Thermal hazard assessment of TKX-50-based melt-cast explosive
2022-09-22JunfengWngShusenChenShohuJinQinghiShuFengleiHungJinRunXioKunChen
Jun-feng Wng ,Shu-sen Chen ,Sho-hu Jin ,Qing-hi Shu ,Feng-lei Hung ,Jin Run ,Xio M ,Kun Chen ,*
a School of Materials Science and Engineering,Beijing Institute of Technology,Beijing,China
b State Key Laboratory of Explosion Science and Technology,Beijing Institute of Technology,Beijing,China
c Gansu Yinguang Chemical Industry Group Co.Ltd,Baiyin,China
Keywords:Dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate(TKX-50)2,4-Dinitroanisole(DNAN)Thermal safety performance Accelerating rate calorimeter(ARC)Cook-off
ABSTRACT In the present study,thermal hazards of TNT and DNAN used as the molten binder in TKX-50-based meltcast explosives were comparatively studied through accelerating rate calorimeter(ARC)and Cook-off experiments.Two kinds of ARC operation modes were performed to investigate the thermal safety performance under adiabatic conditions(HWS mode)and constant heating(CHR mode).The obtained results demonstrated that at both heating modes,DNAN/TKX-50 outperformed TNT/TKX-50 from the thermal safety point of view.However,the sensitivity to heat of the samples was reverse because of the different heating modes.In addition,the results of thermal hazard assessment obtained from the cookoff experiment complied with ARC analysis which indicated the molten binder TNT replaced by DNAN would reduce the hazard of the TKX-50 melt cast explosive.Furthermore,the results of cook-off experiments also showed that DNAN/TKX-50 outperformed TNT/TKX-50 from the aspect of thermal stability,which was consistent with the result of CHR mode because of the similar heating process.
1.Introduction
Considering excellent detonation performance and low sensitivity of Dihydroxylammonium 5,5-bistetrazole-1,1-diolate(TKX-50),this substance has been widely investigated in the last few years[1—7].As a new kind of energetic compound,it is necessary to be converted into composite explosives to promote its application.Undoubtedly,the melt-cast explosive is a preferable option,which is widely used in explosive weapons and warheads[8].Molten binder is the most important ingredient in melt-cast explosives.Worldwide,the melt-cast explosives which use trinitrotoluene(TNT)as a molten binder are widely applied to military explosives such as Octol(75% HMX,25% TNT)and Composition B(60% RDX,40%TNT).In this regard,Badgujar[9]and Yu[10]prepared the TNT/TKX-50 melt-cast explosives and studied their thermal decomposition.
As insensitive munitions(IM)has gained more attention,some alternatives have been discovered to reduce the risks of accidental initiation of using TNT.As one of the most promising candidates,2,4-dinitroanisole(DNAN)[11,12]has been used as the molten binder of IMX-101(43% DNAN,20% NTO,37% NQ)[13—15].By its application prospects,in this study,DNAN is used as a molten binder to prepare the TKX-50-based melt-cast explosives for the first time.IM is asked to exhibit less hazard to high temperatures which is one of the commonest causes of ammunition safety accidents in the process of manufacturing,storing and using[16,17].Therefore,thermal hazard evaluation of explosive is necessary to investigate before its application.
Reviewing the literature indicates that the thermal hazard of TKX-50-based melt-cast explosives has not yet been evaluated.Accordingly,it is intended to perform a comparative study on the thermal safety of these explosives.To this end,TNT and DNAN are used as the molten binder in our studies.In addition,the thermal hazard was evaluated according to Accelerating Rate Calorimeter(ARC)[18—21]and Cook-Off apparatus,which provide the confined space.
2.Experiment
2.1.Formulation and explosives
The chemical composition of the studied materials was adopted from Composition B(40%TNT,60%RDX).In addition,the explosives with 40% TNT and 40% DNAN are hereafter called CTX and CDX,respectively.Fig.1 presents the composition of each explosive.
TKX-50,TNT and DNAN with a purity of more than 99.5% were prepared from Gansu Yinguang Chemical Industry Co.,China.
