A New Brown Frog of the Genus Rana (Anura,Ranidae) from North China,with a Taxonomic Revision of the R.chensinensis Species Group
2022-09-27HuijunSHENMengyuXUXinyueYANGZhuoCHENNengwenXIAOandXiaohongCHEN
Huijun SHEN ,Mengyu XU ,Xinyue YANG ,Zhuo CHEN* ,Nengwen XIAO and Xiaohong CHEN*
1 College of Life Sciences,Henan Normal University,Xinxiang 453007,Henan,China
2 The Observation and Research Field Station of Taihang Mountain Forest Ecosystems of Henan Province,Xinxiang 453007,Henan,China
3 State Environmental Protection Key Laboratory of Regional Eco-process and Function Assessment,Chinese Research Academy of Environmental Sciences,Beijing 100012,China
Abstract The Rana chensinensis species group is widely distributed throughout North China.However,its taxonomy and composition remain controversial.In recent field investigations of the Taihang Mountains,a series of Rana specimens were collected,which were once identified as R.chensinensis.However,these samples showed significant differences from R.chensinensis of the type locality (Shaanxi Province in the Qinling Mountains) in both morphology and genetics.In this paper,based on analyses of seventeen geographic populations from the Taihang and Qinling Mountains,we describe a new species (namely R.taihangensis sp.nov.) in the R.chensinensis species group.A phylogenetic analysis of the R.chensinensis species group based on mitochondrial genes-COI,16S rRNA and Cytbrevealed the monophyly of the cryptic species,which formed the sister taxon to R.kukunoris.Morphological comparisons indicated that the cryptic species can be distinguished from its congeners by a combination of characteristics.Additionally,the distribution patterns of the Rana species in North China were clarified.The populations of the southwestern Taihang Mountains,Xiaoqinling Mountains,and Funiu Mountains in Henan Province remain R.chensinensis,whereas the populations recorded as R.chensinensis in Beijing City,Hebei Province,and the southeastern Taihang Mountains of Henan Province should be revised as R.taihangensis sp.nov.
Keywords morphology,phylogeny,Rana taihangensis sp.nov.,Rana chensinensis species group, taxonomic revision
1.Introduction
The common brown frogs (genusRanaLinnaeus,1758),consisting of fifty-five known species,are widely distributed throughout Eurasia and North America,and twenty-six species among them have been found in most parts of China(Amphibia Web,2021;Frost,2021).However,the taxonomy and classification of this genus are still controversial.Because of the highly similar morphological features of theRanaspecies (Liu and Hu,1961;Tanaka-Uenoet al.,1998;Yuanet al.,2016),accurate taxonomic identification and geographic delimitation are particularly difficult in the genus.Based on morphological and biogeographical data,the Chinese brown frogs were divided into the following three species groups:R.chensinensisspecies group,R.longicrusspecies group andR.amurensisspecies group(Feiet al.,2009).Phylogenetic analyses based on molecular data revealed more species and indicated the presence of a fourth species group-theR.johnsispecies group (Cheet al.,2007;Yuanet al.,2016;Wanet al.,2020).Feiet al.(2009) listed four species-R.chensinensis,R.kukunoris,R.dybowskii,andR.asiatica-in theR.chensinensisspecies group based on morphological similarities.However,molecular evidence has suggested thatR.huanrensisshould be included in theR.chensinensisspecies group (Cheet al.,2007;Wanet al.,2020),which was previously classified into theR.amurensisspecies group because of the absence of vocal sacs(Feiet al.,2009).
Because of the lack of morphologically diagnosable characteristics,the common brown frogs in northern China with the characteristic of a curving dorsolateral fold were all recognized asR.chensinensis(Liet al.,2014;Liu and Hu,1996).However,a comparative analysis of morphology and karyotype regarded the populations distributed in the northeastern and northwestern China asR.dybowskiiandR.kukunoris, respectively (Xieet al.,1999,2000).In addition,populations from Liaoning Province,Jilin Province,and the adjacent Korean peninsula were classified asR.huanrensis(Amphibia Web,2021;Frost,2021).Thus,theR.chensinensissensu stricto was mainly distributed in northern and central China,with plenty of geographic records in Beijing,Hebei,Henan,Ningxia,Shannxi,and Gansu,etc.(Feiet al.,2009;Frost,2021;Quet al.,1995;Wanget al., 1995;Wanget al.,2019;Wuet al.,2009).However,analyses of the phylogenetics and biogeography of theR.chensinensisspecies group have suggested the existence of the cryptic species in theR.chensinensisspecies group and that populations from the Loess Plateau should be an unnamed species (Zhouet al.,2012).It is unclear whether the possible existence of cryptic species can be revealed by morphological data and extensive sampling.In addition,the geographic distributions of the potential cryptic species and theR.chensinensissensu stricto remain unresolved.
In this study,a series ofRanaspecimens were collected during the recent herpetofaunal surveys of the Taihang Mountains area in North China (Figure 1).They showed significantly morphological differences fromR.chensinensisof the type locality (Qinling Mountains in Shaanxi Province)and were distinct from all known congeners.A molecular analysis based on three mitochondrial gene fragments-COI,16S rRNA and Cytb-well supported the morphological differentiation and suggested the existence of a cryptic species in theR.chensinensisspecies group.Therefore,we investigated the taxonomic status of these samples and described a new species.Additionally,we proposed a taxonomic revision of theR.chensinensisspecies group in North China.
2.Materials and Methods
2.1.SamplingA total of 159 adult specimens were collected from seventeen localities of Beijing City,Hebei Province,and Henan Province throughout the Taihang Mountains (localities 1-15) and the eastern Qinling Mountains (localities 16 and 17)(Figure 1).Among them,ninety-one samples were used in a molecular analysis and 138 were measured for morphological analysis (Supplementary Table S1).All samples were kept in the Amphibian and Reptile Laboratory at Henan Normal University.
