酸性介质环境中温度对Ti–Mo合金耐蚀性的影响
2022-09-27魏亚付禹潘志敏王雪飞余伟何石磊苑清英骆鸿李晓刚
魏亚,付禹,潘志敏,王雪飞,余伟,何石磊,苑清英,骆鸿,李晓刚
腐蚀与防护
酸性介质环境中温度对Ti–Mo合金耐蚀性的影响
魏亚1a,付禹1a,潘志敏1a,王雪飞1a,余伟1b,何石磊2,苑清英2,骆鸿1a,李晓刚1a
(1.北京科技大学 a.新材料技术研究院 b.工程技术研究院,北京 100083;2.宝鸡石油钢管有限责任公司,陕西 宝鸡 721008)
研究Ti–Mo合金在不同温度的20% HCl溶液中的腐蚀行为和腐蚀规律,并探究其环境腐蚀机理。采用X射线衍射仪、扫描电子显微镜、电化学工作站以及X射线光电子能谱等对Ti–Mo合金的微观组织结构和不同介质环境中的耐蚀性进行了探究。溶液介质温度对Ti–Mo合金的腐蚀行为具有显著影响。当温度从20 ℃上升到70 ℃时,腐蚀电位从‒548.9 mV(vs. Ag/AgCl)降低到‒593.3 mV(vs. Ag/AgCl),且腐蚀电流密度在20 ℃时最低,为36.925 μA/cm2,维钝电流密度也随温度升高而增加。此外,温度升高不会改变氧化膜的成分,但会使膜内载流子密度升高,导致氧化膜的半导体特性发生n–p型转变。当溶液温度为20、30、50、70 ℃时,腐蚀速率分别为1.138 3、2.931 7、35.217、39.838 6 mm/a,且腐蚀速率随着温度升高而增加。溶液温度升高会使Ti–Mo合金氧化膜内缺陷增多,氧化膜的稳定性和耐蚀性降低。在腐蚀过程中α相与β相会形成微原电池,α相作为阳极更容易发生优先腐蚀,这是由于β相中Mo元素含量较高所致。
Ti–Mo合金;温度;氧化膜;微原电池
随着现代工业体系对石油需求量的增多,石油管道的安全性也越来越引起人们的重视[1-2],管道中的活泼金属元素易与石油介质发生化学反应,从而导致材料发生腐蚀失效现象[3]。石油在开采和运输过程中管道常会受到酸性和Cl‒的侵蚀而产生腐蚀,因此现代工业对石油管道的耐蚀性提出了很高的要求。通常,服役环境的pH值越低,材料的腐蚀速率越高。例如气藏中冷凝水的pH可达3.5以下,这对于管道输送安全是一个巨大的考验[4-5]。此外,Cl‒对金属材料表面的保护膜也有很严重的破坏作用,而且破坏作用随着Cl‒浓度的升高而增大[6-7]。普通金属石油管道由于腐蚀原因造成的各种事故给国民经济带来了极大的损失,因此需要开发新型耐蚀合金来提高石油管道的服役安全性。
钛及钛合金具有密度低[8]、强度高[9]、耐腐蚀性能优异[10]特点,在石油化工、航空航天、生物医疗等领域应用广泛[11]。Ti与O有着很强的亲和力,在与O2、H2O相互作用后会形成亚稳态的Ti(OH)4,由于Ti(OH)4在自然条件下极其不稳定,容易分解为TiO2和H2O,TiO2化学稳定性强。因此,会在钛合金基体表面形成一层致密的保护膜,可以有效阻碍腐蚀介质对基体金属的侵蚀,达到保护钛合金基体的作用,这是钛合金耐蚀性优异的主要原因[12-13]。目前石油管道运输的金属材料主要采用管线钢,管线钢在酸性和中性含Cl‒溶液中不易形成完整的氧化膜。面对如此的服役条件,管线钢容易发生局部或均匀腐蚀,加速其腐蚀失效[14-15]。相比之下,钛合金表面形成的TiO2氧化膜更加致密,TiO2氧化膜在较宽的pH范围内都有很高的稳定性,极大减少各类腐蚀的可能性,而且其表面的氧化膜被破坏后有很强的自修复能力,所以钛合金的耐蚀性能也更好[16-17]。因此,钛合金管道在复杂石油工况环境中的服役时间更长,效果更好[18]。为了进一步提高钛合金的耐蚀性,学者们常常在其中添加Mo、Al等元素。研究表明Mo元素能提高钛合金在Cl‒环境中的耐蚀性,其表面形成的MoO2和MoO3可以作为Cl‒向基材运动的屏障,阻碍腐蚀介质对基体的侵蚀,进一步提升钛合金的耐蚀性[19]。此外,外界服役温度对于石油管道腐蚀有很大的影响,温度升高会使腐蚀动力学反应加速,氧化膜出现溶解,氧化膜破坏之后基材会直接接触侵蚀性环境,造成耐蚀性能下降[20]。
