Effect of Morphology and Age on the Closure Ability of Asian Box Turtles(Cuora)
2022-09-27FanrongXIAOZhenHONGandHaitaoSHI
Fanrong XIAO ,Zhen HONG and Haitao SHI
1 Ministry of Education Key Laboratory for Ecology of Tropical Islands,Key Laboratory of Tropical Animal and Plant Ecology of Hainan Province,College of Life Sciences,Hainan Normal University,Haikou 571158,Hainan,China
2 College of Agriculture and Rural Affairs,Hainan Open University,Haikou 570208,Hainan,China
Abstract The ability of box turtles to close their shell as an antipredation adaptation and the potential impact of the anterior and posterior lobes of the plastron on the closing force and closing time remain relatively unexplored.Here,keeled (Cuora mouhotii)and flowerback (C.galbinifrons) box turtles,whose shell cannot and can be completely closed,respectively,were studied.Anterior and posterior closing forces were measured using a force transducer,and the closing time was recorded.The anterior closing force in both turtle species was substantially greater than the posterior closing force,reaching approximately 4-fold in adults.Moreover,the anterior closing time in adults was significantly longer than the posterior closing time.This closing force difference can be attributed to the substantially smaller anterior plastron lobe than the posterior lobe in the two species.Additionally,the anterior and posterior closing forces in both species positively correlated with body weight and showed no relationship with the length of the bridge and hinge.Interspecies comparison showed that the anterior and posterior closing forces were significantly greater(approximately 2-4-fold) in flowerback box turtle than in keeled box turtles,regardless of age,and the closure time was significantly longer in adult flowerback box turtles than in adult keeled box turtles.Although the closing forces in both species showed negative allometry,the increase rate was significantly higher in flowerback box turtle than in keeled box turtle.The closing forces in both species were observed to be approximately 4-fold stronger in adults than in juveniles.No sexual dimorphism concerning the anterior and posterior closing forces was observed in either species.In summary,this is the first study to comparatively evaluate the anterior and posterior closing ability of box turtles,demonstrating that age,weight,plastron shape,and the degree of shell closure are important factors affecting closing ability.
Keywords anterior plastron lobe length,closing force,closing time,Geoemydidae,shell kinesis
1.Introduction
Chelonians have a weak escape ability owing to their special carapace.They have evolved several passive antipredation strategies relying on the hard carapace to protect them,such as retracting their heads and limbs into the carapace (Pritchard,2008).Among chelonians,box turtles (includingCuora,Terrapene,Pelusios,Emys,Cyclemys,andNotochemys) can not only retract their heads and limbs into the shell,but also possess a special closed structure that allows the plastron and carapace to be either completely or partially closed (Bramble,1974;Dodd,2001).In this closing process,a certain degree of closing force will inevitably be generated.Thus,the closing force of the plastron is an important indicator of box turtle’s closing ability and is critical for successfully using the closed-shell structure to prevent predation (Corderoet al.,2019;Prestonet al.,2020).In the past,more attention has been paid to the anatomy and evolution of the closed-shell structure of box turtles (Bramble,1974;Bramble and Hutchison,1981;Feldman and Parham,2002;Corderoet al.,2018a,2018b,2019),but very few studies have quantified and investigated the closing ability of the closedshell structure (except for Prestonet al.,2020).
There are two types of box turtles,one that can completely close the plastron (most species in the generaTerrapeneandCuora) and other that cannot (Cyclemysspecies andCuora mouhotii).However,it remains unclear whether there is a difference in the closing force between the groups.The anterior and posterior lobes in closed-shell turtles can also be closed;however,it is not clear whether there is any difference in the closing force of the anterior and posterior lobes of the plastron.Available data have only addressed the closing force of the anterior plastron of turtles (Prestonet al.,2020) and have not quantified the closing force of the posterior plastron.As animal morphology determines function,it is reasonable to speculate that,in addition to their size (Prestonet al.,2020),other morphological characteristics of turtles may affect the closing force.For example,the plastron is in motion during carapace closure,and it is unclear whether the shape of the plastron and its attached structures affect the closing force or whether sexual dimorphism exists in closing force.Furthermore,as shell kinesis develops slowly in juveniles (Corderoet al.,2018b,2019),they exhibit a more cautious behavior than adults,and it remains to be explored whether significant differences in the closing force exist between juvenile and adult box turtles.
