Morphology and Histochemistry of Infralabial Glands of Two Species in the Slug-Eating Family Pareidae (Reptilia: Serpentes)
2022-09-27DanWANGJinlongRENKeJIANGWeiWUChangjunPENGHussamZAHERDechunJIANGandJiatangLI
Dan WANG ,Jinlong REN ,Ke JIANG ,Wei WU ,Changjun PENG ,Hussam ZAHER ,Dechun JIANG* and Jiatang LI,4*
1 CAS Key Laboratory of Mountain Ecological Restoration and Bioresource Utilization,Ecological Restoration and Biodiversity Conservation Key Laboratory of Sichuan Province,Chengdu Institute of Biology,Chinese Academy of Sciences,Chengdu 610041,Sichuan,China
2 University of Chinese Academy of Sciences,Beijing 100049,China
3 Museu de Zoologia,Universidade de São Paulo,São Paulo 04263-000,SP,Brazil
4 Southeast Asia Biodiversity Research Institute,Chinese Academy of Sciences,Yezin Nay Pyi Taw 05282,Myanmar
Abstract Pareidae and Dipsadidae,two independently evolved taxa in the Serpentes lineage,both feed exclusively on terrestrial mollusks (snails and slugs).Dipsadid snakes developed hypertrophic infralabial glands in their lower jaw,which are thought to be associated with their specialized feeding behaviors.However,whether a similar gland exists in pareid snakes is unknown.In this study,we examined the morphological characteristics of the infralabial glands in Pareas berdmorei and Pareas chinensis based on comparative anatomical,histochemical,and histopathological analysis.Our results demonstrated that both Pareas species had similar hypertrophied infralabial glands in the lower jaw,which consisted of tubules with mucinous properties and seromucous acini.The secretory granules of the seromucous cells also showed high electron density.The cytoplasm was rich in rough endoplasmic reticulum,mitochondria,and Golgi apparatus,suggesting that these cells may secrete protein substances,and play an important role in digesting mollusks.This study provides evidence of morphological convergence between Pareidae and Dipsadidae due to specialized diet adaptation,which will be the foundation for prospective functional research.
Keywords convergent evolution,histology,Pareas berdmorei,Pareas chinensis,specialized diet
1.Introduction
Snakes display exceptionally diversified feeding habits.Some groups consume a wide variety of animals including both vertebrates and invertebrates,while others exhibit strong dietary specialization (Greene,1997;Lillywhite,2014;Zug,1993) such as crustacean-eating snakes,egg-eating snakes,and ant/termite-eating blind snakes (Scolecophidia) (Broadley,1979;Colemanet al.,1993;Jayneet al.,2018;Webbet al.,2000).Many snakes within the family Dipsadidae are considered specialists,feeding solely on terrestrial mollusks,e.g.,snails of the genusDrymaeusand slugs of the genusSarasinula(Cundall and Greene,2000;Dunn,1951;Peters,1960;Sazima,1989).Some genera within Dipsadidae have developed hypertrophied and specialized infralabial glands in their lower jaw.These glands produce secretory products that are predominantly serous in nature and may be related to the specialized mollusk diet (de Oliveiraet al.,2014;de Oliveiraet al.,2008;Zaheret al.,2014).Notably,injection of infralabial gland extracts in the visceral mass of snails can result in paralysis and,in some cases,death(Laporta-Ferreira and Salomão,1991;Salomão and Laporta-Ferreira,1994).For example,the infralabial glands of the neotropical slug-eating snakeSibynomorphus mikanicontain a high protein content,and extract-injected slugs show an increase in mucus secretion,contortions,and immobilization of the body (Salomão and Laporta-Ferreira,1994).Thus,infralabial gland specialization in some genera of Dipsadidae may be useful for neutralization of mollusk secretions and later digestion.
Snakes in the Southeast Asian family Pareidae are also considered dietary specialists,preying exclusively on terrestrial snails and slugs (Cundall and Greene,2000).The family Pareidae,which is widely distributed across South and Southeast Asia,currently consists of four genera: i.e.,PareasWagler,1830;AplopelturaDuméril,1853;AsthenodipsasPeters,1864;andXylophisBeddome,1878 (Deepaket al.,2018;Wanget al.,2020;Zhao,2006;Poyarkovet al.,2022).These snakes exhibit several morphological changes associated with specialized foraging behavior,such as asymmetry in the number of mandibular teeth (Hosoet al.,2007).Almost all pareid snakes,except for non-snail-eating specialists,have more teeth in the right mandible than the left for functional specialization in feeding on the dextral majority of land snails (Hoso,2017).Fewer teeth in the left mandible may be useful for smoothly biting the sticky tissue of snails,while more teeth in the right mandible may be useful for firmly grasping (Changet al.,2021;Hoso,2017;Hosoet al.,2007).Specialization of skull morphology is also found in some pareid and dipsadid snakes,including short snout and pterygoids,reduced supratemporals,long mandibles,and complete separation of the posterior end of the pterygoid from the quadrato-mandibular joint.This connection makes the mandible more flexible,which can help when preying on terrestrial snails (dos Santoset al.,2017;Kojimaet al.,2020).Although specialized feeding behavior and associated morphological changes are well documented in the family Pareidae (Danaisawadiet al.,2016;Götz,2002),it remains unknown whether well-developed infralabial glands similar to those found in dipsadid snakes also exist in this Asian slugeating group.