从上述分析可知,研究钛合金石油管道在严苛环境下的腐蚀行为和规律,并探究其腐蚀机理有非常重大的理论和现实意义。本文主要探究Ti–Mo合金在不同温度变化的20% HCl溶液中的腐蚀行为和规律,旨在揭示Ti–Mo合金的腐蚀机制,为Ti–Mo合金在石油化工领域的应用提供一定的参考价值。
1 试验
1.1 材料
本试验所用钛合金(Ti–Mo合金)为宝鸡石油钢管有限责任公司生产,其主要成分(质量分数)为:2.99% Mo,0.60% Zr,0.044% Fe,0.014% C,0.005 1% N,余量为Ti。
1.2 组织观察和性能测试
采用X射线衍射(XRD)对Ti–Mo合金进行物相分析。采用Quanta 250扫描电子显微镜(SEM)对Ti–Mo合金的形貌进行观察。电化学测试采用典型的三电极系统,饱和氯化银电极为参比电极,对电极为铂电极,工作电极为钛合金试样,工作电极面积为1 cm2,试验温度为20~70 ℃。电化学试验所用仪器为Princeton VersaSTAT 3F 电化学工作站。为了保证试验结果的准确性,样品在电化学测试之前统一在空气中放置48 h。极化曲线电位范围为‒0.8~2 V(vs. Ag/AgCl),由于扫描范围较大,扫描速率选取为1 mV/s。电化学阻抗谱(EIS)的正弦电位扰动为10 mV,扫描频率为10−2~105Hz。采用美国赛默飞的250Xi X射线光电子能谱仪(XPS)对氧化膜的化学组成进行研究,成膜电位为0.5 V(vs. Ref),时间为7 200 s。采用Mott–Schottky对半导体膜的性质进行探究,Mott– Schottky试验频率为1 000 Hz,扫描电位为2~ ‒1 V,步长为20 mV。选择了20% HCl溶液作为静态浸泡测试溶液,溶液的温度分别保持在20、30、50、70 ℃。由于Ti–Mo合金在常温下腐蚀较慢,为了使腐蚀前后的形貌变化更明显,选取浸泡时间为15 d。浸泡15 d后用蒸馏水和酒精超声清洗,干燥后用精度为±0.1 mg的天平称量,并用3D共聚焦和扫描电子显微镜观察腐蚀后的形貌。
2 结果与讨论
2.1 钛合金原始样品组织结构分析
如图1的Ti–Mo合金的XRD图谱所示,钛合金原始样品主要由α相和β相组成,是典型的双相结构。图2为钛合金原始样品微观结构和元素分布,图中α相和β相相间分布。EDS结果显示,基体Ti元素的整体分布比较均匀,Mo元素和Zr元素主要富集在β相中,在α相中含量较少。

图1 Ti–Mo合金样品的XRD图谱
2.2 电化学分析
2.2.1 开路电位和极化曲线
图3a为Ti–Mo合金在不同温度条件下20% HCl溶液中的开路电位变化曲线。开路电位的下降对应着钛合金表面自然氧化膜的溶解,温度升高使表面氧化膜的溶解速度逐渐加快。图3b所示的阴极区主要为析氢反应[21-22],阳极区由活化溶解区、活化钝化过渡区、一次钝化区、二次钝化区和过钝化区5种区域组成。活化溶解区内钛基体被氧化成可溶的Ti3+并进入到溶液中,电流密度逐渐增大。当活化溶解区的阳极电流达到最大,表明活化钝化过渡区的开始,此区域内TiO2氧化膜生成,电流密度开始减小。活化钝化过渡区之后,在‒0.2~1.2 V之间出现了明显的钝化区,20、30 ℃时钝化区内的电流密度基本保持不变,50、70 ℃时区域内的电流密度出现了波动,发生了亚稳态点蚀,此区域内的电流密度波动是由氧化膜的溶解和生成的竞争作用所致,在发生亚稳态点蚀之后电流密度又逐渐减小,说明氧化膜重新生成,表明Ti–Mo合金有很好的自修复能力[23]。在1.2~1.6 V内出现了二次钝化区,二次钝化区的形成是氧化膜的溶解和竞争重新达到平衡所致。