In addition to the closing force,the closing time of the carapace is also an important indicator of closing ability(Prestonet al.,2020).Although shell closure is beneficial for antipredation,it disrupts respiration in turtles,because when the shell remains completely closed,a sufficient amount of air cannot enter the lungs.The movement of the limbs is an important aspect of lung ventilation in turtles (Johnson and Creighton,2005;Kardong,2015).Oxygen absorption may be hindered when the shell remains closed due to either impaired inhalation or organ compression.Therefore,box turtles have to balance antipredation and normal breathing,which determines the maximum duration of shell closure.For box turtles that cannot completely close their shell,the air that enters is relatively sufficient;however,it remains unclear whether they can maintain their shell closed for longer than fully closed box turtles.Furthermore,as the head of box turtles is in the front,it should also be clarified whether the closing time of the anterior plastron is longer than the posterior lobe of the plastron for them to breathe.
The keeled box turtles (C.mouhotii) and flowerback box turtles (C.galbinifrons) are two species that occupy similar habitats,the tropical and subtropical forests,but differ in their microhabitat selection (Shiet al.,2011;Xiaoet al.,2017).Predators of the two species are primarily mammals such asParadoxurus hermaphroditus(Xiaoet al.,2020).Although keeled and flowerback box turtles belong to the same genus (Stuart and Parham,2004),the former cannot completely close the shell and the latter can completely close the shell (Shiet al.,2011).In fact,the former was once a member of the genusCyclemyscharacterized by incomplete shell closure (Rhodinet al.,2017).This contrasting characteristic makes these two species well suited for a comparative study to understand the differences and the influencing factors of closure ability between the groups with incompletely and fully closed shells,respectively.Therefore,in this study,we focused on these two turtle species and explored the morphological factors influencing the closure ability of box turtles.The closing ability (including closing force and closing duration) of the species was systematically studied to determine both interspecific and intraspecific variations with respect to sex and age,and the relationship between morphology and closing force was investigated.
2.Materials and Methods
2.1.Sample size and ethics statementIn this study,38 keeled box turtles and 38 flowerback box turtles were included,including 6 juveniles,12 subadults,and 20 adults (10 males and 10 females) of each species.Age groups were determined using the carapace length (CL) in both species (Shiet al.,2011) as follows: juveniles (65.15-85.15 mm),subadults (93.41-124.35 mm),and adults (149.39-188.54 mm).All turtles were from captive collections in Qiongzhong,Danzhou,and Lingshui in Hainan Province,China during 2018-2019.Some of them were fresh wild-caught by collectors,whereas others had been kept in captivity for less than a year.
This work was approved by the Animal Research Ethics Committee of Hainan Provincial Education Centre for Ecology and Environment,Hainan Normal University(HNECEE-2019-006) and was carried out in strict accordance with the institutional guidelines.No turtle was sacrificed for this study or incurred injury.