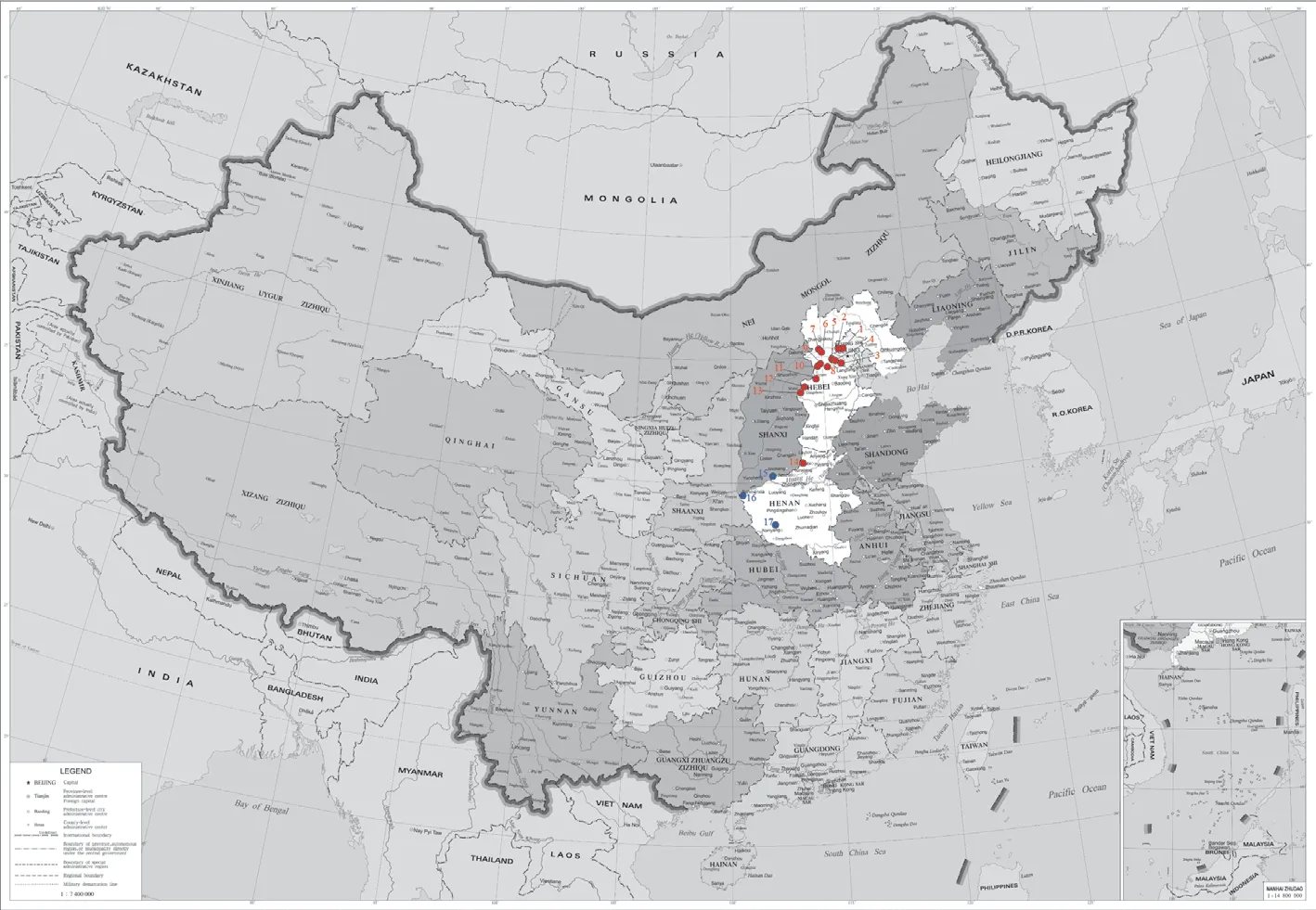
Figure 1 Distribution of sampling localities.The mtDNA subclade T and C are labeled red and blue,respectively.More locality codes and coordinates are presented in Supplementary Table S1.Map approval number: GS (2021)5451.
2.2.Laboratory protocolsFor comparison with the published sequence of otherRanaspecies in our phylogenetic study,three partial mitochondrial DNA (mtDNA) fragments of the cytochrome c oxidase subunit I (COI),cytochrome b (Cytb),and 16S ribosomal RNA (16S rRNA) genes were amplified and sequenced.Total genomic DNA was extracted using a standard phenol-chloroform procedure (Sambrook and Russell,2001).The primers used for polymerase chain reaction (PCR)amplifications and sequencing were summarized in Table 1.The amplification procedures followed Chenet al.,(2013).All new sequences were deposited in GenBank (for GenBank accession numbers see Supplementary Table S1).
2.3.Genetic diversityAll nucleotide sequences were aligned using Clustal W with default parameters and checked manually in MEGA 6.0 (Tamuraet al.,2013).Nucleotide sites with ambiguous alignments were deleted from the analyses.Saturation tests for each gene and gene partitions were conducted using DAMBE (Xiaet al.,2003).The sequences from the respective samples were concatenated (Cytb+COI+16S rRNA,2230 bp) using SequenceMatrix v.1.8 (Vaidyaet al.,2011).The genetic diversity-number of polymorphic sites (S),haplotype number (h),haplotype diversity (H),and nucleotide diversity (π)-was assessed separately for Cytb,COI,16S rRNA,and the combined sequences using DnaSP v.5.10 (Rozaset al.,2003).
2.4.Phylogenetic analyses and genetic distance estimationThe Bayesian inferences (BI) method was used to identify the phylogenetic relationships among the mitochondrial haplotypes of the aligned sequences.The PartitionFinder v2.1.1 (Lanfearet al.,2016) was used to test the best partitioning scheme,and the jModelTest v2.1.2 was used to test the best fitting nucleotide substitution models.The best fit models for 16S rRNA was GTR+G,and that for both COI and Cytb was GTR+I+G.The BI analyses were performed in MrBayes v3.1.2 (Ronquist and Huelsenbeck,2003),using the optimal partitioning strategy and the best-fit nucleotide substitution model for each region.The analyses were performed for 10 million generations,sampling trees every 1000 generations.The first 5000 sampled trees were discarded as burn-in,and the remaining trees were used to generate a majority-rule consensus tree and calculate the Bayesian posterior probabilities (BPP).The sequences ofPelophylax nigromaculatusdownloaded from GenBank (for GenBank Accession numbers see Supplementary Table S1)were used as the outgroup (Cheet al.,2007).A phylogenetic network was computed from the distance matrix using the NeighbourNet approach implemented in SplitsTree version 4(Huson and Bryant,2006),using default parameters.Finally,the uncorrected p-distance model on the Cytb gene was estimated using MEGA v6.0 to evaluate the genetic divergence betweenRanaspecies.
2.5.Morphological analysesA total of 138 specimens (eightythree males and fifty-five females;Supplementary Table S1)were used in the morphological study-eighty-two from theRanasp.,fourteen from theR.chensinensisspecies group,and forty-two from theR.kukunorisspecies group.The sex was determined by examining the pads.External measurements were made with digital calipers to the nearest 0.1 mm.Twentyfour linear measurements were the same ones presented in Shenet al.(2020),with the morphological description following the definition by Feiet al. (2009).
The morphological characteristics of theRanasp.from the Taihang Mountains were compared with those of members in theR.chensinensisspecies group.Comparison characters and morphometric data ofR.chensinensis(from its type locality,N=15),R.kukunoris(N=40),R.dybowskii(N=47),andR.huanrenensiswere taken from Feiet al. (2009) and voucher specimens (Supplementary Table S1).
Raw morphometric data were log-transformed for the following statistical analyses.The canonical discriminant analysis (CDA) was performed to assess the overall patterns of morphological variation amongRanasp.,R.chensinensis,andR.kukunoris(Supplementary Table S1).One-way analysis of variance (ANOVA) was conducted to investigate the detailed interspecific variance.In theRanasp.,the size differences between males and females were examined by ANOVA and the analysis of covariance (ANCOVA) with snout-vent length(SVL) as covariates.The significance level was set at 0.05.Theseanalyses were carried out in R (R Development Core Team,2010).