当电压大于1.6 V后出现了过钝化现象,此时钝化膜的溶解速度加快,电流密度加速增大。采用Tafel外推法从动电位极化曲线得到corr和corr值见表1。结果显示,Ti–Mo合金在20 ℃时的腐蚀电位corr最高(‒554.889 V),同时腐蚀电流密度corr也最低(36.925 μA/cm2),20 ℃时耐蚀性最好。维钝电流密度能够较好地反映钝化膜的环境稳定性,在极化曲线的钝化区内,选取0.5 V对应的电流密度为维钝电流密度(pass),维钝电流密度随温度升高逐渐增大,表明耐腐蚀性能随温度升高逐渐下降。
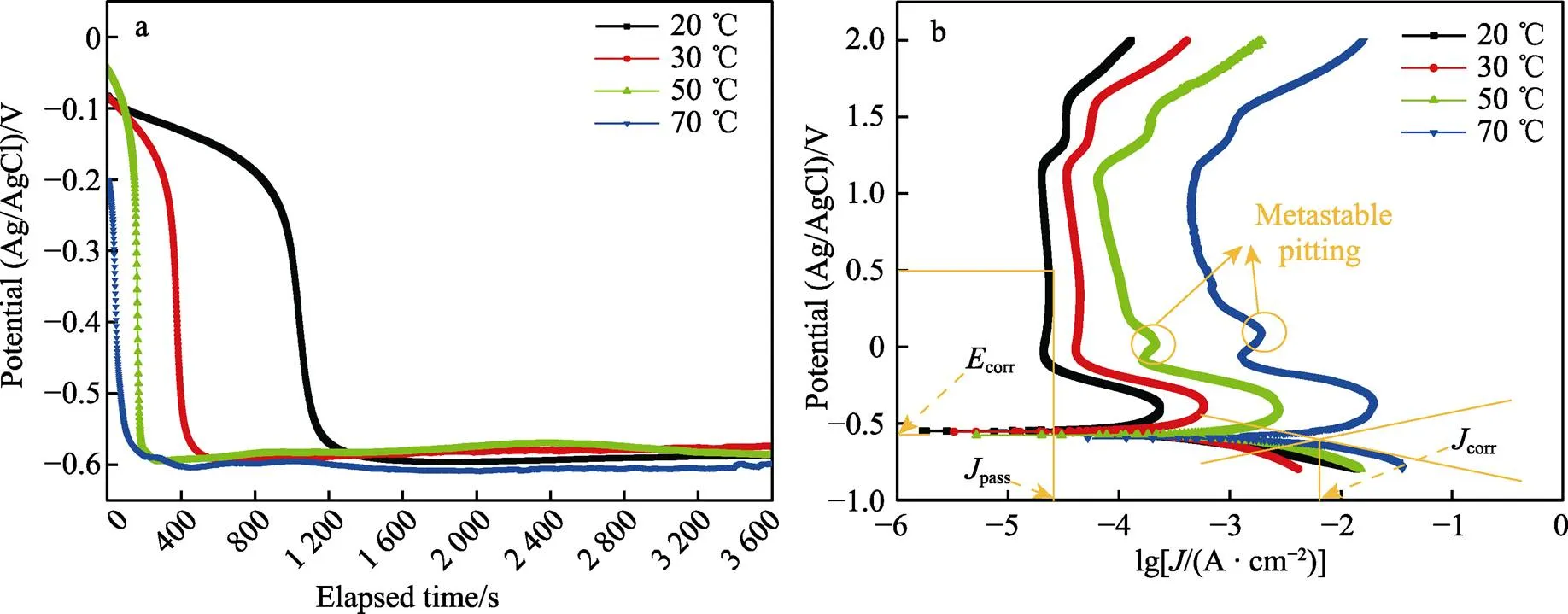
图3 Ti–Mo合金在20~70 ℃的20% HCl溶液中的开路电位和极化曲线
表1 由动电位极化曲线推导出的参数
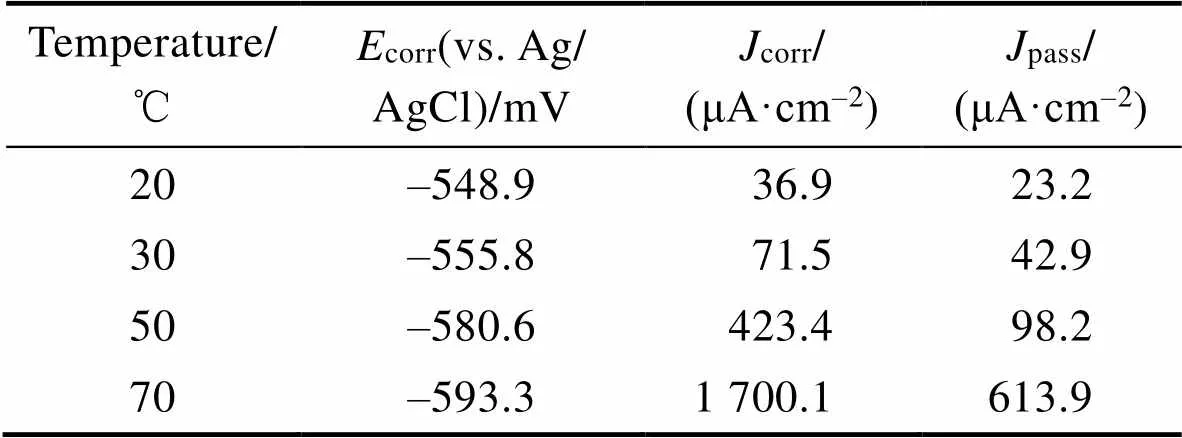
Tab.1 Parameters derived from the potentiodynamic polarization curve
2.2.2 电化学阻抗谱(EIS)分析
为了进一步阐明介质温度变化对Ti–Mo合金腐蚀性能的影响,在20% HCl溶液中进行EIS测试,相应的Nyquist和Bode图如图4所示。图4a的Nyquist图出现2个容抗弧,表明存在2个时间常数。高频电容回路对应于电解液与Ti–Mo合金基体界面处的双电层,低频电容回路对应于表面的氧化膜。高频电容回路半径减小说明电荷转移电阻减小,低频电容回路半径减小表明表面膜电阻减小。图4b是在20% HCl溶液中测量的Bode图,Bode图中相位角随着溶液温度的升高逐渐减小,相位角越大表明氧化膜越致密,在70 ℃时相位角接近30°表明形成的氧化膜已经非常不致密[24-25]。
采用图5所示的等效电路对阻抗谱进行拟合,在等效电路中,S是溶液电阻,1和ct分别是与双电层电容和电荷转移电阻有关的元件,2和f分别是与氧化物/腐蚀产物膜电容和氧化物/腐蚀产物膜电阻有关的元件[26]。拟合的电化学阻抗谱数据如表2所示,极化电阻(p)可以用来评估耐蚀性[27],其中p=f+ct。经过计算,20、30、50、70 ℃时,Ti–Mo合金在20% HCl溶液中的极化电阻分别为314.20、111.29、27.29、8.76 Ω·cm2。极化电阻随着溶液温度的升高逐渐减小,Ti–Mo合金的耐蚀性逐渐变差。
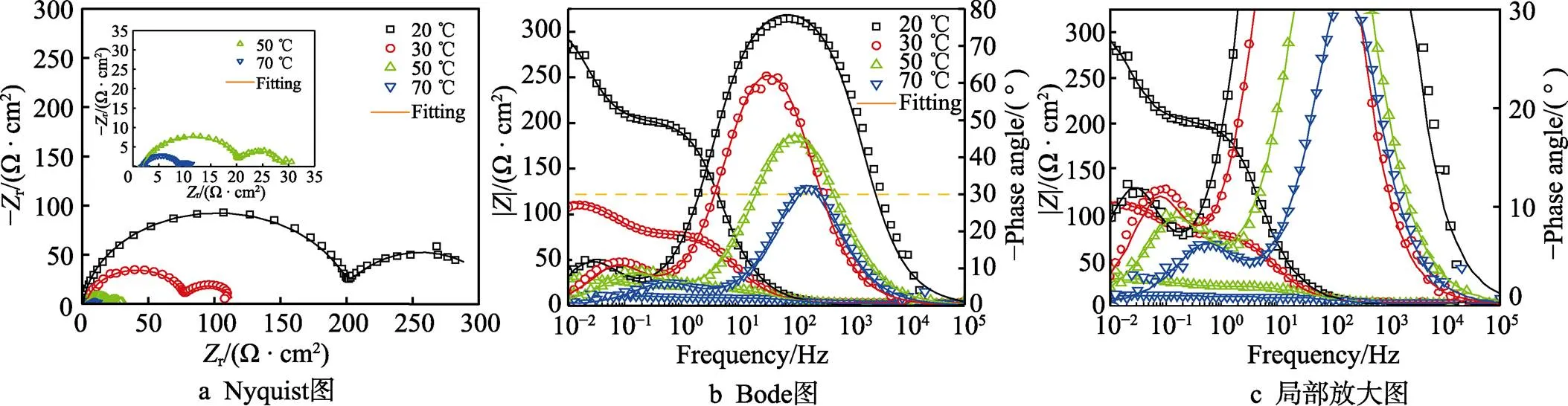
图4 Ti–Mo合金在20~70 ℃的20% HCl溶液中的阻抗谱

图5 Ti–Mo合金在20% HCl溶液中阻抗谱的等效电路图
2.3 XPS分析
Ti–Mo合金的耐蚀性与表面氧化膜的成分密切相关,因此采用XPS对Ti–Mo合金在溶液中形成的氧化膜的成分进行分析。从图6所示的XPS全谱中,可以看到O 1s、Ti 2p、C 1s、Mo 3d、Zr 3d的存在,其中C 1s的存在是由于检测过程中引入的污染物C。
Ti 2p的拟合图谱如图7所示,458.7 eV的峰位可以拟合成双峰,分别对应于459.1 eV的TiO2和457.0 eV的Ti2O3,453.8 eV的峰位对应于金属Ti[28]。图8所示的O1s的峰位也可拟合成3种物质,分别是530.4 eV的O2‒、532.3 eV的OH‒和533.0 eV的 O2‒(H2O)[29]。从图9a可以看到,Mo 3d在229.1 ev和232.2 eV处有2个明显的峰位,分别对应于 MoO2和 MoO3氧化物[30]。Zr 3d在182.4 ev和184.1 eV处也有ZrO2峰的出现,但峰位较弱,表明含量较少[31]。通过对拟合的Ti 2p峰进行计算可知,20、30、50、70 ℃下,氧化膜中TiO2的占比分别为81.9%、88.8%、78.8%和84.2%,Ti2O3的占比分别为13.0%、8.2%、13.6%和11.6%,Ti的占比分别为5.1%、3.0%、7.6%和4.2%,对比各相的比例得不出明显规律。通过XPS分析可知氧化膜的成分由钛合金的氧化物、氢氧化物以及Mo和Zr的氧化物组成。但XPS不能获得氧化膜的半导体特性和膜内载流子密度,所以采用Mott–Schottky测试继续分析合金表面氧化膜的特性。