2.2.Closing force and closing timeThe closing force was measured using a force transducer (type S2-500NHL-001,range± 500 N;Nanjing Bioinspired Intelligent Technology,Nanjing,China) that was connected to a charge amplifier (NBIT-DSU-2404A;Nanjing Bioinspired Intelligent Technology) and a portable computer.Closing force is categorized as anterior closing force (ACF) and posterior closing force (PCF).ACF refers to the closing force of the anterior plastron,whereas PCF refers to the closing force of the posterior plastron.A force transducer was placed between the carapace and the anterior or posterior plastron to measure ACF and PCF.We measured the closing force of the anterior or posterior plastron when they were completely closed with the carapaces in the case of flowerback box turtle.In keeled box turtle,the closing force was measured at the maximum closure of the anterior or posterior plastron.The ACF and PCF in each turtle were measured three times at an interval of 15 min,and the highest value was designated as the closing force in each individual.Additionally,we measured the closing time of the anterior plastron (ACT) and posterior plastron (PCT),which is the time from the beginning to the end of closure.The end of closure time was considered as the time point at which the first visual sign of movement occurred to open the shell.The ACT and PCT of each turtle were measured three times at an interval of 15 min,and the highest value was used as the closing time of each individual.
2.3.Morphological measurementsThe following morphological measurements were obtained from all experimental individuals using a digital caliper (accuracy 0.02 mm;Tricle Brand,Shanghai,China): CL (straight distance from the front of the cervical scute to the rear of the supracaudal scute),hinge length (HL,straight distance between both ends of the bridge),bridge length (BL,straight distance between the front and rear edges of the bridge),anterior plastron length(APL,straight distance from the front of the plastron to the hinge),and posterior plastron length (PPL,straight distance from the rear of the plastron to the hinge).In addition,body mass (BM) was measured using a digital scale (accuracy 0.1 g;Guangdong Senssun,Zhongshan,China).CL and BM represented the body size of the turtle.BM was also used as an indicator of muscle mass,because there is a significant correlation between BM and muscle mass in many vertebrates,including turtles and rats (Abdalaet al.,2008;Müller,1975;Schwartz,1953).
2.4.Statistical testsAll statistical procedures were performed in SPSS 16.0 (SPSS,Inc.,Chicago,IL,USA),and the plots were designed using Sigma Plot 12.5 (Systat Software,San Jose,CA,USA);all data are expressed as mean ± SE.Before running the analyses,Kolmogorov-Smirnov test was used to test the normality of the closing force,closing time,and the morphological measurement data.Results withP<0.05 were considered statistically significant in all statistical analyses.For closing forces,a two-way ANOVA was used to test for a statistically significant main effect between species and age groups in ACF and PCF.Moreover,multiple comparisons among mean age groups were performed via Games-Howell post-hoc test.A pairwiset-test was used to assess the statistically significant differences between ACF and PCF.One-way ANCOVA with CL as a covariate was performed to examine sex-related differences in the ACF and PCF of adult turtles.With respect to the closing time,two independent samplest-test was used to assess the interspecies differences in ACT and PCT,and pairwiset-test was used to assess the significant differences between ACF and PCF in each species.
With respect to the morphological measurements,twoway ANOVA was used for BM and CL to test the difference between the species or among three age groups,and two-way ANCOVA with CL as a covariate was used for the remaining parameters.Multiple comparisons among mean age groups were performed via the least significant difference (LSD) posthoc test.Similarly,one-way ANOVA and ANCOVA,using the ln transformation value of the original measurements,were performed to assess the sexual differences among the adults of each species.Moreover,a pairwiset-test was used to examine the differences between APL and PPL in each age group for each species.
Unary linear regression analysis was performed to evaluate the correlations between the closing force (ACF or PCF) and the BM in each species,and stepwise multiple linear regression analysis was used to evaluate the correlations between ACF or PCF and CL,BL,HL,and APL in each species.
To assess the possible allometric relationship between the closing force and the body size (BM),log closing forces were regressed against log BM in a linear regression,with equations in the form of:y=a+bx(wherex=log BM,y=log closing force,a=intercept,andb=slope).Positive allometry was indicated by slopes significantly greater than one,negative allometry by slopes significantly less than one,and isometry by slopes not significantly different from one (Schmidt-Nielsen,1993).The null hypothesis ofb=1 was tested with at-test in the form of:ts=b-1/SEb(whereb=slope,SEb=standard error of the slope and d.f.=n-2) (Zar,1996).Moreover,one-way ANCOVA (log BM as the covariate) was performed to test whether the two species differed in the regression slope,which is between log closing force and log BM.The same method was used to test whether there was a difference in the regression slope of the intraspecific anterior and posterior closing forces.