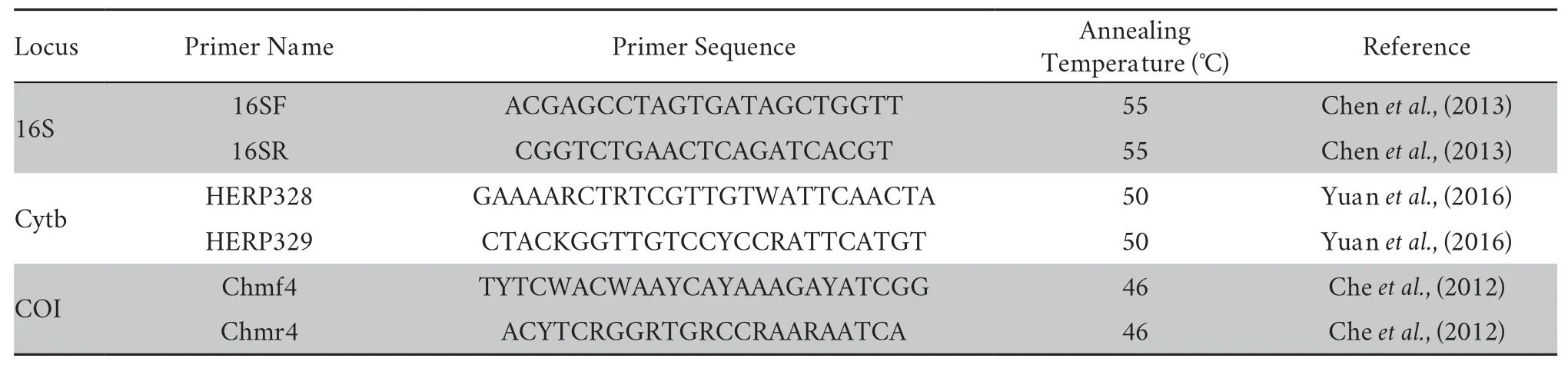
Table 1 PCR primer information of three mitochondrial gene markers.
3.Results
3.1.Genetic diversityA total of 312 mtDNA sequences were obtained for analyses-fifty-three sequences of the ChineseRanafrom GenBank (see Supplementary Table S1) and 259 newly obtained sequences (eighty-four for 16S rRNA,eighty-four for Cytb,and ninety-one for COI) from ninety-one individuals.The genetic diversity indexes were calculated and summarized in Table 2.A total of thirty-eight polymorphic sites and twentytwo haplotypes were detected for the 16S rRNA (966 bp) data set with a haplotype diversity of 0.874 ± 0.001 and nucleotide diversity of 0.009 ± 0.000 (Table 2).Thirty-two haplotypes (S=53,H=0.945 ± 0.011,π=0.029 ± 0.001) were generated for the COI (558 bp) data set.Thirty-seven haplotypes (S=96,H=0.934 ± 0.000,π=0.043 ± 0.001) were identified for the Cytb sequences.A total of fifty-two haplotypes (S=182,H=0.976 ±0.008,π=0.025 ± 0.001) were identified for the concatenated 16S rRNA+Cytb+COI data set.
3.2.Phylogenetic analyses and genetic divergenceThe Bayesian tree revealed four major clades (denoted Clade I-IV)with strong support:R.chensinensisspecies group,R.amurensisspecies group,R.longicruspecies group,andR.johnsispecies group,respectively (Figure 2A).TheR.chensinensisspecies group(Clade I) consisted of five highly supported lineages: subclade T,R.kukunoris,subclade C,R.huanrensis, andR.dybowskii.In Clade I,Rana dybowshiiappears as the sister taxon to a clade comprising all other members of theR.chensinensisspecies group with strong support (BPP=1.00).The well-supported subclade C (BPP=1.00) comprised the individuals from the southwestern edge of the Taihang Mountains (locality 15) and the eastern Qinling Mountains (locality 16-17) as well as those from the type locality ofR.chensinensis.The subclade C formed a sister-group relationship withR.huanrensi, but it is not well supported (BPP=0.88).Nearly all theRanasamples from the Taihang Mountains (localities 1-14) were recovered as a highly supported monophyletic group (subclade T)-the sister taxon toR.kukunoriswith strong support (BPP=1.00).The phylogenetic network generated by SplitsTree based on the concatenated dataset was similar to the mtDNA gene tree (Figure 2B),both strongly supported the existence of a cryptic lineage in theR.chensinensisgroup.
Furthermore,the genetic divergence (uncorrected p-distance)of the Cytb gene among the studiedRanaspecies is shown in Table 3.The sequence divergences within subclade T and subclade C were 1.5% and 0.2%,respectively.The mean pairwise distances between the subclade T andR.kukunoris, subclade T and subclade C,and subclade T andR.chensinensiswere 4.8%,8.4%,8.2%,respectively.The pairwise distance among the five clades in theR.chensinensisspecies group was obviously higher than that betweenR.culaiensisandR.zhenhaiensis(2.5%) as well as betweenR.culaiensisandR.longicrus(4.4%).
3.3.MorphologyThe results of the one-way ANOVA and ANCOVA showed that males and females ofRanasp.differed in many traits,whether controlled for body size or not (Table 4).Therefore,the morphological divergence between species was examined in males and females,respectively.The canonical discriminant analysis based on twenty-four morphometric characters significantly differentiatedRanasp.from subclade C (i.e.,R.chensinensis) andR.kukunoris-for both males and females (Figure 3).The univariate assessments by one-way ANOVA showed that both male and femaleRanasp.were significantly different from subclade C andR.kukunorisin many morphometric characteristics (allP<0.05;Table 4).For males,Ranasp.was significantly different fromR.chensinensisin head length (HL),tympanum-eye distance (TED) and lower arm diameter (LAD) as well as different fromR.kukunorisin snout length (SL),nostril-snout tip distance (NSD),nostrileye length (NEL),maximum width of upper eyelid (UEW),tympanum diameter (TD),TED,LAD,hand length (HAL),tibia length (TL),and inner metatarsal tubercle width (IMTW) (allP<0.05).For females,the new species differed fromR.chensinensisin SL and TED,and it was separated fromR.kukunorisin SL,NSD,NEL,UEW,TED,and IMTW (allP<0.05).