表2 Ti–Mo合金在20% HCl溶液中的电化学阻抗谱拟合参数
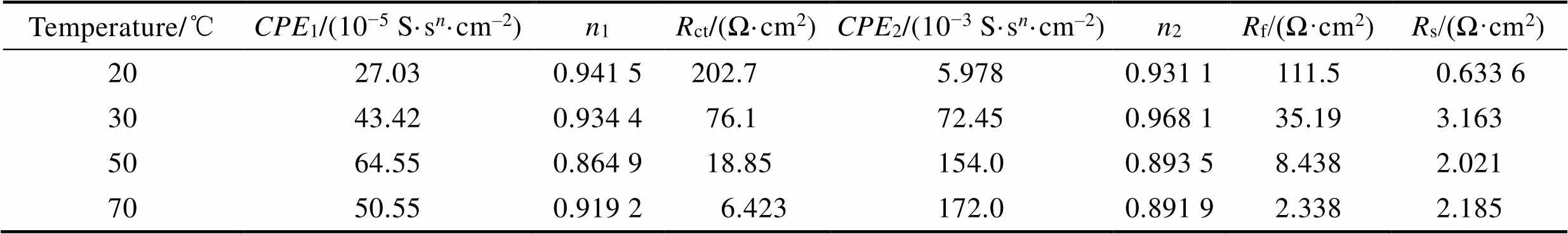
Tab.2 Fitting parameters of Ti-Mo alloy in 20wt.% HCl solution

图6 Ti–Mo合金在不同温度的20% HCl溶液中表面氧化膜的全谱图

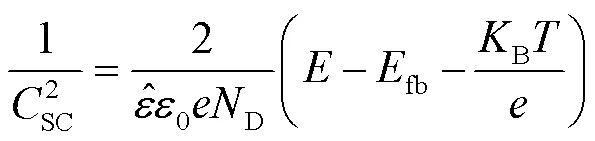

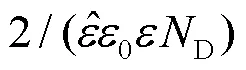

图7 XPS结果中的Ti 2p峰拟合图

图8 XPS结果中的O 1s峰拟合图

图9 XPS结果中的Mo 3d峰和Zr 3d峰
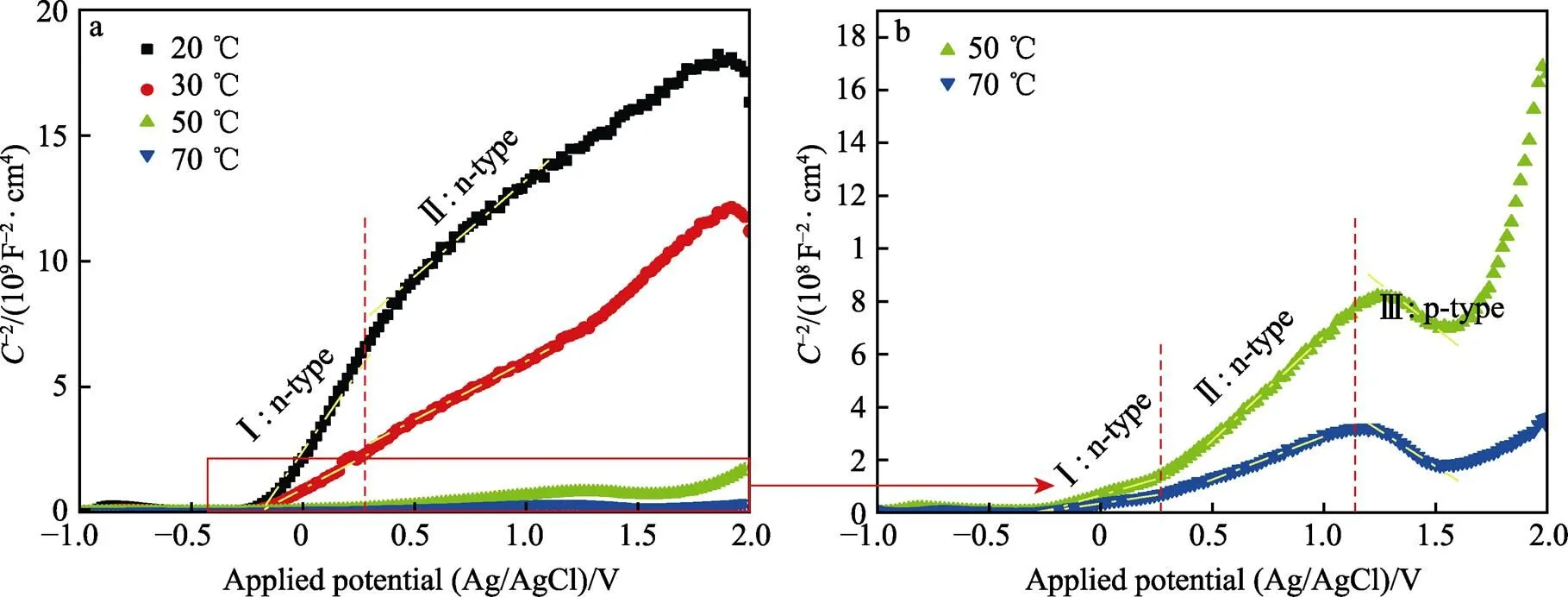
图10 Ti–Mo合金在20~70 ℃的20% HCl溶液中的Mott–Schottky结果
表3 由Mott–Schottky曲线拟合得到的载流子密度和平带电位

Tab.3 ND/A and flat-band potential derived from Mott-Schottky in 20wt.% HCl solution
2.4 静态浸泡试验
由图11a可知,Ti–Mo钛合金的单位面积失重从20 ℃的21.051 2 mg/cm2增加到70 ℃的736.742 2 mg/cm2。采用公式(3)对钛合金的腐蚀速率做出了直观的计算,式中代表钛合金腐蚀速率(mm/a),1是钛合金腐蚀前质量(g),2是钛合金腐蚀后质量(g),代表钛合金试样表面积,代表试验时间(h),代表钛合金密度(4.5 g/cm3)[37],经过计算得到20、30、50、70 ℃时,腐蚀速率分别为1.138 3、2.931 7、35.217 4、39.838 6 mm/a。
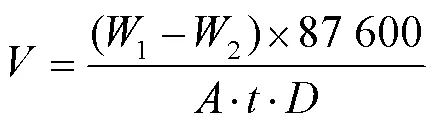
图12a—d为腐蚀后样品的3D共聚焦表面形貌及沿图中白线分布的腐蚀坑深度。从图12的3D共聚焦显微镜的结果可以看出,浸泡后样品表面的沟壑逐渐增多,70 ℃甚至出现了开裂现象。3D共聚焦结果显示,20、30、50、70 ℃时,最大腐蚀深度依次为12.426、13.601、23.957、41.492 μm。图13为Ti–Mo合金浸泡15 d之后的腐蚀形貌,可以看到Ti–Mo合金已经严重腐蚀,说明此时的氧化膜已经破裂。图13a为20 ℃浸泡15 d后的腐蚀形貌,Ti–Mo合金呈现出典型的不均匀腐蚀,α相腐蚀较为严重并出现了尖锐的棱角,β相腐蚀程度相对较轻,在浸泡过程中α相和β相会形成微原电池,这是因为β相中Mo含量较高,在腐蚀过程中作为阴极,α相作为阳极会加速腐蚀[38]。当温度升高至30 ℃时,可以清楚看到α相与β相交错分布,此时β相形状为细条状,相比20 ℃时腐蚀进一步加剧。50 ℃浸泡15 d后可以看到α相已无尖锐棱角,70 ℃浸泡15 d后样品已经明显疏松,腐蚀逐步加重。浸泡结果与之前的电化学测试结果保持一致。
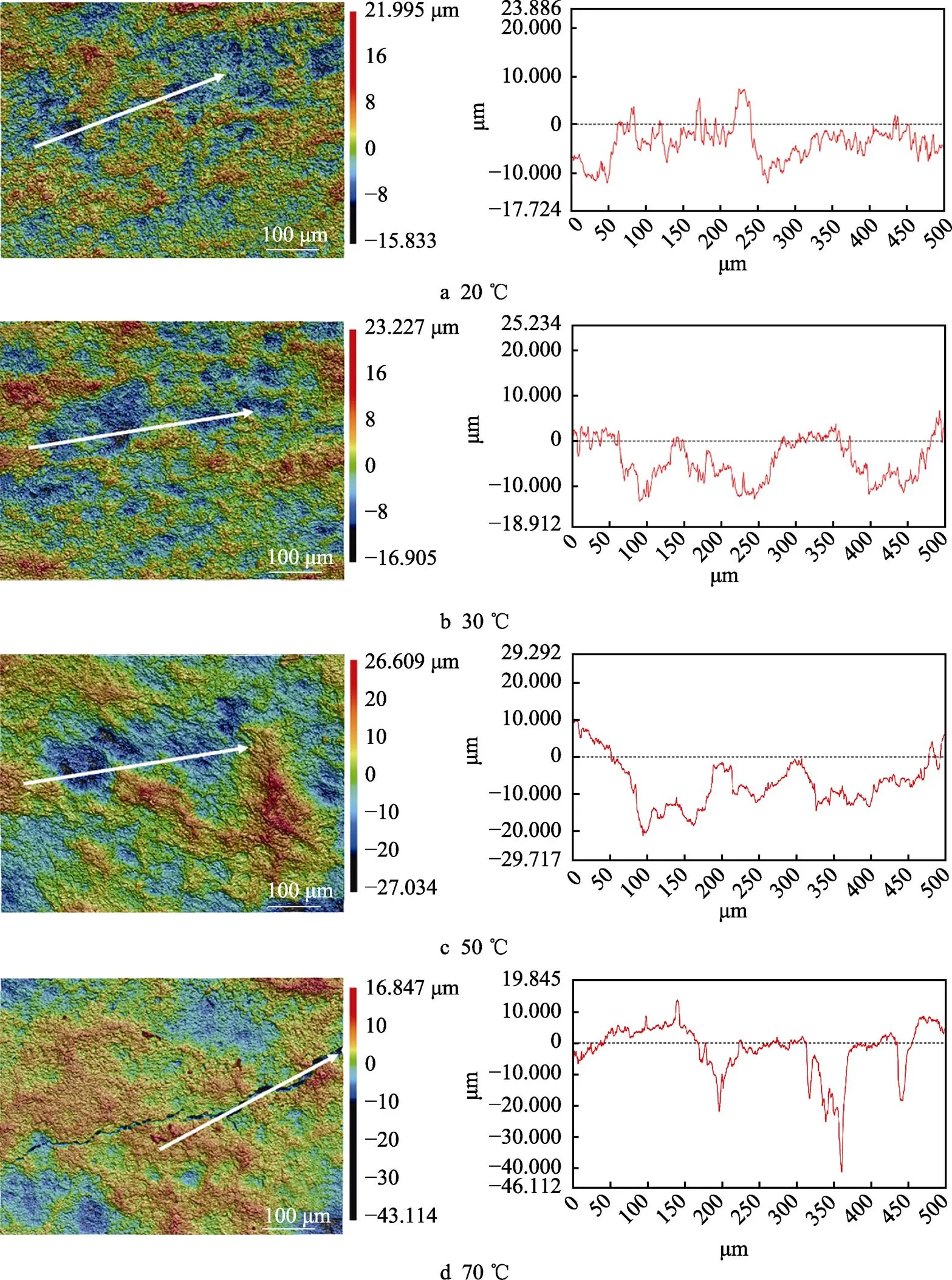
图12 Ti–Mo合金在20~70 ℃的20% HCl溶液中浸泡15 d的3D共聚焦形貌

图13 Ti–Mo合金在20% HCl溶液浸泡15 d后腐蚀形貌