3.Results
3.1.Closing force and closing timeThe ACF and PCF of flowerback box turtles were found to be greater than those of keeled box turtles (ACF,F=484.504,P<0.001;PCF,F=262.423,P<0.001) (Figure 1),and differed significantly among the age groups (ACF,F=158.956,P<0.001;PCF,F=149.522,P<0.001).Adults had the strongest ACF and PCF,whereas juveniles had the weakest ACF and PCF in both species (Figure 1).The ACF was greater than the PCF in each age group in both species(Table 1).Furthermore,no sexual difference was observed in ACF in both keeled box turtles (female=13.87 ± 1.41 N,male=14.37±2.06 N;one-way ANCOVA,F=0.008,P=0.932) and flowerback box turtles (female=56.50±5.59 N,male=54.25± 8.53 N;one-way ANCOVA,F=0.003,P=0.959).Similarly,no sexual difference in PCF was observed in either species(keeled box turtle: female=12.90±1.77 N,male=12.68±1.86 N;one-way ANCOVA,F=0.527,P=0.479;flowerback box turtle: female=41.25±5.76 N,male=41.67±6.487 N;one-way ANCOVA,F=0.103,P=0.752).

Figure 1 Anterior closing force (A) and posterior closing force (B) in different age groups of two Cuora spp.Values and error bars indicate mean ± SE of the closing force.Asterisks and lowercase letters indicate significant differences between species (**P<0.001) and within species (P<0.001),respectively,according to two-way ANOVA and Games-Howell post-hoc test.

Table 1 Differences between the anterior closing force (ACF) and posterior closing force (PCF) in Cuora spp.
Both ACT and PCT of the adult flowerback box turtle (n=8;ACT=238 ± 42 s;PCT=213 ± 26 s) were significantly greater than those of the adult keeled box turtle (n=11;ACT=195 ±31 s,t=2.577,P=0.02;PCT=166 ± 28 s;t=3.768,P=0.002).Moreover,ACT was significantly greater than PCT in both flowerback box turtle (pairwiset-test,t=2.863,P=0.024) and keeled box turtle (pairwiset-test,t=3.857,P=0.003).
3.2.Morphological measurementsKeeled box turtles had larger CL than flowerback box turtles,whereas the BM was similar between the species (Table 2).Both BM and CL differed significantly among the juvenile,subadult,and adult individuals in each species (Table 2).Two-way ANCOVA with CL as a covariate further showed that the BL,APL,and PPL did not vary between the species or among the age groups,whereas the HL differed significantly between the species and among the age groups (Table 2).In addition,keeled box turtles had longer HL than flowerback box turtles.Adults had the longest HL,whereas juveniles had the shortest HL in both species (Table 2).The pairwiset-test showed that the PPL was greater than the APL in each age group in keeled box turtles (juvenile:t=3.035,P=0.029;subadult:t=6.498,P<0.001;adult:t=11.334,P<0.001) and flowerback box turtles (juvenile:t=4.889,P=0.005;subadult:t=13.059,P<0.001;adult:t=15.554,P<0.001).Except for HL in flowerback box turtles,no sexual differences in morphology was observed in either species (Table 3).