In the current study,we investigated infralabial glands and associated structures in pareid snakes and correlated gland structure with secretion biochemistry and the peculiar feeding habits of this group.We focused on morphology,histochemistry,and arrangement of different cell types within the secretory units of the infralabial glands of two species ofPareas.This work will provide a foundation for further research on the functional mechanisms of these specialized glands.
2.Materials and Methods
2.1.Samples collection and processingThree individuals ofP.berdmoreiwere collected from Mengla county,Xishuangbanna,Yunnan Province,China and three individuals ofP.chinensiswere from Mt.Emei,Leshan city,Sichuan Province,China in this work.An adult male ofP.berdmorei(total length 620 mm)was used to predation experiment.The snake was starved for three days before the predation experiment,and the snake was then placed on a dissecting tray with dead leaves (41.5 cm length× 31.2 cm width × 3.5 cm height),three snails of equal size were placed in front of the snake.At a distance of one meter from the snake,we used the camera equipped in the laboratory to film the whole process of preying on the snail.On the basis of existing specimens in the laboratory,we selected snakes with different feeding habits.A total of other four families,including nine genera and 16 species were used for comparison of infralabial gland (Table 1).The specimens were euthanized via an intraperitoneal injection of sodium thiopental,and then we removed the entire head from the specimens at the first cervical vertebra and skinned off the head.The infralabial glands were dissected individually for histological and histochemistry study.All specimens in this work were preserved in the Herpetological Museum,Chengdu Institute of Biology (CIB),Chinese Academy of Sciences (CAS),Chengdu,Sichuan,China.

Table 1 Information on voucher numbers and localities of 16 snake species used in this study.
The entire head was fixed in 10% neutral formalin fixative solution for 24 h and subsequently submitted to decalcification in 4.13% aqueous EDTA,pH 7.2,renewed every other three days,and kept in constant stirring for a month.After head decalcification was completed,the entire head was divided into left and right parts from the middle,put the head in gradient ethanol for dehydration,and embedded in paraffin,and submitted to serial sagittal or transversal sectioning.We used pathology slicer of Shanghai Leica Instruments Co.,Ltd,model RM2016 to obtain sections of 4 μm.Dissected infralabial glands were fixed in 4% paraformaldehyde with PBS 0.1 M,pH 7.2 for 24 h,and in gradient ethanol for dehydration,and embedded in paraffin,and conducted to serial sagittal or longitudinal sectioning.We used same pathology slicer to obtain glandular sections of 4 μm.
2.2.Histology and histochemistryAll sections of the head were stained with hematoxylin-eosin (HE) staining for general study of the tissues.A group of three sections of infralabial glands were stained with hematoxylin-eosin (HE) staining for general study of the tissues.The remaining four groups glandular sections were used to following histochemical staining steps,the alcian blue (AB) pH 2.5,periodic acid-Schiff(PAS) and combined alcian blue (AB) pH 2.5 and periodic acid-Schiff (PAS) for identification of acid and natural mucosubstances,and Coomassie brilliant blue R250 for identification of protein (Kiernan,2015).
2.3.Transmission electron microscopyFresh tissues were selected to minimize mechanical damage such as pulling,contusion and extrusion.We used a sharp blade to quickly cut and harvest fresh tissue blocks within 1-3 min.The size of tissue block should be no more than 1 mm3.The removed tissue block was immediately put into 2.5% glutaraldehyde fixative solution for 24 h (Sabatiniet al.,1963),which was fixed at 4 ℃for preservation and transportation.Tissues were fixed in 1%OsO4with PBS 0.1 M,pH 7.4 for 2 h at room temperature and kept from light,and then dehydrated in ethanol and embedded in resin (EMBed 812).The resin block was cut to 60-80 nm ultrathin sections on the ultramicrotome,2% uranium acetate saturated alcohol solution avoid light and 2.6% Lead citrate avoid CO2staining.The ultrathin sections are observed and recorded under TEM (Transmission Electron Microscope).