2.2.Processing of the explosive formulations
The composite explosives were prepared according to the standard procedure.In this regard,the melt-cast technique was applied to add TKX-50 to molten TNT and DNAN,respectively.The mixture was stirred in a blade mixer with a steam jacketed anchor.Before molding,the mixture was stirred for 20 min.Then it is left to be cooled to ambient temperature,and machined to the designed dimensions.
2.3.Apparatus and measurement
The thermal safety performance of the two melt-cast explosives was evaluated by using ARC(NETZSCH Company)and Slow Cook-Off apparatus.ARC was designed to measure the temperature and pressure which were produced by the decomposition of the sample.In addition,two kinds of explosives were placed in a highpressure resistant Hastelloy bomb on a larger scale than DSC and TG.In the present study,Constant-Heating-Rate(CHR)and Heat-Wait-Seek(HWS)modes of ARC were performed to concentrate on studying the thermal safety of CTX and CDX powders by temperature and pressure parameters.In HWS mode,120 mg samples were initially heated to 100C.Subsequently,20 min equilibrium was carried out followed by 10 min of an exothermic signal,which can be detected when the self-heat rate(HR)of samples was higher than the exothermic threshold.Under this circumstance,the temperature increases by 5C until the exothermic signal was detected and the adiabatic decomposition occurred simultaneously.Differently,100 mg samples were heated in CHR mode with a heating rate of 1 K/min.Moreover,every single ARC measurement in this paper was started at 100.5 kPa and 30C(ambient condition).During the experiment,sampling was carried out every 0.01 min.
Generally,the thermal vulnerability of the ammunition is evaluated in the slow cook-off test based on the military standard for explosives.In the present study,explosive cylinders were loaded in a stainless steel slow cook-off bomb.Fig.2 illustrates the prepared cylinders with a diameter and height of 40 mm.Moreover,heat tapes and thermocouples were attached to the bomb to adjust the external temperature of specimens.At each test,two specimens were heated until the bomb responds.The heating rate was set to 1C/min.
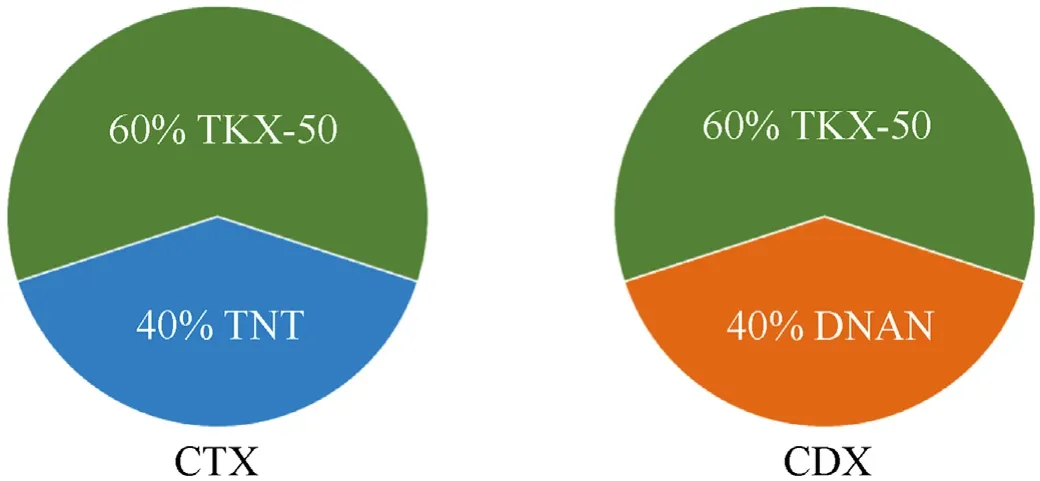
Fig.1.Composition of CTX and CDX explosives.