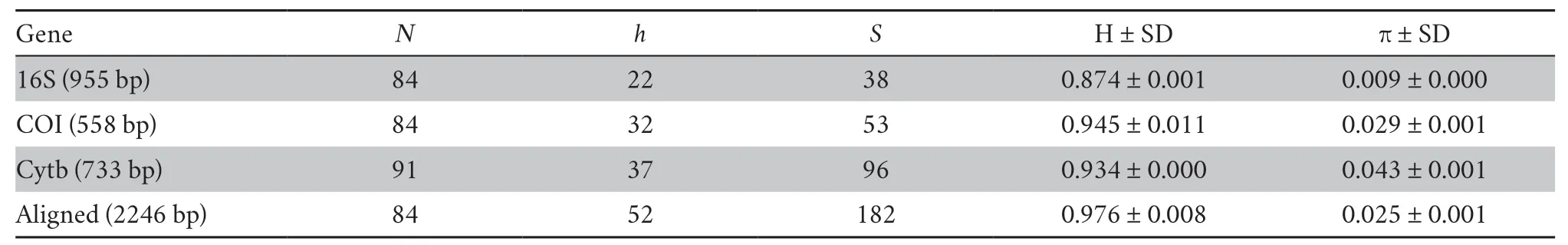
Table 2 The DNA polymorphism and genetic diversity based on the three mitochondrial genes of all populations.
Evidence of both molecular and morphological analyses supported the species status ofRanasp.(subclade T in Figure 2A) collected from Beijing City,Hebei and Henan Provinces(located in the east of the Taihang Mountains).We identified itasRana taihangensisChen,sp.nov.

Figure 2 A: The Bayesian consensus tree inferred from the haplotypes of the combined 16S rRNA,COI,and Cytb partial sequences.Numbers near the branches represent Bayesian posterior probabilities (BPP).B: Phylogenetic network generated by SplitsTree based on the concatenated data set.
3.4.Species accounts
Rana taihangensis Chen,sp.nov.(Figures 4-9;Table 5)
HolotypeHNNU2104II018,an adult male (Figures 4A,B,E;5A,B),was collected by M.Y.Xu and Y.J.Zhu on April 9,2021,from Huixian City (35°37’43.60’N,113°36’24.84’E,1025 m a.s.l.)in Henan Province,China.
AllotypeHNNU2104II019,an adult female (Figures 4C,D,E;5C,D),was collected with the holotype (HNNU2104II018).

Figure 3 Results of canonical discriminant analysis of samples representing subclade T,subclade C and R.kukunoris in males (A) and females (B),respectively.Different lineages or species are denoted with different colors and shapes.
ParatypesEighty adult specimens (52 males,28 females) from the Taihang Mountain area in Beijing City,Hebei and Henan Provinces were collected by X.H.Chen,M.Y.Xu,X.Y.Yang,Y.Q.Lu,Q.Q.Feng,D.Liu,C.Ke,Y.M.Zhang,S.X.Gao,Y.J.Zhu,Y.Liu,G.L.Hu,W.K.He and T.H.Wang during 2003 and 2021(Supplementary Table S1).All the type specimens were deposited in the Herpetological Research Laboratory,College of Life Science,Henan Normal University.
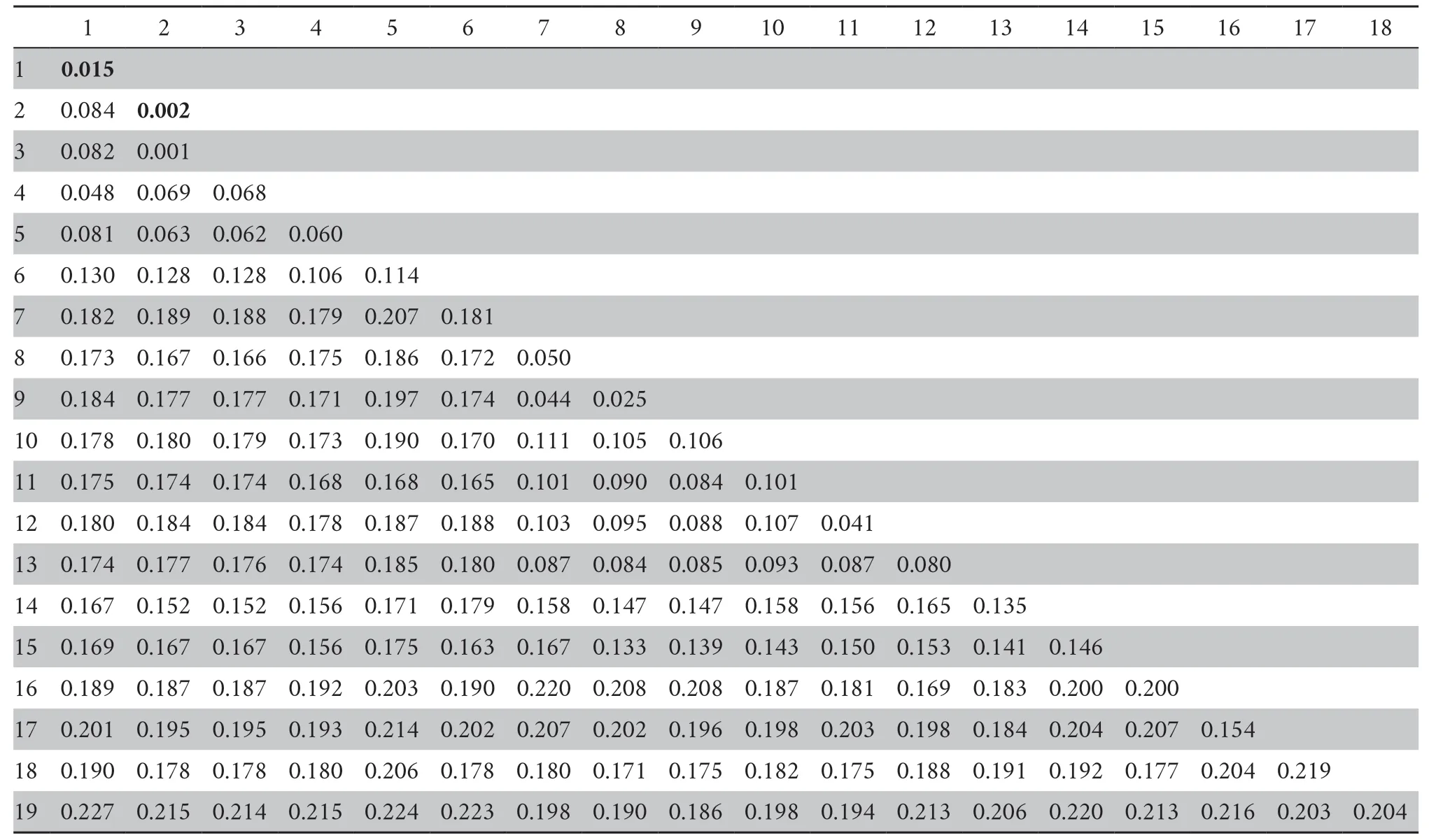
Table 3 The pairwise uncorrected p-distance (%) of the Cytb partial sequence (733 bp) used in this study.