2.4.Photomicrograph acquisitionPhotomicrographs were obtained with a Cnoptec B302 microscope and this microscope equipped with a digital camera (Sony ICX285AQ CCD) and with Image acquisition software the ImageView in computer.
3.Results
3.1.Feeding behavior and presence of infralabial glandsIn this work,we observed the predatory processes and relevant behaviors ofP.berdmoreithrough feeding experiments,demonstrating similar results to previous studies (Danaisawadiet al.,2016;Götz,2002),i.e.,pre-capture,feeding,and post-feeding stages (Figure 1).Based on comparative anatomy,our results showed that,compared with other snake species with different feeding habits,members of the genusPareashad hypertrophied infralabial glands in the lower jaw (Figure 2).
The infralabial gland inP.berdmoreiwas whitish,extended,and hypertrophied.The gland was attached to the mandible,from the anterior tip of the dentary to the anterior portion of the compound bones,and the entire gland was wrapped and dispersed in muscle (Figure 3).
3.2.General morphology of infralabial glandWe investigated the glandular structure,cell types,and physiological and biochemical properties of cell contents inP.berdmoreiandP.chinensisusing histological and histochemical methods.Results showed that the infralabial glands were wrapped in a thin layer of connective tissue,which divided the glandular body into acinar lobules of varying size in a mesh formation (Figures 4A,5A).The acini were typically arranged with multiple polygonal cells,predominantly mucous and seromucous cells,with large ducts formed in the middle of the mucous cells (Figures 4A,5A).The acini contained multiple ducts formed by long columnar mucous cells surrounded by seromucous cells,which were confined to the peripheral area of the mucous cells (Figures 4A,5A).
InP.berdmorei,the long columnar mucous cells usually formed ducts with mucinous properties in the central region of the acini,which were characterized by cytoplasmic secretory granules and flat basal nuclei pressed to the edge of the cell by cellular content (Figure 4C,4D).The seromucous cells were irregular polygonal cells with round basal nuclei and cytoplasm full of secretory granules (Figure 4B,4D).Two different types of secretory granules were observed in the seromucous cells (sm1and sm2).Seromucous cell type 2 (sm2) showed stronger reaction to periodic acid-Schiff (PAS) than seromucous cell of type 1(sm1).The former was less abundant,while the latter was more abundant (Figure 4E,4F).

Figure 1 Process and behavior displays of P.berdmorei in predation on a snail.A: When the snail fully stretches its soft body,the snake begins to approach the snail,tilting its head and pursues the snail.B: After determining the appropriate attack position,the snake attacks and bites the snail.C: At the moment of strike,the snake inserts its mandibles into the aperture (pink arrowhead) and to lean the upper jaws against the ventral outer surface of the aperture (black arrowhead).D: After extracting the soft part of the snail,extracts the mandible from the shell,remaining snail shell and viscous mucus (black arrowhead).
InP.chinensis,the glandular body was mainly composed of mucous and seromucous cells,with different-sized lumina.The seromucous cells were more dominant than the mucous cells in the acini (Figure 5B-E).Columnar mucous cells formed different-sized lumina,with flat basal nuclei and cytoplasm full of secretory granules (Figure 5C,5D).We observed two different types of mucous cells (i.e.,m1and m2) based on the staining properties of their secretory granules: i.e.,cells with medium granules (m1) and cells with strong-stained granules(m2),indicating different mucosubstances (Figure 5D).The seromucous cells formed different-sized vesicles (v),with round basal nuclei and cytoplasm containing secretory granules.We also observed two different types of seromucous cells,i.e.,the more abundant type 1 (sm1) and less abundant type 2 (sm2),similar toP.berdmorei(Figure 5E,5F).
3.3.Histochemistry of infralabial glandInP.berdmorei,most secretory cells of the acini were positive to both periodic acid-Schiff (PAS) and Coomassie brilliant blue R250,and negative to Alcian blue pH 2.5,indicating the seromucous nature of these cells (Figure 4B-E).The seromucous cells showed stronger positive reaction to PAS than the mucous cells,and the secretory granules in the sm2cells showed a stronger positive reaction to periodic acid-Schiff (PAS) than the sm1cells,indicating differences in protein content (Figure 4E,4F).Duct and columnar mucous cells around the duct were strongly positive to Alcian blue pH 2.5 and negative to Coomassie brilliant blue R250,indicating their mucinous properties (Figure 4B,4D).
InP.chinensis,most secretory cells were positive to both periodic acid-Schiff (PAS) and Coomassie brilliant blue R250,and negative to Alcian blue pH 2.5,similar to that observed inP.berdmorei(Figure 5B-E).The m2cells showed stronger positive reaction to Alcian blue pH 2.5 than the m1cells,indicating differences in mucosubstance content (Figure 5D).