3 结论
1)电化学测试结果显示,溶液介质温度会使得Ti–Mo合金的腐蚀电流密度和维钝电流密度增加,同时减小电荷转移电阻和膜电阻。
2)结合XPS和Mott–Schottky分析结果可知,溶液介质温度升高会造成Ti–Mo合金表面氧化膜内的缺陷增多,氧化膜半导体类型会发生转变。
3)浸泡结果显示,Ti–Mo合金的腐蚀速率和腐蚀坑深度都随着溶液温度升高而增大。在浸泡过程中α相和β相会形成微原电池,β相中Mo含量较高,在腐蚀过程中作为阴极,α相作为阳极,两相耦合会加速钛合金的腐蚀。
[1] 王正泉, 徐玮辰, 周子扬, 等. X65管线钢在成品油管道沉积物中的微生物腐蚀行为[J]. 表面技术, 2020, 49(7): 245-254.
WANG Zheng-quan, XU Wei-chen, ZHOU Zi-yang, et al. Microbial Corrosion Behavior of X65 Pipeline Steel in Product Pipeline Sediments[J]. Surface Technology, 2020, 49(7): 245-254.
[2] 王丹, 袁世娇, 吴小卫, 等. 油气管道CO2/H2S腐蚀及防护技术研究进展[J]. 表面技术, 2016, 45(3): 31-37.
WANG Dan, YUAN Shi-jiao, WU Xiao-wei, et al. Research Progress of CO2/H2S Corrosion in Oil and Gas Pipelines and the Protection Techniques[J]. Surface Technology, 2016, 45(3): 31-37.
[3] TSAY L W, LIN Y J, CHEN C. The Effects of Rolling Temperature and Sensitization Treatment on the Sulfide Stress Corrosion Cracking of 304L Stainless Steel[J]. Corrosion Science, 2012, 63: 267-274.
[4] AZAM M A, SUKARTI S, ZAIMI M. Corrosion Behavior of API-5L-X42 Petroleum/Natural Gas Pipeline Steel in South China Sea and Strait of Melaka Seawaters[J]. Engineering Failure Analysis, 2020, 115: 104654.
[5] IANNUZZI M. Environmentally Assisted Cracking (EAC) in Oil and Gas Production[M]. Amsterdam: Elsevier, 2011: 570-607.
[6] QIU Zhi-chao, XIONG Chun-ming, CHANG Ze-liang, et al. Major Corrosion Factors in the CO2and H2S Coexistent Environment and the Relative Anti-Corrosion Method: Taking Tazhong I Gas Field, Tarim Basin, as an Example[J]. Petroleum Exploration and Development, 2012, 39(2): 256-260.