3.Result and discussion
3.1.HWS mode
The measured thermal curves for CTX and CDX under adiabatic conditions were shown in Fig.3 and Fig.4,respectively.Furthermore,adiabatic decomposition parameters of CTX and CDX were presented in Table 1.It was observed that the self-decomposition reaction of CTX started at 195.78C(T),which was much higher than that of CDX(165.09C).It implied that CTX exhibited good thermal insensitive compared with CDX under adiabatic conditions.In addition,the Tof CTX and CDX were less than that of pure TKX-50(200.22C)[18,20],which meant that the addition of TNT and DNAN reduced the thermal stability of TKX-50 under the adiabatic condition.Moreover,TKX-50 had been proved existing two independent decomposition stages which took place in 200.22—222.88C and 240.26—244.96C.And in the first stage,TKX-50 was transformed into diammonium 5,5-bistetrazole-1,1-diolate(ABTOX)[20,22,23].However,only a continuous decomposition process was revealed both in CTX and CDX under adiabatic conditions.Undoubtedly,it was the reflection of the decomposition of TKX-50-TNT and TKX-50-DNAN.
As for estimating the thermal safety performance,self-heating rate(HR)and pressure change rate(dP/dt)were for analysis.As depicted in Fig.5,the maximum value of HR(m)of CTX was up to 0.42C/min at 220.58C.The appearance of mindicated that the fast rate of decomposition occurred while the heat was generated rapidly.Consequently,the higher mindicated that the decomposition reaction of the sample was more violent.Compared with CTX,a much lower mwas presented in CDX(0.19C/min).The obtained results demonstrated that the decomposition of CTX was stronger than that of CDX.
Explosives released a lot of gas during its thermal decomposition,which made the pressure in the confined space rose.Fig.6 illustrated the pressure distributions in CTX and CDX specimens.Compared with Fig.5,we could conclude that,in the adiabatic condition,the trend of temperature curves and pressure curves of the samples were approximately the same,which signified that the gas released of the samples was almost simultaneous with the exothermic reaction.As shown in Fig.6,the maximum pressure increasing speed(dP/dt)of the CTX(5.39 kPa/min)was much higher than that of CDX(2.24 kPa/min).It was concluded that CTX exhibited a serious thermal hazard compared with CDX,which complied with the foregoing analysis.
Mechanism Functions Method(Eq.(1))indicated that with an appropriate reaction mechanism function f(a),ln(m/(f(a))had a linear correlation with 1/T.In this case,the activation energy(E)and pre-exponential factor(A)of the main decomposition stage in CTX and CDX could be obtained.

where mis the HR at arbitrary temperature of adiabatic system;ΔTis the measured adiabatic temperature rise.
Table 2 showed that the adiabatic thermal decomposition of CTX and CDX were both obeyed the(1-α)mechanism function.Obviously,Efor the CTX was much higher than that of CDX,reflecting better thermal insensitivity and a high-energy barrier in the thermal decomposition of CTX.
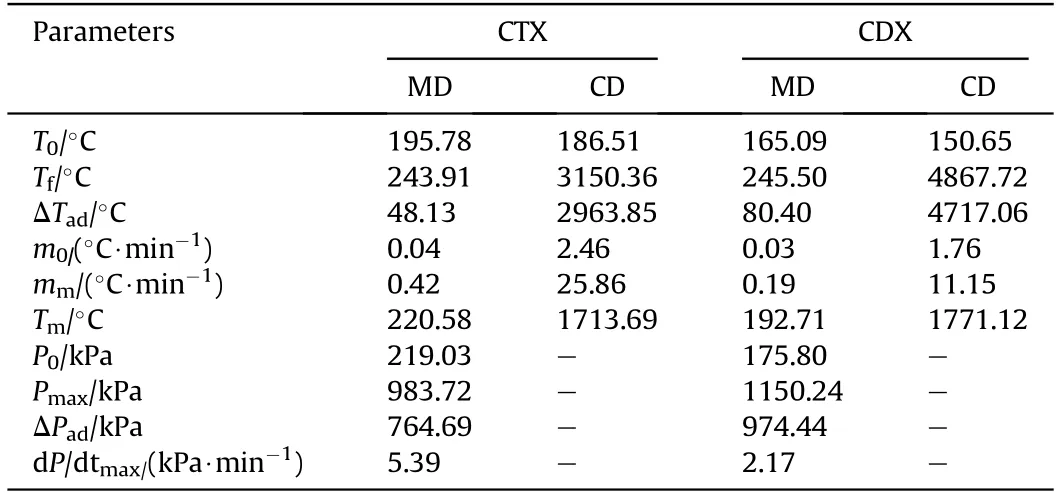
Table 1The adiabatic decomposition parameters of CTX and CDX.