3.4.Ultrastructure of infralabial glandThe ultrastructure can reflect the subcellular components of secretory cells.Ultrastructural criteria are mainly based on the electron density of secretory granules,with high electron density indicating rich protein content (related to serous glands) and low electron density indicating poor protein and rich mucous content(related to mucous cells) (Kardong and Luchtel,1986;Yoshieet al.,1982).The ultrastructure of the infralabial gland inP.berdmoreishowed that the acini were predominantly composed of secretory granules with different electron densities and polygonal cells of different sizes.A large duct was formed in the middle of the secretory cells,and lumen-facing microvilli protuberances were found near the bottom of the secretory cells(Figure 6A).These microvilli protuberances may participate in exuding secretions to the outside of the cell.The electron density of the seromucous cells was higher than that of the mucous cells.The seromucous cells contained unique spherical or elliptical granules,which were more electron-dense and heterogeneous when compared to mucous cells,indicating differences in cell content between the cell types (Figure 6C).Mucous cells around the duct contained uniform secretory granules with medium electron density (Figure 6B),whereas the seromucous cells contained secretory granules of varying size with higher electron density.Cytoplasm of the perinuclear region of the seromucous cells was rich in mitochondria (mi)and rough endoplasmic reticulum (er),typical characteristics of protein-secreting cells.Desmosomes were found at the junction of adjacent cells (Figure 6D).
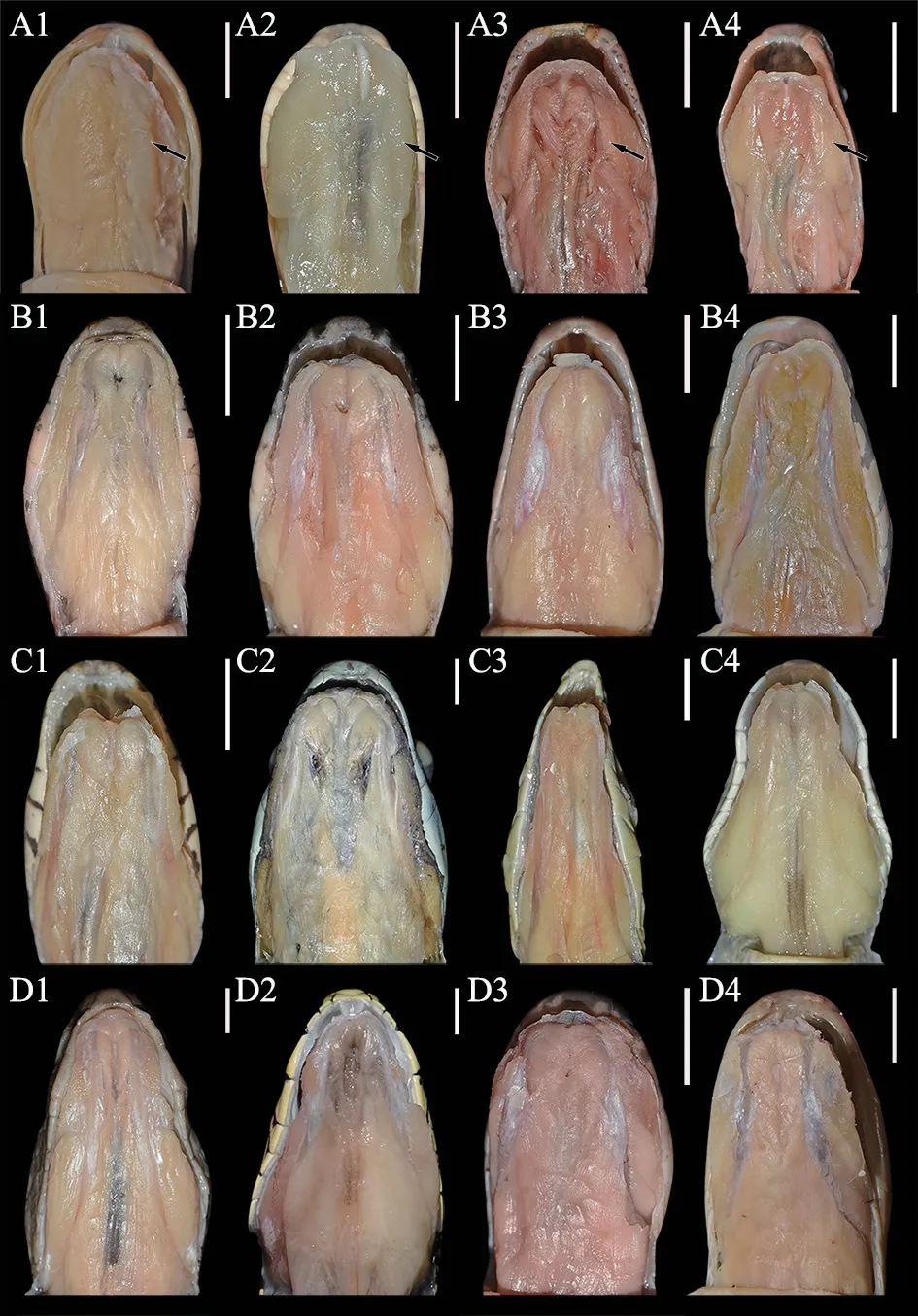
Figure 2 The lower jaw anatomy of the genus Pareas snakes and other snakes with different dietary habits.(A1-A4) The first row is P.berdmorei (CIB 119346),P.chinensis (CIB 119351),P.formosensis (CIB 119336),P.nigriceps (CIB 119350),respectively;(B1-B4)the second row is Lycodon rufozonatus (CIB 119349),Boiga multomaculata (CIB 119344),Oreocryptophis porphyraceus (CIB 119342),Pseudoxenodon macrop (CIB 119337),respectively;(C1-C4) the third row is Boiga multomacula (CIB 119347),Boiga cyanea (CIB 119340),Ahaetulla prasina (CIB 119339),Trimeresurus guoi (CIB 