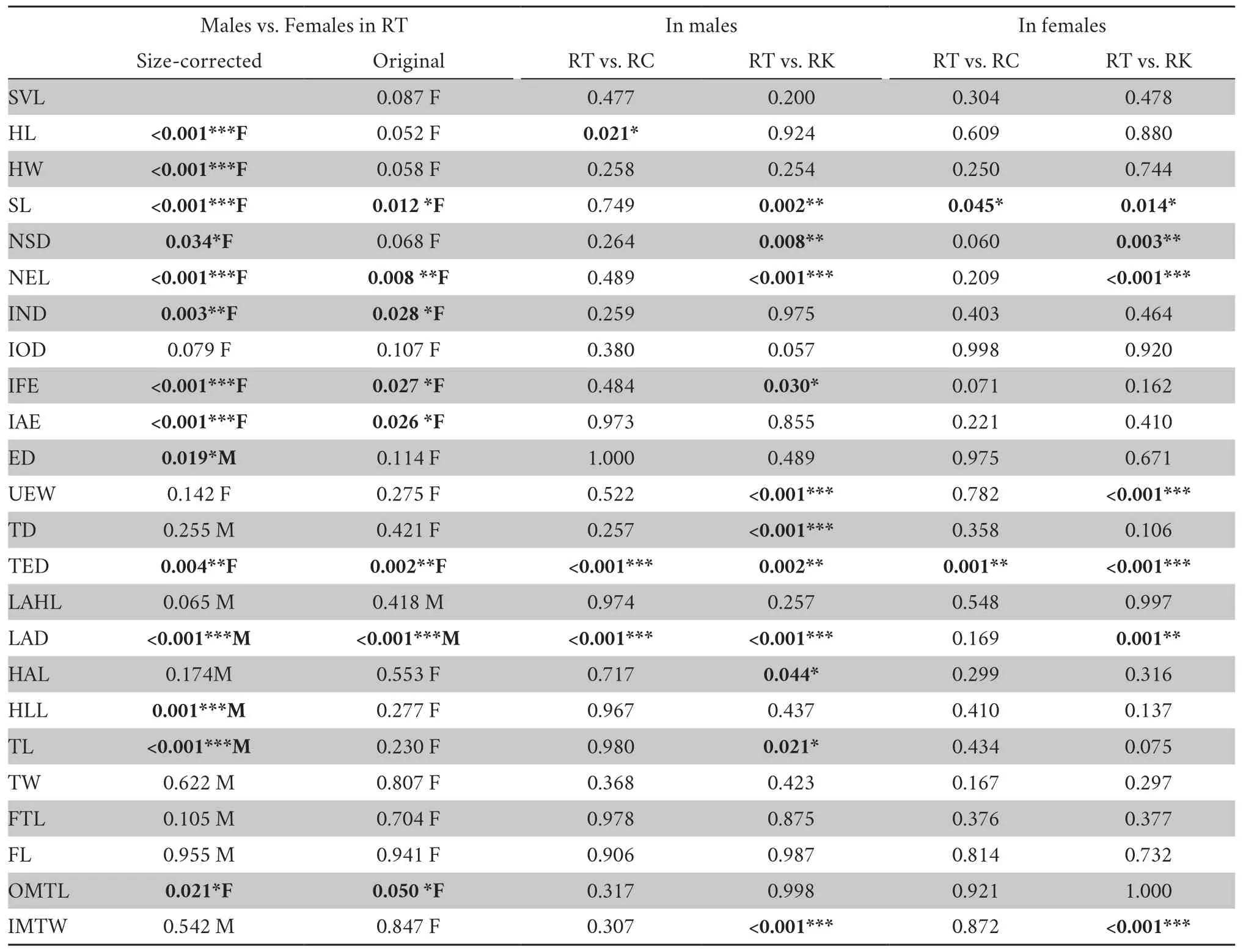
Table 4 P values of one-way ANOVA (applied to log-transformed original data) and ANCOVA (using SVL as covariates) for morphometric comparisons between the sexes and between species in R.taihangensis sp.nov.,R.chensinensis and R.kukunoris.
EtymologyThe specific name taihangensis is in reference to the type localities in Beijing City,Hebei and Henan Provinces of the Taihang Mountains,North China.
DiagnosisRana taihangensissp.nov.differs from its congeners by the following combination of characteristics: (1) body medium-sized,SVL=37.06-55.42 (44.87 ± 4.03,N=52) mm in adult males,38.41-63.30 (46.96 ± 6.02,N=28) mm in adult females;(2) snout obtusely rounded,canthus rostralis obtuse and raised prominently;(3) interorbital distance (IOD) less than internarial distance (IND) and the width of upper eyelid(UEW);(4) tympanum diameter equal (TD) less than half of the eye diameter;(5) vomerine teeth in two short oblique series,anterior edges suited in the posterior border of the choanae;(6) supratympanic fold extending from the eye to over the shoulder;(7) dorsolateral fold narrow,extending from the posterior canthus to crotch,slight curving above the tympanic membrane;(8) dorsal and ventral skin smooth;(9) the color of the dorsal surface changing with environment from reddishbrown,light pink-orange,light brown,grayish-white to olive patterns;(10) dorsum of limbs with black-brown transverse bars;(11) dorsum of limbs with black-brown transverse bars;(12) heels overlapping when hindlimbs flexed at right angles to axis of body;(13) fingers unwebbed,relative finger lengths:III>I>IV>II;(14) toes two-thirds webbed,relative toe lengths:IV>V≈III>II>I;(15) internal subgular vocal sacs and red lineae musculinae present in males;(16) nuptial pad dark brown,developed into two groups with an obvious gap on the ventralside of finger I in males;and (17) fertilized eggs dark brown with grayish brown poles,mean diameter of 1.9 mm.
Description of holotypeSVL 47.31 mm.Head slightly longer than broad (HL/HW=1.06),snout obtusely rounded in dorsal view,projecting beyond the lower lip;canthus rostralis obtuse and raised prominently;nostril closer to tip of snout than eye;loreal region concave,sloping outwards;interorbital distance(IOD) less than internarial distance (IND) and the widthof upper eyelid (UEW);tympanum diameter shorter than half of eye diameter (TD/ED=0.47);tongue deeply notched posteriorly;vomerine teeth in two short oblique series,anterior edges suited in the posterior border of choanae (Figure 4A,B).
Forelimbs: Forearm robust,length of lower arm and hand less than half of SVL (LAHL/SVL=0.45);relative finger lengths: III>IV>I>II;fingers slender,without web nor narrow fringe;one prominent subarticular tubercle on fingers I and II,two tubercle on fingers III and IV;prominent supernumerary tubercles below the base of fingers III and IV;inner metacarpal tubercle ovoid,two outer metacarpal tubercles distinctly separated,slightly larger,long elliptic;dark brown nuptial pad on the ventral side of finger I,without spines,clearly divided into two parts,the larger one close to the tip of finger I extending beyond the supernumerary tubercles,the basal one smaller (Figures 4B;5A).