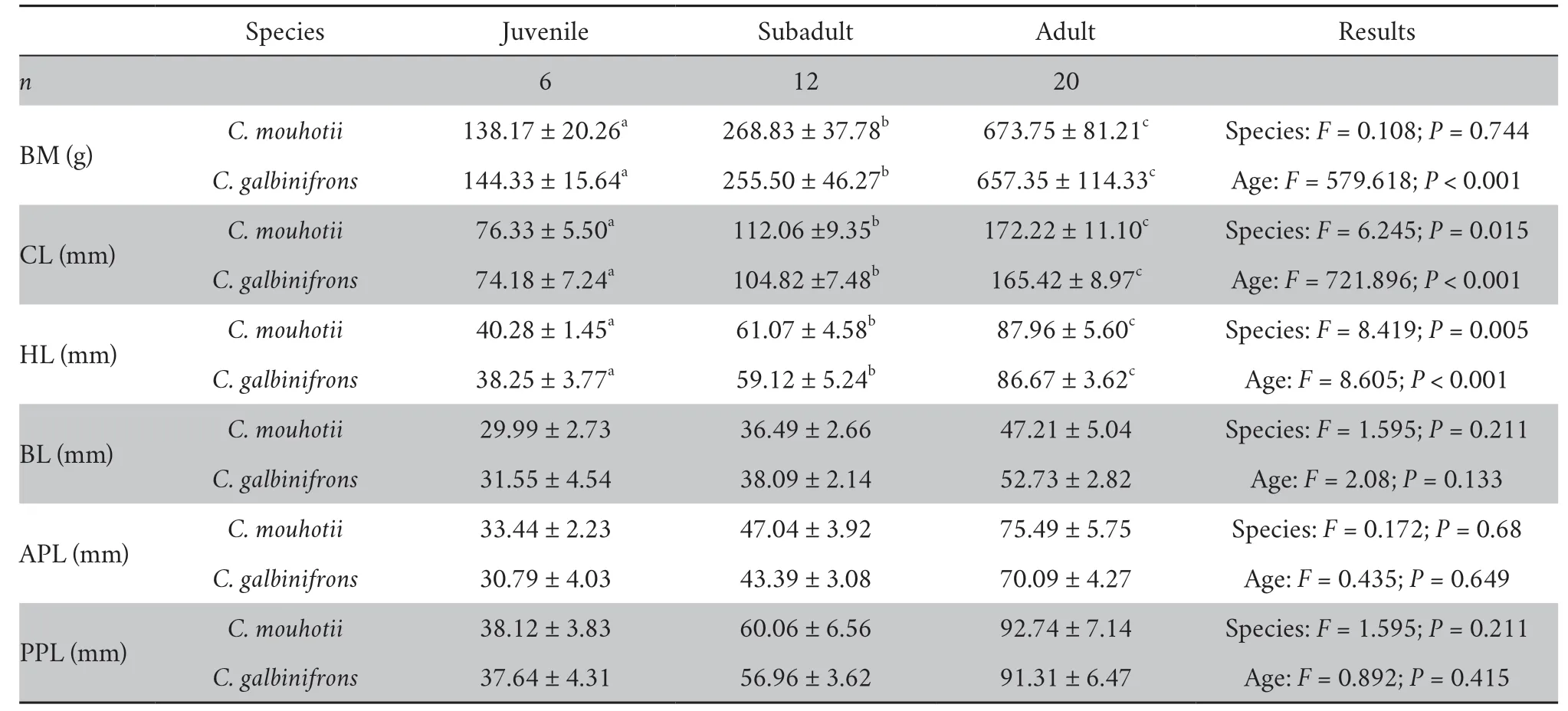
Table 2 Morphological measurements of the two Cuora spp.