119348),respectively;(D1-D4) the fourth row is Protobothrops mucrosquamatus (CIB 119341),Protobothrops jerdonii (CIB 119343),Bungarus multicinctus (CIB 119338),Bungarus wanghaotingi (CIB 119345),respectively.The first raw is the species of Pareas,we observed hypertrophic infralabial glands in the lower jaw (black arrowhead),whereas other snakes with different dietary habits have not hypertrophic infralabial glands in the lower jaw.Scale bar=5 mm.

Figure 3 Histological sections of the head of P.berdmorei.A: Lateral view of the head of P.berdmorei show the hypertrophy of the lower jaw.Not to scale.B: Sagittal section of the head stained with HE,showing that the infralabial gland is attached to mandible,from the anterior tip of the dentary to the anterior portion of the compound bones,occupying approximately 80% of the total head length,the entire gland wrapped by and dispersed in associated muscle.C: Longitudinal section of the mandible,showing the entire infralabial gland wrapped by the muscle adductor mandibulae externus medialis (aem).Hg: harderian gland;oc: oral cavity;e: eye;mx: maxillary;sl:supralabial gland;cp: compound bone;d: dentary bone;il: infralabial gland;aem: the muscle adductor mandibulae externus medialis.Scale bar=5 mm.
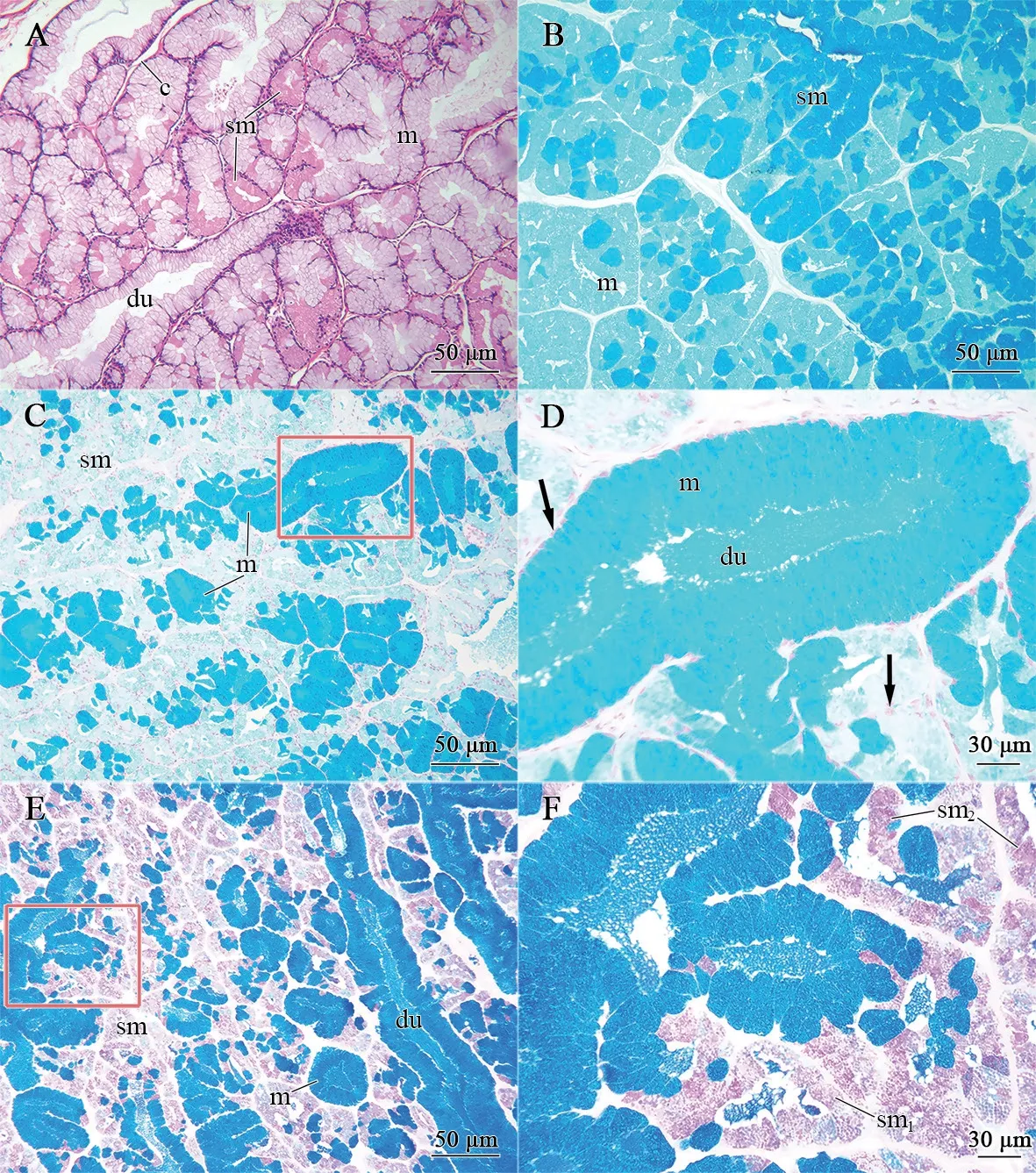
Figure 4 Morphological and histochemical condition of the infralabial glands in P.berdmorei.A: Sagittal section stained by Hematoxylin-eosin,showing general structure of the glandular body,mainly constituted of the duct (du) with mucous cells (m) and acini with seromucous cells (sm),divided by connective tissue (c) into acinar lobules of varying sizes.B: Transverse section stained by Coomassie brilliant blue R250 histochemical method,two types of cells have different reaction intensity,showing a strong positive reaction in seromucous cells (sm) whereas a weak positive reaction in mucous cells (m),indicating the difference in protein content between seromucous cells and mucous cells.C: Method of alcian blue,pH 2.5,showing a positive result for the mucous cells (m),indicating the presence of acid mucous,and contrasting with the seromucous cells (sm),negative to the method.Scale bar=50 μm.D: Higher magnification of the region marked in C,stained by alcian blue,pH 