Subsequently,the kinetic parameters of CTX and CDX adiabatic thermal decomposition were used for estimating the Self-Accelerating Decomposition Temperature(SADT),which was an appropriate index to investigate thermal hazard during the storage and transport.This index reflected the decomposition temperature for different substances,and could be expressed in the form below[24,25]:
Fig.2.The explosive cylinders and bomb.
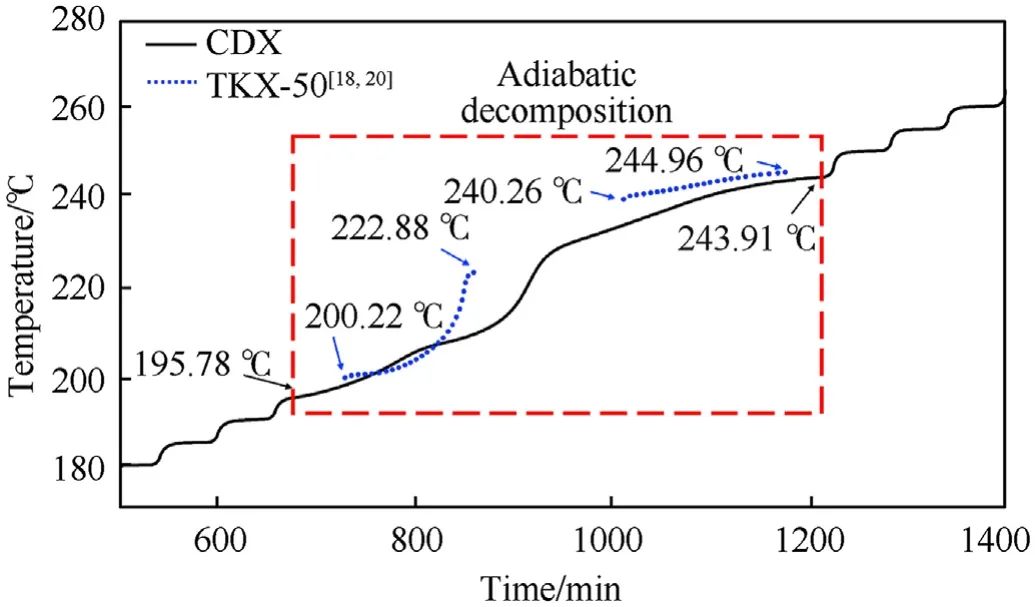
Fig.3.The adiabatic decomposition process of CTX.
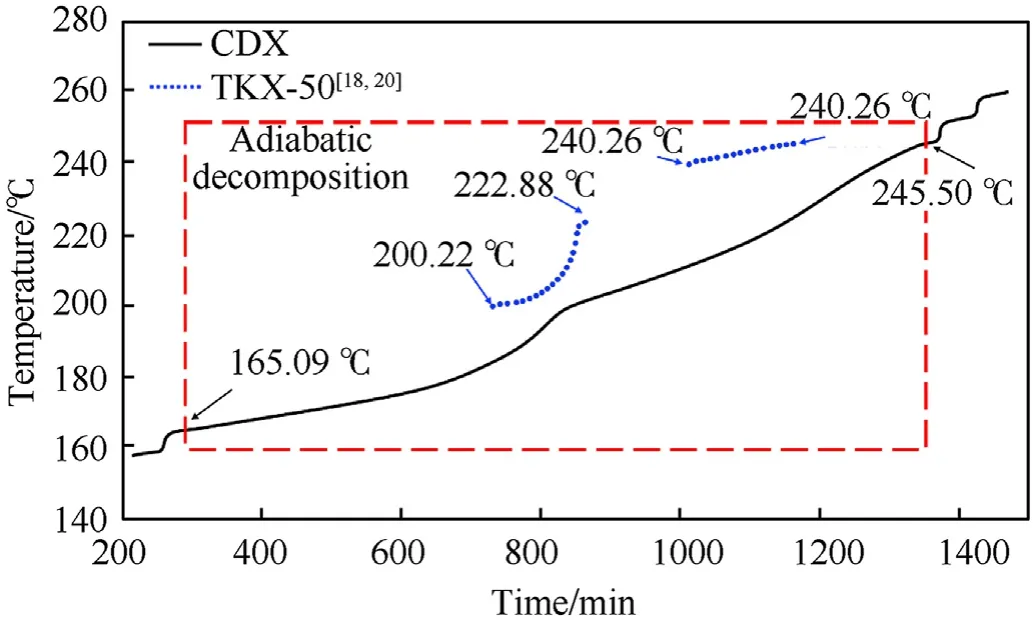
Fig.4.The adiabatic decomposition process of CDX.