[7] SUNABA T, ITO T, MIYATA Y, et al. Influence of Chloride Ions on Corrosion of Modified Martensitic Stainless Steels at High Temperatures under a CO2Environment[J]. Corrosion, 2014, 70(10): 988-999.
[8] SALEEM A, KHADIJA G, AKHTAR Z, et al. Short-Term Exposure to Titanium, Aluminum and Niobium (Ti-6Al- 4Nb) Alloy Powder can Disturb the Serum Low-Density Lipoprotein Concentrations and Antioxidant Profile in Vital Organs but not the Behavior of Male Albino Mice[J]. Drug and Chemical Toxicology, 2020, 43(3): 298-306.
[9] BLACKWELL P L, WISBEY A. Laser-Aided Manufacturing Technologies; Their Application to the Near-Net Shape Forming of a High-Strength Titanium Alloy[J]. Journal of Materials Processing Technology, 2005, 170(1-2): 268-276.
[10] 许爱军, 万海峰, 梁春祖, 等. 低温钛合金材料应用现状及发展趋势[J]. 精密成形工程, 2020, 12(6): 145-156.
XU Ai-jun, WAN Hai-feng, LIANG Chun-zu, et al. Application Status and Development Trend of Cryogenic Titanium Alloy[J]. Journal of Netshape Forming Engineering, 2020, 12(6): 145-156.
[11] 陈正云. 钛在石油化工中的应用[J]. 钛工业进展, 2000, 17(4): 35-37.
CHEN Zheng-yun. Application of Titanium in Petrochemical Industry[J]. Titanium Industry Progress, 2000, 17(4): 35-37.
[12] HE X, NOËL J J, SHOESMITH D W. Temperature Effects on Oxide Film Properties of Grade-7 Titanium[J]. Corrosion, 2007, 63(8): 781-792.
[13] NADY H, EL-RABIEI M M, SAMY M. Corrosion Behavior and Electrochemical Properties of Carbon Steel, Commercial Pure Titanium, Copper and Copper-Aluminum-Nickel Alloy in 3.5% Sodium Chloride Containing Sulfide Ions[J]. Egyptian Journal of Petroleum, 2017, 26(1): 79-94.
[14] 高文平, 吕祥鸿, 谢俊峰, 等. 苛刻环境中钛合金石油管材的抗腐蚀性能研究[J]. 稀有金属材料与工程, 2018, 47(1): 151-156.