2.5,showing long columnar mucous cells and flat basal nuclei (arrowhead) and ducts (du) formed in the middle.Scale bar=30 μm.E: Longitudinal section conjugated reaction to the methods alcian blue,pH 2.5 and periodic acid-Schiff (PAS),showing mucous cells and duct have positive reaction for alcian blue,pH 2.5,while seromucous cells have lightly positive reaction for PAS.Scale bar=50 μm.F: Higher magnification of E,showing the differences in reaction intensity of seromucous cells.The most reactive cells seem to be the seromucous type 2 (sm2) whereas response strength of seromucous type 1 (sm1) is relatively weak.Scale bar=30 μm.
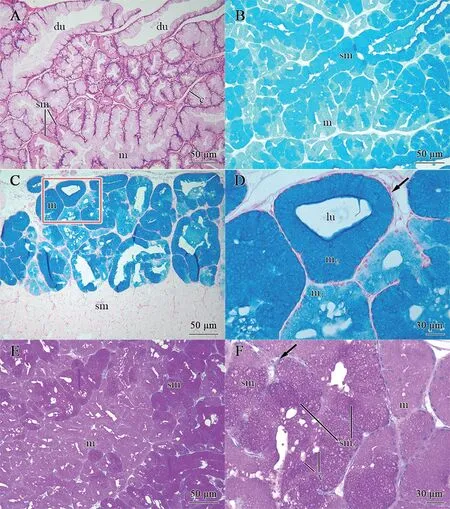
Figure 5 Morphological and histochemical condition of the infralabial glands in P.chinensis.A: General structure of the glandular body,mainly constituted of the duct (du) with mucous cells (m) and acini with seromucous cells (sm),divided by connective tissue (c) into lobules of varying sizes.B: Sagittal section stained by Coomassie brilliant blue R250 histochemical method,showing a strong positive reaction in seromucous cells (sm) whereas a weak positive reaction in mucous cells (m).C: Alcian blue,pH 2.5 histochemical method,showing positive results for two types of mucous cells (m) and a negative result for the seromucous cells (sm),indicating a different type of mucous content in these cells.Scale bar=50 μm.D: Higher magnification of glandular body marked in C,showing a few large acini,which are constituted of mucous cells (m),with flat basal nuclei (arrowhead),two types of mucous cells (m),i.e.,m2 have more strongly positive reaction to alcian blue,pH 2.5 than m1.Scale bar=30 μm.E: Sagittal section stained by method of periodic acid-Schiff (PAS),showing all types of cells are positive result to PAS,only varying in intensity,in each type.Scale bar=50 μm.F: Higher magnification of E,showing the differences in reaction intensity of each cell type.The seromucous cells have stronger reaction to PAS than mucous cells,and have round basal nuclei (arrowhead).The seromucous type 2 (sm2) have stronger positive reaction whereas seromucous type1 (sm1)has a weaker reaction intensity.Some vesicles (v) of varying sizes filled with secretory granules among these cells.Scale bar=30 μm.