In particular,Twas the temperature at the time of no returnobtained from the curves of the temperature at the time to maximum rate(T)vs.temperature by the time constant(τ).In addition,M was the mass of packaged samples;Cwas the specific heat capacity of samples;U was the heat transfer coefficient of the package;S was the contact area of the system and environment;Ewas the activation energy and R was the gas constant.
50 kg CTX and CDX were separately assumed to be stored in a wooden cylinder with 60 cm height and 30 cm diameter,the S and U were 0.5652 mand 5 W∙m∙K,respectively.Moreover,as the input value,the specific heat capacity of CTX(1.19 J∙g∙K)and CDX(1.25 J∙g∙K)were obtained by using DSC at 25C.
As shown in Fig.7,Tof samples was obtained.Then the SADT of CTX and CDX were determined to be 199.33C and 162.60C,respectively(Table 3).In summary,CTX would possess better thermal stability than CTX and even compared with TKX-50.
3.2.CHR mode
In this section,CHR mode was applied to monitor the thermal decomposition of CTX and CDX powders.In this mode,the pressure data were used to evaluate the thermal hazard.Fig.8 and Fig.9 illustrated the pressure distributions in CTX and CDX specimens.In this regard,parameters were presented in Table 4.
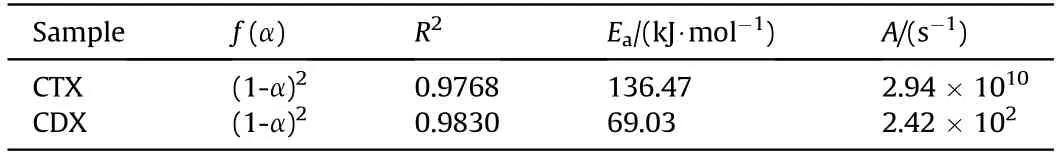
Table 2The kinetic parameters of CTX and CDX under adiabatic condition.

Table 3TNR and SADT for CTX and CDX.
In Fig.8,the pressure was increasing abruptly and forming the overpressure(P)while the temperature and pressure reached 223.02C(T)and 225.09 kPa(P),respectively,which was shown in the red dotted line and enlarged in the inset.It indicated that CTX was undergoing autocatalysis and a large amount of gas appeared.Afterward,the pressure in the vessel dropped to certain pressure(P)along with the completion of autocatalysis and thermal decomposition.After this stage,the pressure had a direct correlation with temperature.This was a common feature in ideal gases.