GAO Wen-ping, LV Xiang-hong, XIE Jun-feng, et al. Corrosion Resistance of Titanium Alloy OCTG in Severe Environment[J]. Rare Metal Materials and Engineering, 2018, 47(1): 151-156.
[15] 高文玲, 高嘉喜, 尹志福, 等. 20和L245NS钢在模拟低含硫饱和CO2油田污水中的电化学耐蚀行为研究[J]. 材料保护, 2021, 54(5): 29-34, 62.
GAO Wen-ling, GAO Jia-xi, YIN Zhi-fu, et al. Study on the Electrochemical Corrosion Resistance Behavior of 20 and L245NS Steels in Simulated Low Sulfur-Containing and CO2-Saturated Oilfield Sewage[J]. Materials Protection, 2021, 54(5): 29-34, 62.
[16] 李冲, 陶欢, 宋德军, 等. 钛合金在高含硫化氢工况中的腐蚀性能研究[J]. 材料开发与应用, 2020, 35(6): 19-23.
LI Chong, TAO Huan, SONG De-jun, et al. Study on Corrosion Properties of Titanium Alloy in Gathering Line with High Sulfur Content[J]. Development and Application of Materials, 2020, 35(6): 19-23.
[17] GARFIAS-MESIAS L F, ALODAN M, JAMES P I, et al. Determination of Precursor Sites for Pitting Corrosion of Polycrystalline Titanium by Using Different Techniques[J]. Journal of the Electrochemical Society, 1998, 145(6): 2005-2010.
[18] UEDA M, KUDO T, KITAYAMA S, et al. Corrosion Behavior of Titanium Alloys in a Sulfur-Containing Environment[J]. Corrosion, 2006, 48(1): 596.
[19] KASEEM M, CHOE H C. The Effect of In-Situ Reactive Incorporation of MoOon the Corrosion Behavior of Ti-6Al-4V Alloy Coated via Micro-Arc Oxidation Coating[J]. Corrosion Science, 2021, 192: 109764.
[20] FEKRY A M. The Influence of Chloride and Sulphate Ions on the Corrosion Behavior of Ti and Ti-6Al-4V Alloy in Oxalic Acid[J]. Electrochimica Acta, 2009, 54(12): 3480-3489.
[21] CUI Zhong-yu, WANG Li-wei, ZHONG Ming-yuan, et al. Electrochemical Behavior and Surface Characteristics of Pure Titanium during Corrosion in Simulated Desulfurized Flue Gas Condensates[J]. Journal of the Electrochemical Society, 2018, 165(9): C542-C561.
[22] SU Bao-xian, LUO Liang-shun, WANG Bin-bin, et al. Annealed Microstructure Dependent Corrosion Behavior of Ti-6Al-3Nb-2Zr-1Mo Alloy[J]. Journal of Materials Science & Technology, 2021, 62: 234-248.
[23] WANG D B, WU J, WANG Q, et al. Temperature- Dependent Corrosion Behaviour of the Amorphous Steel in Simulated Wet Storage Environment of Spent Nuclear Fuels[J]. Corrosion Science, 2021, 188: 109529.
[24] WANG Z B, HU H X, LIU C B, et al. The Effect of Fluoride Ions on the Corrosion Behavior of Pure Titanium in 0.05 M Sulfuric Acid[J]. Electrochimica Acta, 2014, 135: 526-535.
[25] GONZÁLEZ J E G, MIRZA-ROSCA J C. Study of the Corrosion Behavior of Titanium and some of Its Alloys for Biomedical and Dental Implant Applications[J]. Journal of Electroanalytical Chemistry, 1999, 471(2): 109-115.
[26] LUO Hong, WANG Xian-zong, DONG Chao-fang, et al. Effect of Cold Deformation on the Corrosion Behaviour of UNS S31803 Duplex Stainless Steel in Simulated Concrete Pore Solution[J]. Corrosion Science, 2017, 124: 178-192.
[27] ARGADE G R, PANIGRAHI S K, MISHRA R S. Effects of Grain Size on the Corrosion Resistance of Wrought Magnesium Alloys Containing Neodymium[J]. Corrosion Science, 2012, 58: 145-151.
[28] MILOŠEV I, METIKOŠ-HUKOVIĆ M, STREHBLOW H H. Passive Film on Orthopaedic TiAlV Alloy Formed in Physiological Solution Investigated by X-Ray Photoelectron Spectroscopy[J]. Biomaterials, 2000, 21(20): 2103-2113.
[29] LUO Hong, SU Huai-zhi, YING Guo-bing, et al. Effect of Cold Deformation on the Electrochemical Behaviour of 304L Stainless Steel in Contaminated Sulfuric Acid Environment[J]. Applied Surface Science, 2017, 425: 628-638.
[30] PARK P Y, AKIYAMA E, HABAZAKI H, et al. The Corrosion Behavior of Sputter-Deposited Mo-Ti Alloys in Concentrated Hydrochloric Acid[J]. Corrosion Science, 1996, 38(10): 1649-1667.
[31] OLSSON C O A, LANDOLT D. Atmospheric Oxidation of a Nb-Zr Alloy Studied with XPS[J]. Corrosion Science, 2004, 46(1): 213-224.
[32] BEHNAMI FAR V, JAFARZADEH K, SHOOSHTARI GUGTAPEH H, et al. A Study on Electrical Properties of Thermally Grown TiO2Film at the Interface of Ti/RuO2- IrO2-TiO2Anode Using Mott-Schottky and Electrochemical Impedance Spectroscopy Techniques[J]. Materials Chemistry and Physics, 2020, 256: 123756.
[33] LI D G, FENG Y R, BAI Z Q, et al. Influence of Temperature, Chloride Ions and Chromium Element on the Electronic Property of Passive Film Formed on Carbon Steel in Bicarbonate/Carbonate Buffer Solution[J]. Electrochimica Acta, 2007, 52(28): 7877-7884.
[34] BAUMANIS C, BAHNEMANN D W. TiO2Thin Film Electrodes: Correlation between Photocatalytic Activity and Electrochemical Properties[J]. The Journal of Physical Chemistry C, 2008, 112(48): 19097-19101.