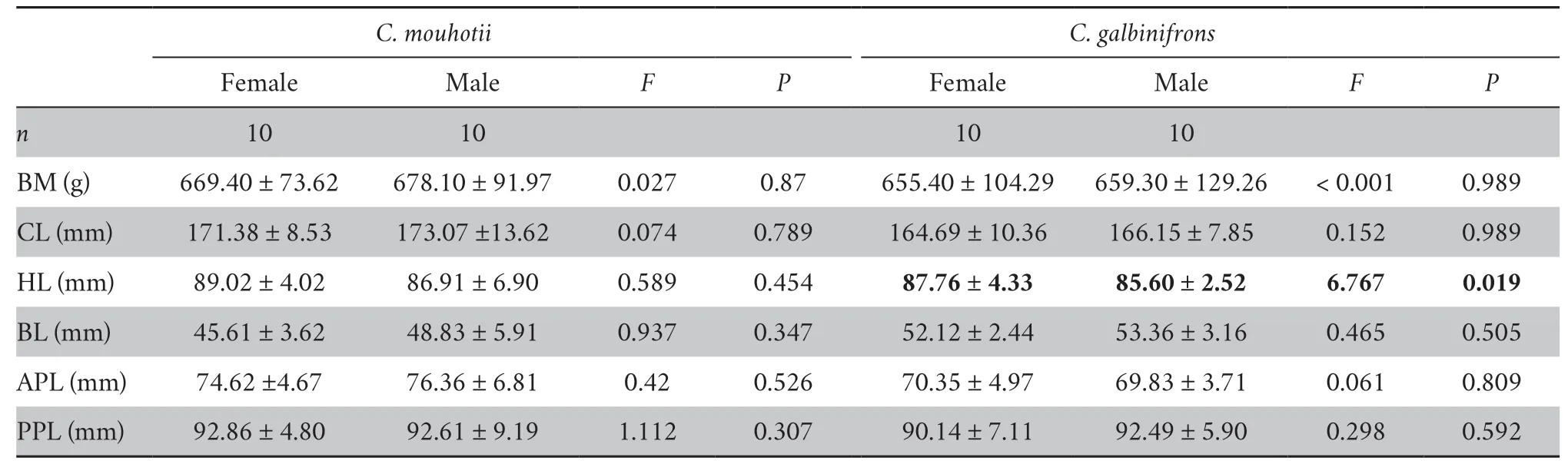
Table 3 Differences between the anterior closing force (ACF) and posterior closing force (PCF) in Cuora spp.
3.3.Relationship between closing force and morphologyThe ACF and PCF positively correlated with BM in both species(Figure 2).Stepwise multiple linear regression showed that the ACF and PCF positively correlated with APL (n=38;r2=0.904,F=337.437,P<0.001) and PPL (n=38;r2=0.891,F=294.925,P<0.001) in flowerback box turtle.In keeled box turtle,the PCF also positively correlated with the PPL (n=38;r2=0.718,F=91.792,P<0.001),whereas the ACF positively correlated with the CL (n=38;r2=0.695,F=82.053,P<0.001).

Figure 2 Relationship between body mass (BM) and anterior and posterior closing forces (ACF and PCF,respectively) in C.mouhotii (A;n=38) and C.galbinifrons (B;n=38) assessed via unary linear regression analysis.
The regression slope between log closing force and log BM was significantly less than 1 in flowerback box turtle (ACF:b=0.79,95% CI=0.68 -0.90,t=-17.39,P<0.001;PCF:b=0.83,95% CI=0.71-0.95,t=-16.122,P<0.001) and keeled box turtle (ACF:b=0.32,95% CI=0.24-0.40,t=-25.995,P<0.001;PCF:b=0.37,95% CI=0.29 -0.45,t=-24.631,P<0.001).This indicated the existence of negative allometry in the increase in closing force,that is,the increase rate of closing force was significantly slower than the growth rate of body size in both species.Moreover,ANCOVA showed that the regression slope of flowerback box turtle was greater than that of keeled box turtle (ACF:F=50.384,P<0.001;PCF:F=41.384,P<0.001);however,the regression slope of ACF was similar to that of PCF in flowerback box turtle (F=0.203,P=0.654) and keeledbox turtle (F=0.755,P=0.388).This indicated that the increase rate of both ACF and PCF in flowerback box turtle was significantly higher than that of keeled box turtle.
