) during the field surveys.Numbers above the bar represent observed times of lizards perching above backwater pools.Letters inside the bar represent results of pairwise z-tests for independent proportions.Different letters between two age groups represent significant difference of the frequencies of lizards observed perching above backwater pools (P<0.05 after Bonferroni’s correction).
4.Discussion
Our individual-based surveys provided monitoring data of lizards in the wild for three years.Individual growth and age structure of lizards in the wild were then quantified.The dispersal distances of lizards were also tracked and compared between age groups.Captive lizards could breed after two growing seasons (Liet al.,2019;Luoet al.,2018).We found that most lizards in the wild could also reach sexual maturity(judged by SVL) at 2.5 years old,which is much earlier than the previously reported 3.5-4 years (Zhang,2002).However,the mating season of crocodile lizards starts immediately after they emerge from hibernation in late March or early April,and ends in late May.Therefore,although female lizards reach maturity at 2.5 years old,reproduction begins at three years old.Two-year fluctuation cycles of age structure in wild lizards indicate that crocodile lizards likely reproduce biennially.Most female lizards may skip the following reproductive season after giving birth in the spring.More direct data of the reproductive cycle of lizards in the wild could be collected using other noninvasive methods,such as abdominal palpation,to assess reproductive conditions (Holmes and Cree,2006). In addition to a seemingly biennial reproductive cycle,crocodile lizards have prolonged gestation,resulting in parturition in the spring,which possibly increases the survival rate of newborn lizards (Liet al.,2019;Olsson and Shine,1998).These reproductive characteristics are often associated with lizards living in cold environments (Stewart and Blackburn,2014),but rarely possessed by lizards in temperate environment such as crocodile lizards (crocodile lizards inhabit subtropical montane forests),which raises the question about their adaptive origin.Our data also reveal that the populations of the three streams are reaching their carrying capacity.The number of adults is relatively stable in each stream,despite the birth of many lizards every two years (Figure 3),indicating that recovering of the crocodile lizard’s wild populations requires preservation of suitable habitats and recolonization of populations,which is being carried out by the reintroduction of captive-bred individuals to their former habitat (Tanget al.,2019).
Our data show that non-adults have longer dispersal distances than adults,and juveniles and subadults are less frequently observed in backwater pools.Animal dispersal can be triggered by various factors,including competition for resources (Ebrahimi and Bull,2014;Pavlovet al.,2021),escaping from predators (Gilliam and Fraser,2001;Winandyet al.,2019),and avoidance of inbreeding (Huet al.,2017).The crocodile lizards showed preference for backwater pools,which has been described in other studies (Ninget al.,2006;van Schingenet al.,2016;Yuet al.,2006),and is largely supported by our observation data (Figure 4B).Our data also show that individuals observed near backwater pools have a better body condition.This could be explained by the better quality of territory around backwater pools and covariation between individual quality and territory quality (i.e.,the best territories are occupied by the strongest individuals through competition) (Sergioet al.,2009;Zabala and Zuberogoitia,2014).Backwater pools may have a higher abundance of food because we have observed that several crocodile lizards inhabited the same large backwater pool.Nevertheless,whether backwater pools offer better food resources or other benefits to crocodile lizards should be further investigated.Our results suggest that competition for better resources,such as backwater pools,facilitated the dispersal of juvenile lizards.
The use of tail stripes as IDs enabled us to conduct an individual-based study on the population dynamics of crocodile lizards in the wild.The limited dispersal ability of crocodile lizards also facilitated the identification of individuals through tail stripe pictures from different field surveys,because most lizards did not migrate much between two surveys.However,the identification of possible migration between different streams demands exponential growth of labor as data grow.The development of automated computer programs to identify lizard individuals through tail stripes or other natural marks may enable large scale monitoring of the wild populations and exploring inter-stream migrations in future studies.
AcknowledgementsWe thank Weiguo DU for helpful advice on data analyses and manuscript preparation.We thank Jiasong HE,Yaohuan CHEN,Wei ZHANG and other staff in Daguishan National Nature Reserve for help in field surveys.We also thank Mingxian HE,Rui CHENG,Chunying ZHONG,and Guoshuai TANG for help in field surveys.This project was supported by National Natural Science Foundation of China(31901223 and 32170528) and National Key Wildlife Protection Project of Central Finance of China (450000215020340001327).
杂志排行
Asian Herpetological Research的其它文章
- Elevational Variation in Reproductive Strategy of a Widespread Lizard:High-Elevation Females Lay Fewer but Larger Eggs
- Morphology and Histochemistry of Infralabial Glands of Two Species in the Slug-Eating Family Pareidae (Reptilia: Serpentes)
- Partial Masquerading and Background Matching in Two Asian Box Turtle Species (Cuora spp.)
- Effect of Morphology and Age on the Closure Ability of Asian Box Turtles(Cuora)
- A New Brown Frog of the Genus Rana (Anura,Ranidae) from North China,with a Taxonomic Revision of the R.chensinensis Species Group