[35] CÁMARA O R, DE PAULI C P, VASCHETTO M E, et al. Semiconducting Properties of TiO2Films Thermally Formed at 400 ℃[J]. Journal of Applied Electrochemistry, 1995, 25(3): 247-251.
[36] AMRI J, SOUIER T, MALKI B, et al. Effect of the Final Annealing of Cold Rolled Stainless Steels Sheets on the Electronic Properties and Pit Nucleation Resistance of Passive Films[J]. Corrosion Science, 2008, 50(2): 431-435.
[37] YANG Fen-fen, KANG Hui-jun, GUO En-yu, et al. The Role of Nickel in Mechanical Performance and Corrosion Behaviour of Nickel-Aluminium Bronze in 3.5wt.% NaCl Solution[J]. Corrosion Science, 2018, 139: 333-345.
[38] MENG Kang, GUO Kai, YU Qiang, et al. Effect of Annealing Temperature on the Microstructure and Corrosion Behavior of Ti-6Al-3Nb-2Zr-1Mo Alloy in Hydrochloric Acid Solution[J]. Corrosion Science, 2021, 183: 109320.
The Influence of Temperature on Corrosion Resistance of Ti-Mo Alloy in Acidic Medium
1a,1a,1a,1a,1b,2,2,1a,1a
(1. a. Institute for Advanced Materials and Technology, b. Institute of Engineering and Technology, University of Science and Technology Beijing, Beijing 100083, China; 2. CNPC Baoji Petroleum Steel Pipe Co., Ltd., Shaanxi Baoji 721008, China)
Titanium alloy pipeline is playing an increasingly important role in petrochemical industry. This paper mainly explores the corrosion behavior of Ti-Mo alloy in 20wt.% HCl solution with different temperature changes, aiming at revealing the corrosion mechanism of Ti-Mo alloy and providing certain reference value for the application of Ti-Mo alloy in petrochemical industry.
The titanium alloy used in this experiment is produced by CNPC Baoji Petroleum Steel Pipe Co., Ltd. The main components (wt.%) are as follows: 2.99 Mo, 0.60 Zr, 0.044 Fe, 0.014 C, 0.005 1 N, and the balance is Ti. X-ray diffraction (XRD) and Quanta 250 scanning electron microscope (SEM) were used to observe the phase and morphology of Ti-Mo alloy. Electrochemical test adopts typical three-electrode system, with saturated silver chloride electrode as reference electrode, platinum electrode as counter electrode, titanium alloy sample as working electrode with the area of 1 cm2. The electrochemical experiment temperature were 20, 30 50 and 70 ℃. The potential range of polarization curve was ‒0.8 V (vs. Ag/AgCl) to 2 V (vs. Ag/AgCl) and the scanning frequency of electrochemical impedance spectroscopy (EIS) was 10−2Hz to 105Hz. The chemical composition of the oxide film was studied by 250Xi X-ray photoelectron spectroscopy. The oxide film was formed at 0.5 V (vs. Ref) for 7 200 s. Mott-Schottky was used to explore the properties of semiconductor films. The experimental frequency of Mott-Schottky was 1 000 Hz, the scanning potential was 2 to ‒1 V with the step size of 20 mV. 20wt.% HCl solution was selected as the static immersion test solution, and the temperature of the solution was kept at 20 ℃, 30 ℃, 50 ℃ and 70 ℃ respectively. After soaking for 15 days, ultrasonic cleaning with distilled water and alcohol, drying, weighing with a balance with accuracy of 0.1 mg, and observing the morphology after corrosion with 3D laser confocal and scanning electron microscope.
The solution medium temperature produced a great influence on the corrosion behavior of Ti-Mo alloy. The corrosion potential decreased from ‒548.9 mV (vs. Ag/AgCl) to ‒593.3 mV (vs. Ag/AgCl) in the range of 20 to 70 ℃. Ti-Mo alloys had the lowest corrosion current density at 20 ℃, i. e., 36.925 μA/cm2, and the passive current density decreased as the temperature enhanced. In addition, the content of the oxide film had no obvious difference at various temperature, but the donor concentration in the film increased at elevated temperature. Besides, it caused the n-p type transition of the semiconductor characteristics. The corrosion rate of the Ti-Mo alloy in 20wt.% HCl solution were 1.138 3, 2.931 7, 35.217 and 39.838 6 mm/a when solution temperature were 20, 30, 50 and 70 ℃, which indicated that the corrosion rate accelerated with the temperature went by.
An increase in the temperature of the solution will promote the formation of defects and reduce the stability and corrosion resistance of the oxide film. The α and β phases form microgalvanic cells in the corrosion process, and the α phase is more prone to corrode acting as the anode, which is due to the higher content of Mo in the β phase.
Ti-Mo alloy; temperature; oxide film; microgalvanic cells
2021-07-09;
2022-01-25
WEI Ya (1995-), Male, Postgraduate, Research focus: titanium alloy corrosion and protection.
骆鸿(1980—),男,博士,教授,主要研究方向为先进金属、钢铁材料的设计和服役安全。
LUO Hong (1980-), Male, Doctor, Professor, Research focus: design and service safety of advanced metal and steel materials.
魏亚, 付禹, 潘志敏, 等.酸性介质环境中温度对Ti–Mo合金耐蚀性的影响[J]. 表面技术, 2022, 51(9): 168-177.
tg174
A
1001-3660(2022)09-0168-10
10.16490/j.cnki.issn.1001-3660.2022.09.000
2021–07–09;
2022–01–25
中国石油科技创新基金(2019D–5007–0308);国家重点研究开发项目(2016YFB0301204);中央高校基本科研业务基金(FRF– MP–20–51,FRF–BD–20–28A2)
Fund:PetroChina Innovation Foundation (2019D-5007-0308); National Key Research and Development Program of China (2016YFB0301204); Fundamental Research Funds for the Central Universities (FRF-MP-20-51, FRF-BD-20-28A2)
魏亚(1995—),男,硕士研究生,主要研究方向为钛合金腐蚀与防护。
WEI Ya, FU Yu, PAN Zhi-min, et al. The Influence of Temperature on Corrosion Resistance of Ti-Mo Alloy in Acidic Mediu[J]. Surface Technology, 2022, 51(9): 168-177.
责任编辑:万长清