Hindlimbs: Hindlimbs 171% of SVL,tibio-tarsal articulation reaching to the anterior corner of eyes when leg stretched forward;foot length and tibia length larger than half of SVL;heels overlapping when the limbs are held at right angles to body axis;the relative toe lengths: IV>V≈III>II>I;toe tips rounded,unexpanded,with narrow fringes;subarticular tubercles small and distinct with two tubercles on toes I and II,three tubercle on toes III and V,four on toe IV;inner metatarsal tubercle large,ovoid,outer small,round;toes two-thirds webbed,reaching the base of the disk,except toe IV webbed to the distal subarticular tubercle (Figures 4A,B;5A,B).

Figure 4 A-B: Dorsal and ventral views of the holotype (HNNU2104II018,male);C-D: Dorsolateral and ventral views of the allotype(HNNU2104II019,female);E: Dorsolateral view of the amplexus of the holotype (upper) and allotype (lower);F: Eggs of female R.taihangensis sp.nov.
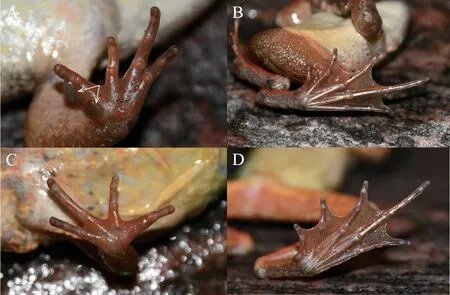
Figure 5 Ventral views of the left hand (A) and foot (B) of the holotype in life (HNNU2104II018,male).Ventral views of the right hand (C)and the left foot (D) of the allotype (HNNU2104II019,female).
Skin rather smooth,irregular tubercles sparsely scattered on dorsum surface;several small tubercles on flank;supratympanic fold well developed and extending from the eyes to over the shoulder;a triangular brown patch behind the eyes and anterior to the temporal fold;dorsolateral fold distinct and thin,extending from the posterior canthus to crotch,slight curving above the tympanic membrane;a mass of grannules on throat and belly,tiny whitish grannules around vent.
Coloration in lifeDorsal side reddish brown with blurred dark brown spots;on dorsum a x-shaped elongated tubercle with blurred dark blots,reaching posterior of upper eyelid;lip greyish brown with light yellow spots;a black spot in front of each nostril;dorsolateral fold light reddish;nuptial pad dark brown;color of forelimbs and hindlimbs light reddish;four faint brown bars in the forearm,five clear brown bars in both thigh and tibia;throat and chest creamy white,belly greyish white;ventral side of hands,limbs,and foot webbing reddish;metacarpal tubercles,distal subarticular tubercle,supernumerary tubercles and foot webbing red;grannules on throat and belly yellowish or whitish;light yellowish markings on throat;light reddish markings on chest;yellowish mottled markings on belly.
Coloration in preservativeDorsal side grayish brown,tubercles on dorsum light reddish,dorsolateral fold and upper eyelid light reddish brown;nuptial pad black;strips on limbs brown;venter dark gray,dark brown spots on throat;ventral surface of hindlimbs light reddish brown;metacarpal tubercles,distal subarticular tubercle and supernumerary tubercles reddish brown.
Variation in the type seriesThe variations in morphometric measurements of the type series from fourteen populations across its distribution range are listed in Table 5.The type series exhibit high intraspecific variation in color and spot patterns (Figure 6).The dorsal surface is greyish white in male HNNU2104I006 (Figure 6A),light pinkish orange in female HNNU2104I004 and male HNNU2104I008 (Figure 6C,D),and greenish brown in male HNNU20TI06057 (Figure 6E).Although black spots are present on the dorsum in most specimens,HNNU2104II008 and HNNU20TI06033 lack spots on the dorsum (Figure 6D,F).In 119 adult male specimens,the number of nuptial pads on finger I is two in most males (72%,Figure 6G);moreover,some individuals have another one (19%)or two (9%) small,unclear nuptial pads on the tips of their fingers (Figure 6H,I).

Figure 6 Variation of color patterns in living R.taihangensis sp.nov. Dorsal view of HNNU2104II006 (male;A);dorsolateral view of HNNU2104II003 (male;B),dorsolateral view of HNNU2104II004 (male;C);and dorsal view of HNNU2104II008 (female;D) from the type locality,Huixian City,Henan Province,China.E: Dorsal view of HNNU20TI06057 (male) from Fuping County,Hebei Province,China.F: Dorsal view of HNNU20TI06033 (female) from Pingshan County,Hebei Province,China.G-I: The varied number of nuptial pads from two to four in HNNU20TI08012 from Yixian County and HNNU1907VI028 and HNNU1907VI034 from Huailai County,Hebei Province,China.
Sexual dimorphismThebody size of the females (SVL,mean 44.87 ± 4.03 mm,N=28) is slightly smaller than that of the males (SVL,mean 46.72 ± 6.16 mm,N=52).Females are significantly larger than males in HL,head width (HW),SL,NSD,NEL,internarial distance (IND),distance betweenanterior corner of eyes (IFE),distance between posterior corner of eyes (IAE),TED,and outer metatarsal tubercle length(OMTL) (allP<0.05,Table 5).The males are significantly larger than the females in LAD,length of hind leg (HLL),and TL (allP<0.01,Table 5).