4.Discussion
This study showed for the first time that the ACF ofC.mouhotiiandC.galbinifronsjuveniles,subadults,and adults was significantly greater than their PCF,and that the anterior closing time of adults was also significantly longer than the posterior closing time.These findings demonstrated that the closing ability of the anterior lobe of the plastron of the two species was stronger than that of the posterior lobe,suggesting that both species use shell closure as an antipredation strategy to protect the front of the body more successfully than the rear.The head is the most important part of the animal body;hence,the anterior closure of the box turtle can better protect the head from being attacked by predators.Moreover,it has been observed that,in box turtles,the posterior area,where the legs are located,is potentially less exposed because it does not open as wide as the anterior when the turtles feed,and this could be the reason for the weaker posterior closing force.
The reason for the shorter closing time of the posterior lobe of the plastron than that of the anterior lobe of the plastron in both species could be morphological or physiological constraints.For instance,the presence of large fat stores or eggs may result in a shorter closing time of the posterior lobe of the plastron(Dodd,2001).Alternatively,movement of the limb girdles is known to help turtles respire (Johnson and Creighton,2005;Kardong,2015),and breathing will be affected if they keep the shell closed for a long time because shell closure could compress organs and abolish limb movement.
The present study also revealed that the ACF and PCF of keeled and flowerback box turtles are influenced by individual size (body weight or length of the dorsum) and the length of the anterior and posterior plastron,but not by the length of the hinge or of the bridge.Indeed,the ACF and PCF of both species increased with the increase in body weight,which is consistent with the results of Prestonet al.’s (2020) study in eastern box turtles (Terrapene carolina carolina).These results prove that the body weight is an important factor that influences the closing force of box turtles,as heavier individuals may have relatively more developed muscles and can produce greater muscle tension during shell closure (Randallet al.,2001).Bramble and Hutchison (1981) considered that the closing mechanism of the anterior lobe of the carapace of box turtles could be compared to a simple bent-lever system,in which the greatest potential mechanical advantage (such as the ACF) is determined by the lever ratio,that is,in-lever:out-lever (equivalent to the length of the anterior plastron).Thus,the smaller the anterior lobe length of the plastron,the greater the lever ratio and the greater the ACF,implying that the two are negatively correlated.If the closure mechanism of the posterior lobe of the plastron is similar to that of the anterior lobe of the plastron,the closure of the posterior lobe of the plastron should show similar results.However,the collected data showed that the ACF and PCF of the flowerback turtle positively correlated with the length of the anterior and the posterior lobes of the plastron,and the PCF of keeled box turtle also positively correlated with the posterior plastron,which contradicted the lever system hypothesis.To date,it has been assumed that the relationship between the ACF and PCF and the length of the anterior and the posterior plastrons is primarily affected by weight,as the size of the individual determines the length of the plastron.Interestingly,in both species,the length of the anterior lobe of the plastron was significantly shorter than the length of the posterior lobe of the plastron,and the ACF was significantly larger than the PCF,which concurs with the abovementioned lever system.In addition,the difference in the ACF and PCF may also be related to the different bones and muscles involved in the closure of the anterior and posterior lobes of the plastron (Bramble,1974).
Here,intraspecies comparisons showed that the ACF and the PCF of the two species were the largest in adults and the lowest in juveniles,which indicated that adult box turtles have a better antipredation response than the juveniles.In flowerback box turtle,the ACF and the PCF of the adults were approximately 4-times those of the juveniles.Studies on eastern box turtles have shown that shell kinesis presents delayed development (3-5 years post-hatching) (Corderoet al.,2018b;2019).Therefore,the incompletely developed shell kinesis of the juvenile box turtle may result in a weaker closing force.The increase rate of the closing force of the two species was slower than the growth rate of body size,which supported this conclusion.Moreover,the muscles and the bones of adults are more developed than those of juveniles and subadults,which may lead to a stronger closing force.Notably,no sex differences were observed in the two species in terms of the closing force,suggesting that this feature is primarily the result of natural selection (antipredation) and is not affected by sexual selection.