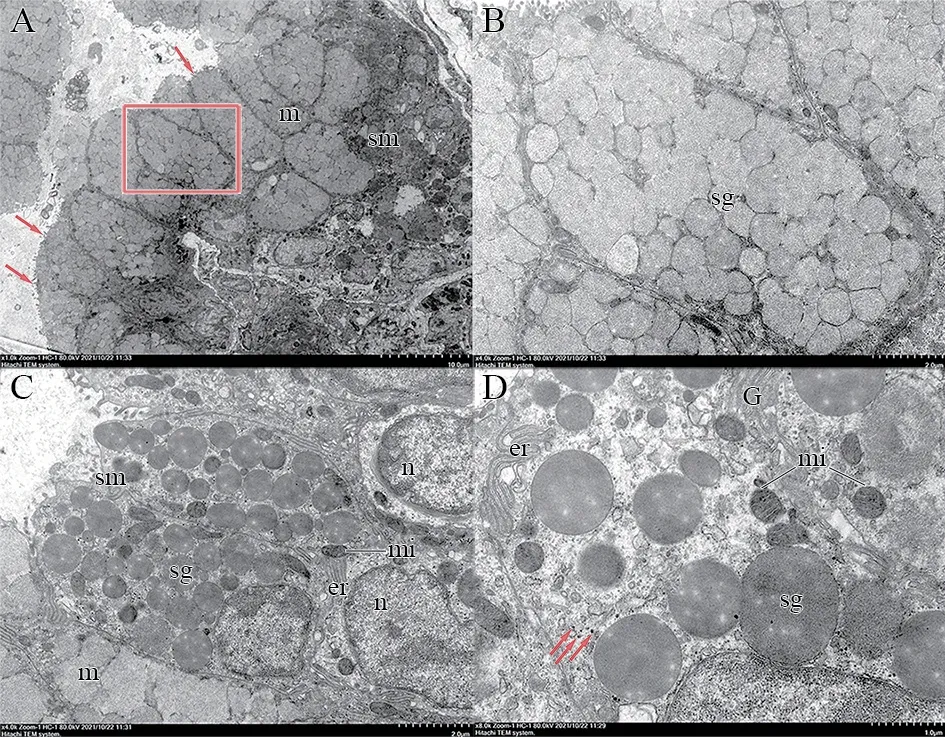
Figure 6 Transmission electron microscopy of the infralabial gland of P.berdmorei.A: Sagittal section of the gland show that acinus is composed of most polygonal cells,packed with medium electron dense homogeneous secretory granules in mucous cells (m) and other type of cell present on the periphery of mucous cells,cytoplasm packed with elliptical high electron dense secretory granules in seromucous cells (sm),and in the apical membrane of all types of secretory cells have microvilli facing the lumen.m: mucous cells;sm: seromucous cells;arrowhead: microvilli.Scale bar=10 μm.B: Higher magnification of mucous cells and cytoplasm packed with medium-electrodense granules (sg).C: Detail of cytoplasm of the seromucous cells (sm),show difference of electron density secretory granules between mucous and seromucous cells,among them,the seromucous cells contain high electron density granules of varying sizes and irregular basal nucleus (n),rich in endoplasmic reticulum and mitochondria.n: nuclei;mi: mitochondria;er: endoplasmic reticulum;sg: secretory granules.Scale bar=2 μm.D: Higher magnification of seromucous cells shows that have abundant mitochondria,rough endoplasmic reticulum and Golgi apparatus.There are round or oval desmosomes between adjacent cells.G: Golgi apparatus;arrowhead: desmosomes.Scale bar=1 μm.