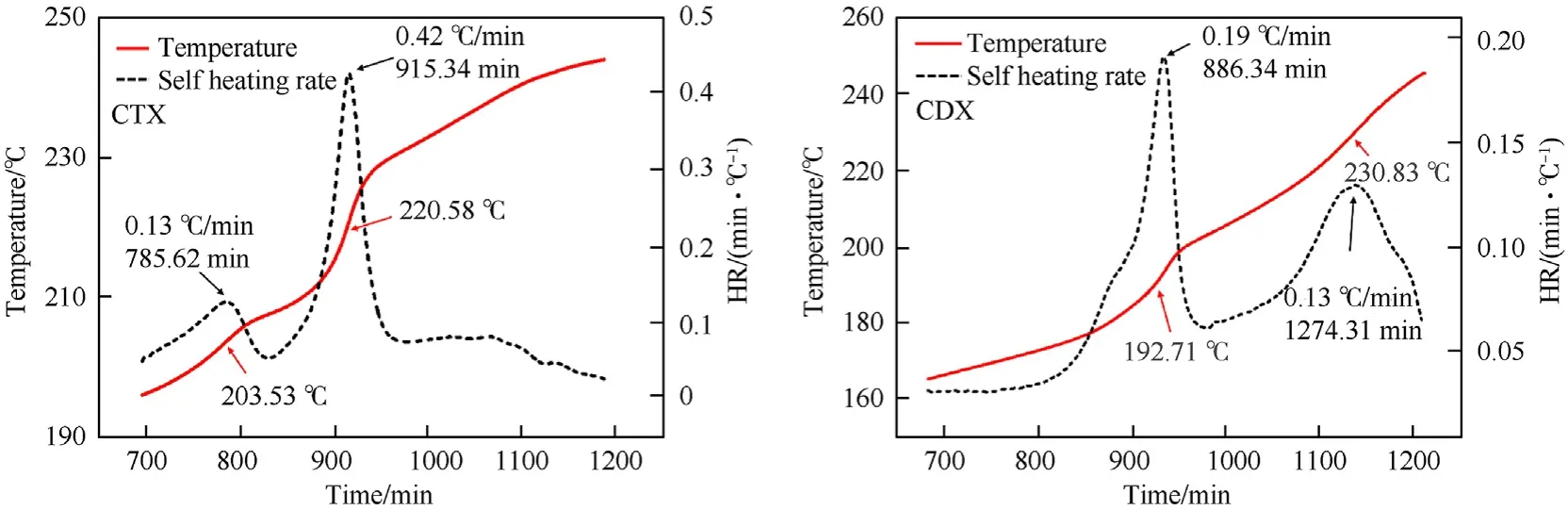
Fig.5.Temperature and HR distributions of CTX and CDX specimens.
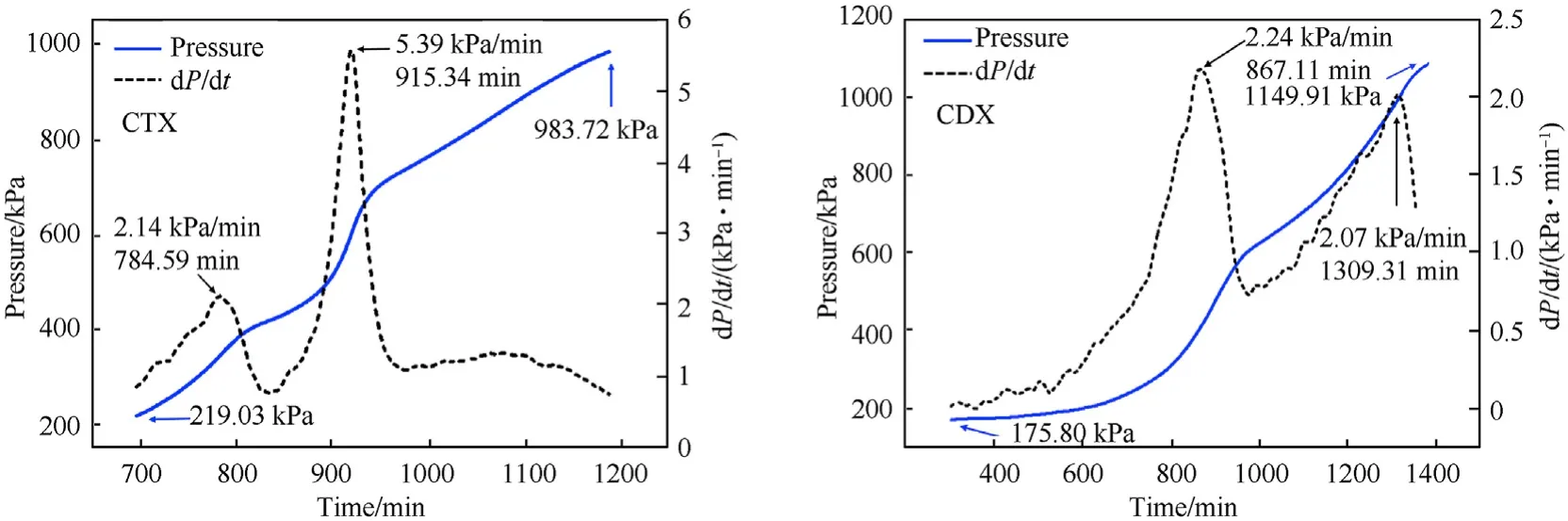
Fig.6.Pressure distributions in CTX and CDX.