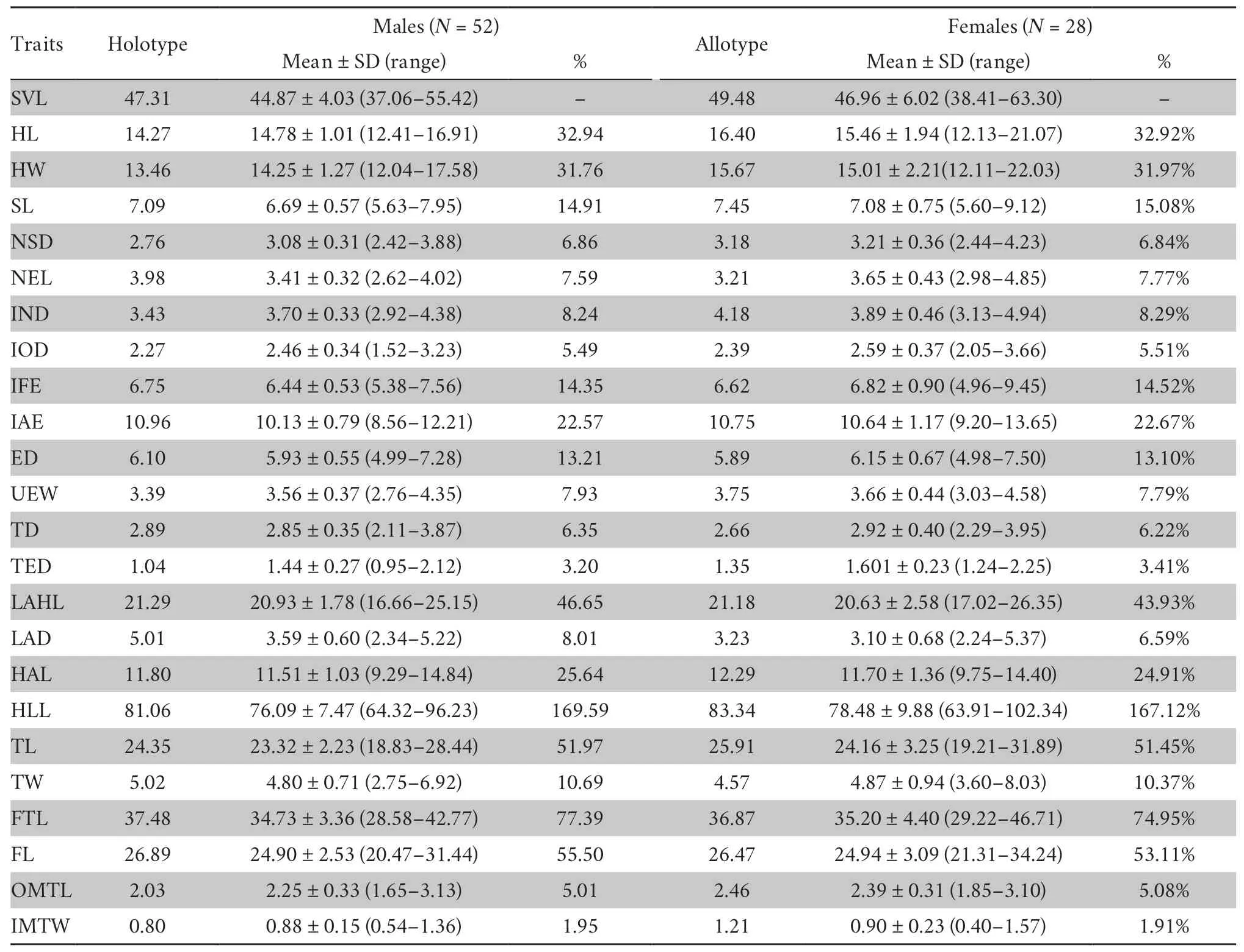
Table 5 Morphometric measurements (in mm) of the type series of R.taihangensis sp.nov. with mean ± SD (range) and ratio of each measurement to the SVL (%).
Distribution and ecologyRana taihangensissp.nov.is known from the east slope of the Taihang mountain area in Beijing City,Hebei Province,and Henan Province.All specimens were found around water pools or slow streams at 116-1 943 m elevations surrounded by shrubs and small hardwoods (Figure 7).At the type locality,Huixian City of Henan Province (113.61°E,35.63°N,1 073m a.s.l),females began to spawn on April 6,and amplexus pairs could be found as late as on April 24.Adult females spawn 331-707 eggs (N=6,mean 483) at one time with a mean diameter of 1.9 mm (N=7,range 1.7-2.0 mm).The fertilized eggs are dark brown with grayish brown poles(Figure 4F).The breeding season was approximately from early to late April.The tadpoles are yellowish-brown on the dorsal side (Figure 8A,B),with tail fins lightly colored and small golden spots scattered on the whole body.At the Gosner stage 25,the average total length (TOL) is 13.26 mm,and the tail is 144% of SVL.At the thirty-fifth stage the average TOL is 28.21 mm,and the tail is 232% of SVL.The dorsal fin arises behind the origin of the tail.The tail muscles are strong,and the tip of the tail is blunt.The nostrils are near snout,and the eyes are positioned dorsally (Figure 8A,B).The keratodont row formula is I: 3+3/III: 1+1 (Figure 8C).The papillae on the mouth angles around upper labium (the anterior corner of the mouth) and lower labium is in one row,with some additional tubercles at the angles of the mouth (Figure 8C).

Figure 7 Typical habitat of R.taihangensis sp.nov. in Huixian City,Henan Province,China.

Figure 8 Tadpole of Rana taihangensis sp.nov. Lateral (A) and dorsal (B) views of a Gosner stage 35 individual in life.C: The structure of the mouth of a Gosner stage 40 individual in life.
Comparisons Within theR.chensinensisspecies group,R.taihangensissp.nov.differs fromR.kukunorisby having smaller size (inR.taihangensissp.nov.,males SVL mean 44.9 mm,females mean 47.0 mm;inR.kukunoris,males SVL mean 56.3 mm,females mean 61.9 mm),longer hindlimbs 171% of SVL (versus 160% of SVL),tibio-tarsal articulation reaching to the anterior corner of eyes when leg stretched forward(versus tibio-tarsal articulation reaching to tympanum when leg stretched forward),relative finger lengths: III>IV>I>II(versus III>I>IV>II),two distinct nuptial pads with an obvious gap (versus four nuptial pads with a narrow gap) (Figure 9A),dorsal skin smooth (versus rough dorsum).The new species differs fromR.chensinensisby having two distinct nuptial pads(versus four nuptial pads) (Figure 9B),hindlimbs 170% of SVL(versus 185% of SVL),head length 33% of SVL (versus 34% of SVL),lower arm diameter 8% of SVL (versus 9% of SVL),tibiotarsal articulation reaching to the anterior corner of eyes when leg stretched forward (versus tibio-tarsal articulation reaching to nostrils or beyond tip of snout when leg stretched forward),toes two-thirds webbed (versus toes one half webbed).The new species differs fromR.huanrenensisby having internal subgular vocal sacs (versus absent),the anterior edges of vomerine teeth in line with the center of the choanae (versus the vomerine teeth located behind the posterior level of the choanae),having two distinct nuptial pads (versus four nuptial pads) (Figure 9C),heels overlapping when limbs are held at right angles to body axis (versus heels just meeting when the limbs are folded at right angle to the body),foot length longer than tibia length(versus foot length shorter than tibia length),toes two-thirds webbed (versus toes nearly fully webbed).The new species differs from The new species differs fromR.dybowskiiby having smaller body size (inR.taihangensissp.nov.,males SVL mean 44.9 mm,females mean 47.0 mm;inR.dybowskii,males SVL mean 62.6mm,females mean 67.1 mm),the tympanum diameter shorter than half of the eye diameter (TD/ED=0.47)(versus the tympanum diameter larger than half of the eye diameter),hindlimbs 171% of SVL (versus 175% of SVL),tibiotarsal articulation reaching to the anterior corner of eyes when leg stretched forward (versus tibio-tarsal articulation reaching to the anterior corner of eyes or nostrils when leg stretched forward),having two distinct nuptial pads with an obvious gap(versus four nuptial pads with a narrow gap) (Figure 9D),toes two-thirds webbed (versus toes nearly fully webbed).The new species differs fromR.huanrenensisby having internal subgular vocal sacs (versus absent),the anterior edges of vomerine teeth in line with the center of the choanae (versus the vomerine teeth located behind the posterior level of the choanae),heels overlapping when limbs are held at right angles to body axis(versus heels just meeting when the limbs are folded at right angle to the body),foot length longer than tibia length (versus foot length shorter than tibia length),toes two-thirds webbed(versus toes nearly fully webbed).