4.Discussion
Convergent evolution usually refers to the evolution of the same or similar phenotypes from independent lineages under similar selective pressure (Christinet al.,2010;Penget al.,2022;Stern,2013;Storz,2016).In Serpentes,the South American dipsadid snakes (Dipsadidae) and Asian pareid snakes (Pareidae)have evolved independently to feed exclusively on terrestrial mollusks (snails and slugs) (Götz,2002;Sazima,1989;Zaheret al.,2019).The Pareidae family is one of the basal groups of Colubroidea,which split with other Colubroidea snakes in~62.5 million years ago (Ma),whereas Dipsadidae is a recently evolved family,which diverged~32.4 Ma (Liet al.,2020).Previous studies have indicated that snakes in Dipsadidae developed hypertrophied infralabial glands as an adaptation to their specialized feeding behaviors (de Oliveiraet al.,2014;de Oliveiraet al.,2008;Taub,1966).Based on comparative anatomy,our results confirmed that hypertrophic infralabial glands similar to those found in dipsadid snakes also exist inPareas,suggesting that these two groups evolved convergent phenotypes to target their specialized diets.
In Dipsadidae snakes that feed exclusively on mollusks,e.g.,DipsasandSibynomorphus,the infralabial glands are primarily composed of mucous and seromucous cells (Dunn,1951;Kochva,1978;Taub,1966).In contrast,in the dipsadid genusAtractus,which feeds predominantly on earthworms,the infralabial glands are primarily composed of mucous cells (de Oliveiraet al.,2008).These results suggest that different dietary behaviors in these predators may cause differences in the composition of the infralabial glands.In our study,the two species ofPareasshowed an infralabial gland composition similar to that of dipsadid snakes (de Oliveiraet al.,2008),suggesting that the seromucous cells in the infralabial glands may be specialized to snakes that feed exclusively on terrestrial mollusks.In addition,based on ultrastructural analysis,the seromucous cells were rich in cell structures correlated with protein secretion,indicating that such cells may secrete abundant proteases to aid in the digestion of food (Ichikawa and Ichikawa,1975;Yoshieet al.,1982).This is consistent with previous research reporting high protein content in infralabial gland extracts of the neotropical slug-eating snakeS.mikani(Salomão and Laporta-Ferreira,1994).Therefore,we speculate that seromucous cells are unique in the infralabial glands of snakes that feed on terrestrial mollusks in both Dipsadidae and Pareidae,and these cells may be responsible for digesting mollusks.
Previous studies have suggested that the secretions produced by the glands are discharged to the outside through ducts (de Oliveiraet al.,2016).Ducts inside glands and ducts leading to the oral cavity have been observed in neotropical goo-eating snakes (de Oliveiraet al.,2014;Laporta-Ferreira and Salomão,1991).The former are receptacles for secretions generated by secretory cells,with the latter transporting these secretions to mediate physiological functions of the organism (Rosenberg,1967).In our work,the mucous cells of the twoPareasspecies were only found in the ducts inside the glands,thus showing a similar distribution pattern as found in some dipsadid snakes(de Oliveiraet al.,2014;de Oliveiraet al.,2008).This pattern
facilitates the transportation and release of secretions generated by glandular cells (Taub,1967;1966;Yoshieet al.,1982).However,we did not observe ducts leading the oral cavity,possibly due to wrong anatomical methods and sectional angles.Therefore,how seromucous cell secretions are transported through the surrounding ducts inPareasneeds to be further researched.
AcknowledgementsWe thank Junfeng GUO,Chenyang TANG and Hanliu AI for the fieldwork.We thank Zhongliang PENG for his help in the sampling work.We thank Dihao WU and Junjie HUANG for their help in taking the photographs and editing photographs,respectively.We also thank Zeng WANG for her help in reviewing the manuscript.This work was supported by the National Natural Science Foundation of China (32220103004 and 32200363);the International Partnership Program of Chinese Academy of Sciences (151751KYSB20190024);the Sichuan Science and Technology Program (2020YFH0005);the Key Research Program of Frontier Sciences,CAS (QYZDB-SSWSMC058);the Youth Innovation Promotion Association of CAS(2021370);CAS President’s International Fellowship Initiative(2021VBA0003) (Hussam El Dine ZAHER).
杂志排行
Asian Herpetological Research的其它文章
- A New Brown Frog of the Genus Rana (Anura,Ranidae) from North China,with a Taxonomic Revision of the R.chensinensis Species Group
- Effect of Morphology and Age on the Closure Ability of Asian Box Turtles(Cuora)
- Partial Masquerading and Background Matching in Two Asian Box Turtle Species (Cuora spp.)
- Field Surveys Suggest Biennial Reproduction Cycle and Competition-triggered Dispersal of the Endangered Chinese Crocodile Lizard
- Elevational Variation in Reproductive Strategy of a Widespread Lizard:High-Elevation Females Lay Fewer but Larger Eggs