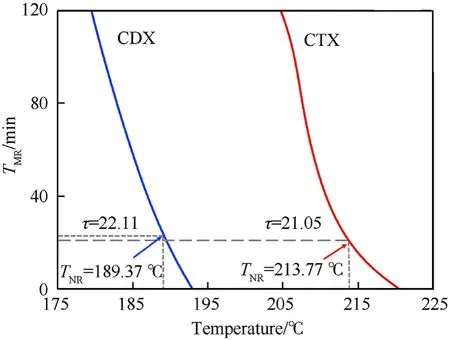
Fig.7.The curve of TMR versus temperature for CTX and CDX.
In Fig.9,the same phenomenon was also founded.However,itwas noteworthy that the pressure plot of CDX showed an obvious increasing trend before autocatalysis and Pwas already reached 341.67 kPa,which was far higher than that of CTX.It implied that a certain amount of gas had already been produced because of partial decomposition.
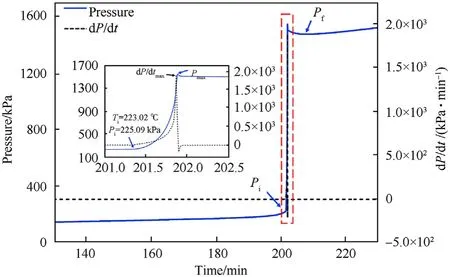
Fig.8.Pressure distributions in CTX.Inset:magnified dot-line area.
Moreover,Tof CDX was higher than that of CTX,reflecting lowersensitivity of CDX to heat in this mode.The result was contrary to the conclusion of HWS mode because samples displayed different decomposition mechanisms with different heat modes.
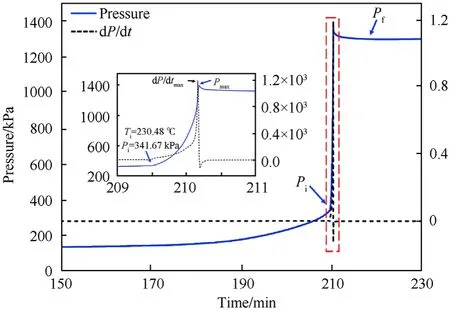
Fig.9.Pressure distributions in CDX.Inset:magnified dot-line area.
The shell faced a larger strain rate so that it was prone to shear cracks when it was subjected to a rapid rise of pressure[26,27].It was concluded that the pressure rise rate was an affecting parameter on the thermal decomposition and thermal safety in a confined space.It was well known that hazard incidents such as explosions often occurred after the ignition of explosives.Fig.10 illustrated the pressure rise rates in CTX and CDX.It was observed that the pressure rise rate of CTX was always higher than that of CDX at the same pressure after the ignition,which implied CTX would cause more severe destruction under the same conditions.Furthermore,dP/dtof CTX was much higher than that of CDX,indicating poor thermal safety in CTX.
It was found that the TPR of CTX and CDX were 0.51 and 0.68 min,respectively.It was worth noting that the higher the TPR,the lower the thermal decomposition intensity.In conclusion,CDX presented better thermal safety than CTX in a constant heating rate.
3.3.Slow cook-off experiment
Slow cook-off experiment was an effective method to evaluate the thermal safety of explosives.In order to assess the violent degree of experiment response,we collected the fragments of the cook-off bomb after the experiment.Fig.11 showed the postexperiment fragments of CTX and CDX specimens.