Interspecies comparisons further showed that regardless of the age group,the ACF and the PCF of flowerback box turtles were significantly greater than those of keeled box turtles,especially in adults,in which the difference was nearly 4-fold.Overall,the ACF and the PCF of both species were found to be primarily related to the body weight and the length of the anterior and posterior lobes of the plastron;however,no significant difference in these morphological characteristics between the species was noted.The biggest morphological difference between the species is that the shell of flowerback box turtle can be completely closed,whereas that of keeled box turtle cannot be completely closed,which may explain the large difference in the closing force of the two species.Therefore,it can be inferred that,in box turtles,the closing force of the species that can fully close their shells is stronger than that of the species that cannot,which could be explained by a systematic comparison of the anatomical structure and the biomechanics of their musculoskeletal system during the closing process.Although the ecological factor that induced the difference in the closing force of the two box turtle species remains to be determined,in flowerback and keeled box turtles,one possible explanation is that they live in different microhabitats (Xiaoet al.,2017).Flowerback box turtles may require a greater closing force to adapt to the microhabitat of hiding in litter.As having their body exposed in the litter microhabitat is easy,resulting in an increased probability of being discovered by predators,they need a greater closing force and a fully closed shell to protect their body.In the case of keeled box turtles,which are more exposed to natural enemies as they cannot completely close their shell,they have to protect themselves from predation by hiding in hard rock crevices for a long time (Xiaoet al.,2017;Xiaoet al.,2020).Although the ACF and the PCF of both species showed negative allometry,the increase rate of the closing force in flowerback box turtle was significantly higher than that of keeled box turtle.This may also be caused by the adaptation of the two species to the different microhabitats mentioned above: flowerback box turtles need a rapidly increasing closing force to resist predation in the litter microhabitat.In addition,the closing time of flowerback box turtles is significantly longer than that of keeled box turtles.A longer duration may be beneficial for flowerback box turtles to protect themselves from predators in an exposed microhabitat.
In summary,the present study showed that both keeled and flowerback box turtles have a stronger ACF than PCF,and a longer anterior closing time than posterior closing time.The difference between the ACF and the PCF is related to a significantly shorter anterior lobe of the plastron than the posterior lobe.Moreover,the ACF and the PCF increase in both species as the individuals grow older.Nonetheless,both ACF and PCF of flowerback box turtles are greater than those of keeled box turtles,due to the difference in the degree of closure between the species.Although the closing force of both species showed negative allometry,the increase rate in flowerback box turtle was significantly higher than that in keeled box turtle.Taken together,we investigated the ACF and the PCF of box turtles for the first time,and proved that age,weight,plastron shape,and degree of shell closure are important factors affecting the closing force.Future studies are required to test the general applicability of this research in other box turtle species.Furthermore,future investigations are necessary to address the difference in the closing force between incompletely and completely closed shells from biomechanics and anatomic perspectives.
AcknowledgmentsWe are grateful to Jichao WANG and Tongliang WANG for their help with this study.This work was supported by the National Natural Science Foundation of China (32170532 and 31772486).
杂志排行
Asian Herpetological Research的其它文章
- Elevational Variation in Reproductive Strategy of a Widespread Lizard:High-Elevation Females Lay Fewer but Larger Eggs
- Field Surveys Suggest Biennial Reproduction Cycle and Competition-triggered Dispersal of the Endangered Chinese Crocodile Lizard
- Morphology and Histochemistry of Infralabial Glands of Two Species in the Slug-Eating Family Pareidae (Reptilia: Serpentes)
- Partial Masquerading and Background Matching in Two Asian Box Turtle Species (Cuora spp.)
- A New Brown Frog of the Genus Rana (Anura,Ranidae) from North China,with a Taxonomic Revision of the R.chensinensis Species Group