Figure 9 Male nuptial pads of R.kukunoris (QZ817) from Shiqu County,Sichuan Province,China (A),R.chensinensis (HNNU1604III002)from Xiaoqinling Mountain in Henan Province,China (B),R.huanrensis (39702) from Hulunbuir City,Inner Mongolia Autonomous Region,China (C),and R.dybowskii (94352) from Benxi City,Liaoning Province,China (D).More voucher information is presented in Supplementary Table S1.
4.Discussion
Because of the phenotypic plasticity and local morphological adaptations to the similar habitats,cryptic species are common in amphibians (Chenet al.,2020;Shenet al.,2020;Stuartet al.,2006).Recently,the integrative taxonomy of morphological comparisons and molecular analyses facilitated the identification of many cryptic amphibian species (Cheet al.,2020;Shenet al.,2020;Shiet al.,2020;Shiet al.,2021;Wanget al.,2020).Phylogenetic and biogeographic analyses suggested the existence of cryptic species in theR.chensinensisspecies group(Zhouet al.,2012).In this study,through extensive sampling,two robust monophyletic subclades-subclade C and T-were revealed for the samples from the Taihang Mountains based on the phylogenetic analysis (BPP=1.00).Subclade C included the haplotypes from the southwest of the Taihang Mountains(locality 15),the east Qinling Mountains (localities 16-17),and the haplotype ofR.chensinensisfrom the type locality-the middle Qinling Mountains in Shaanxi Province.The pairwise divergence within subclade C was lower (0.2%),and based on the aforementioned evidence,subclade C should beR.chensinensissensu stricto.
The highly supported subclade T comprised the haplotypes from the majority area of the Taihang Mountains (localities 1-14),and it has a close affinity withR.kukunoris(BPP=1.00).The pairwise distance between subclade T andR.kukunoris(4.8%) is obviously higher than the divergence threshold of 3% for identifying amphibian cryptic species based on the standard mitochondrial barcoding marker (Chenet al.,2020).In addition,morphological differentiation confirmed the phylogenetic results,and both supported the validity of a new species (R.taihangensissp.nov.) in theR.chensinensisspecies group.According to the sampling sites and the phylogenetic relationship between our study and that of Zhouet al.(2012),the new species (i.e.,R.taihangensissp.nov.) revealed in this study corresponds to the Clade L in the phylogenetic tree of theR.chensinensisspecies group in Zhouet al.(2012).Hence,we proposed that the populations formerly recorded asR.chensinensisin Beijing City,Hebei Province,and the southeastern Taihang Mountains of Henan Province (localities 1-14) should be revised toR.taihangensissp.nov.,whereas the populations of the southwestern Taihang Mountains (locality 15),Xiaoqinling Mountains,and Funiu Mountains in Henan Province (localities 16-17,the eastern foothills of the Qinling Mountains) remainR.chensinensissensu stricto.
Numerous phylogeographic studies have demonstrated that mountain ranges may generate geographic barriers and contribute to the speciation (Wanget al.,2016;Wienset al.,2019;Xuet al.,2018;Yanet al.,2010).The dramatic uplift of the plateau and the associated climatic change of eastern Asia are considered as the major drivers of vicariant speciation for many organisms (Cheet al.,2010;Wanget al.,2016;Wuet al.,2020).With the uplift of the Qinghai-Tibetan plateau(QTP),a cold and arid climate developed in the northeastern QTP,and the three-stepped terrain formed in China.Furthermore,the uplifting of the Qinling Mountains formed the biogeographic boundary between southern and northern China (Rost 1994;Webb and Bartlein,1992;Wuet al.,2020;Zhouet al.,2012).The diversification ofR.taihangensissp.nov.,R.chensinensis,andR.kukunorisalso indicated the effect of longterm geographic isolation and ecological divergence between species because of the orogeny of the QTP.The geographic distributions ofR.taihangensissp.nov.,R.chensinensisandR.kukunorisare primarily located in the Taihang Mountains,Qinling Mountains and the eastern edge of QTP boundaries,respectively,suggesting the role of mountain edges as a potential geographic barrier for their dispersal and divergence.
In addition,the riverine barrier hypothesis (reviewed in Chenet al.,2020) might explain the distribution patterns and genetic variation ofR.taihangensissp.nov.andR.chensinensis.In the southern Taihang Mountains,the distributions ofR.chensinensissensu stricto andR.taihangensissp.nov.were separated by drainage.The populations ofR.chensinensissensu stricto are only distributed in the Yellow River Basin,whereas all the populations ofR.taihangensissp.nov.are located within the Haihe River system (Figure 1).The role of rivers in shaping the range boundaries and genetic structure of species has been investigated in a range of amphibian species (Chenet al., 2020;Liet al.,2009;Nowakowskiet al.,2015;Wanget al.,2015;Yanet al.,2013;Zhanget al.,2010).Regarding theR.chensinensisgroup,barriers to gene flow produced by the complex topography of mountains and/or river systems might contribute to the high level of species diversity in northern and central China.In addition,considering the gene flow previously revealed betweenR.chensinensisandR.kukunoris(Qiet al.,2014;Zhouet al.,2012),it is also possible that gene exchange may occur during or after the speciation of the new species,particularly in populations at the periphery of its distribution range,which is a common mechanism underlying divergence (Smadja and Butlin,2011).Further studies should be conducted to determine the detailed roles of the mountain ridges,river systems and gene flow in the diversification and distribution of theRanaspecies.
AcknowledgementsWe would like to thank Meihua ZHANG from the Chengdu Institute of Biology,Chinese Academy of Sciences for providing samples ofR.kukunorisfor morphological measurements.We are grateful to Yuxiao HE,Youqiang LU,Qiqi FENG,Dian LIU,Can KE,Yimin ZHANG,Shuxuan GAO,Yanjun ZHU,Yao LIU and Guilin HU for their efforts in the field surveys,Xuewei LI for work in measuring samples.This study was supported by the Biodiversity Survey and Assessment Project of the Ministry of Ecology and Environment,China (2019HJ2096001006),China Biodiversity Observation Networks (Sino BON),the National Natural Science Foundation of China (31872220,U21A20192 and 31572245),the Natural Science Foundation of Henan Province (202300410222)and the Second National Survey of Terrestrial Wildlife Resources Project of the National Forestry and Grassland Bureau of China.
杂志排行
Asian Herpetological Research的其它文章
- Effect of Morphology and Age on the Closure Ability of Asian Box Turtles(Cuora)
- Partial Masquerading and Background Matching in Two Asian Box Turtle Species (Cuora spp.)
- Morphology and Histochemistry of Infralabial Glands of Two Species in the Slug-Eating Family Pareidae (Reptilia: Serpentes)
- Field Surveys Suggest Biennial Reproduction Cycle and Competition-triggered Dispersal of the Endangered Chinese Crocodile Lizard
- Elevational Variation in Reproductive Strategy of a Widespread Lizard:High-Elevation Females Lay Fewer but Larger Eggs