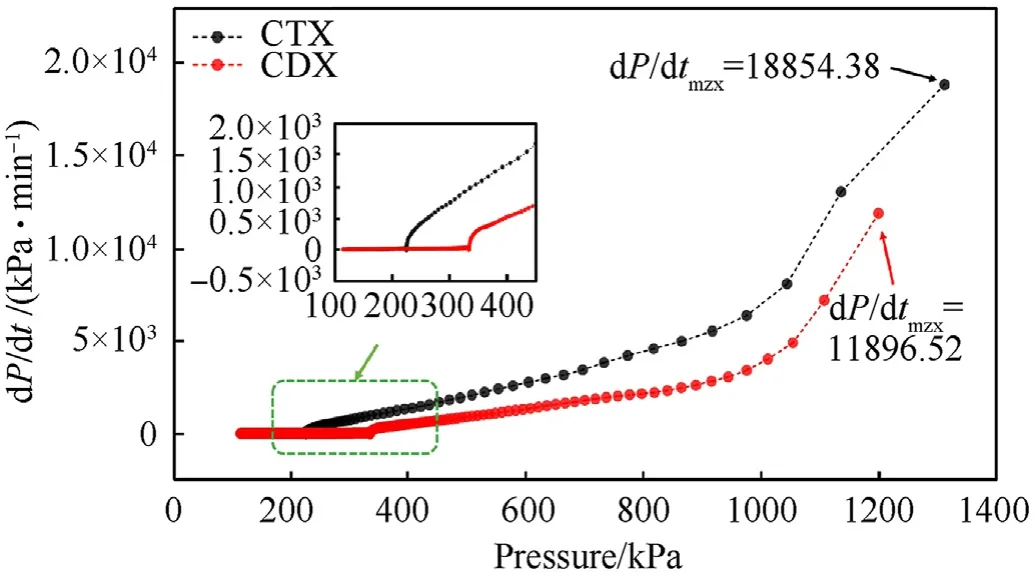
Fig.10.Pressure distributions in CTX and CDX.Inset:magnified dot-line area.
As shown in Fig.11,it was clear that the fragments of the CTX cook-off bomb were produced in quantity and serious deformation contrasted to CDX cook-off fragments,which proved the reaction of CTX was much violent.It could be concluded that the TKX-50-based melt-cast explosives which using DNAN as the molten binder exhibited better thermal safety performance than using TNT as the molten binder.It was in a good agreement with the conclusion of ARC analysis regardless of the modes.
Furthermore,the temperatures(T)were recorded when the bomb respond.As listed in Table 5,CTX was the response at 230C which was much lower than that of CDX(256C).The obtained result demonstrated that the thermal stability of CDX was better than that of CTX,which was consistent with the result of CHR mode.

Table 4The detailed decomposition parameters of explosives.

Table 5Response temperatures in cook-off test.
4.Conclusion
This paper focused on the thermal hazard assessment of two TKX-50-based melt-cast explosives which the molten binder was TNT and DNAN,respectively.The main conclusions were as follows:
(1)Thermal behaviors of CTX and CDX were studied and compared by employing ARC data under adiabatic conditions(HWS mode).The results revealed that CTX was less thermal sensitive than CDX while the reaction was much violent than that of CDX.In addition,the simulation results of SADT for 50 kg samples stored in wooden drums illustrated that the thermal stability of CTX was better than CDX.
(2)Thermal behaviors of CTX and CDX under the constant heating rate(CHR mode)were also studied.Consistent with the conclusion of HWS mode,CDX presented better thermal safety than CTX.However,the sensitivity to heat of samples was reverse because of the different heating modes.

Fig.11.Post-experiment debris for CTX and CDX.
(3)It was observed that the thermal safety performance of CDX was better than CTX,which was in good agreement with ARC analysis regardless of the mode.Therefore,the molten binder TNT replaced by DNAN will improve the thermal safety and reduce the hazard of the TKX-50-melt cast explosive.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors are grateful to the National Defense Foundation of China(3090021322001,3090020221912,3090021211903.)for financial support of this work.
杂志排行
Defence Technology的其它文章
- 3D laser scanning strategy based on cascaded deep neural network
- Damage analysis of POZD coated square reinforced concrete slab under contact blast
- Autonomous maneuver decision-making for a UCAV in short-range aerial combat based on an MS-DDQN algorithm
- The properties of Sn—Zn—Al—La fusible alloy for mitigation devices of solid propellant rocket motors
- The surface activation of boron to improve ignition and combustion characteristic
- Numerical investigation on free air blast loads generated from centerinitiated cylindrical charges with varied aspect ratio in arbitrary orientation
