The surface activation of boron to improve ignition and combustion characteristic
2022-09-22JinWngJunWngYofengMoRufngPengFudeNie
Jin Wng ,Jun Wng ,Yofeng Mo ,Rufng Peng ,Fude Nie *
a State Key Laboratory of Environment-friendly Energy Materials,Southwest University of Science and Technology,No.59,Qinglong Road,Mianyang City,Sichuan,621010,China
b Institute of Chemical Materials,China Academy of Engineering Physics,No.64,Mianshan Road,Mianyang City,Sichuan,621999,China
Keywords:Boron Surface activation Ignition Pressure output Combustion mechanism
ABSTRACT Boron is a very promising and highly attractive fuel because of high calorific value.However,the practical applications in explosives and propellants of boron have been limited by long ignition delay time and low combustion efficiency.Herein,nano-Al and graphene fluoride(GF)as surface activated materials are employed to coat boron(B)particles to improve ignition and combustion performance.The reaction heat of nano-Al coated B/KNO3 and GF coated B/KNO3 are 1116.83 J/g and 862.69 J/g,respectively,which are higher than that of pure B/KNO3(823.39 J/g).The ignition delay time of B/KNO3 could be reduced through nano-Al coating.The shortest ignition delay time is only 75 ms for B coated with nano-Al of 8 wt%,which is much shorter than that of pure B/KNO3(109 ms).However,the ignition delay time of B/KNO3 coated with GF has been increased from 109 to 187 ms.B coated with GF and nano-Al shown significantly influence on the pressure output and flame structure of B/KNO3.Furthermore,the effects of B/O ratios on the pressure output and ignition delay time have been further fully studied.For B/KNO3 coated with nano-Al and GF,the highest pressures are 88 KPa and 59 KPa for B/O ratio of 4:6,and the minimum ignition delay time are 94 ms and 148 ms for B/O ratio of 7:3.Based on the above results,the reaction process of boron coated with GF and nano-Al has been proposed to understand combustion mechanism.
1.Introduction
Highly active metal fuels(Al,B)are used in explosives and propellants can enhance the energy density and combustion performance[1,2].Boron(B)shows great potential applications due to high calorific value(58.28 kJ/g 136.38 kJ/cm),which is 1.9 and 1.66 times of Al[3,4].However,it is limited by low combustion efficiency and long ignition delay time because of BOshell with high boiling point(1860C)and low melting point(450C),which would prevent mass diffusion and interfacial reaction between oxidizer and boron during ignition and combustion process[5—8].Thus,it is important to eliminate effect of BOshell on ignition and combustion performance for practical applications.
Based on previous literatures,surface coating technology could improve ignition and combustion performance of metal fuels[9—14].Metals[15,16],metal anhydrides or oxides[17,18],rareearth metal catalysts[19]and fluorine-containing materials[20]have been used to coat B particles.For B added with Magnesium(Mg),the ignition delay time could be reduced to 48 ms and the maximum combustion efficiency could be increased to 64.2%[21].Grafted 2,4-toluene diisocyanate and 3-amino-1,2,4-triazole were introduced to modify the surface of B,the initial reaction temperature of B/KNOhas been reduced[22].Compared with metal and organic compound,fluorine as oxidizer can react with BOto from BFwould release high heat(188.99 kcal/m)[23—26].In addition,BFas gas could enhance the reaction combustion efficiency of B[27,28].Fluoropolymers(PVDF/Viton/THV)with different fluorine content were used to improve the combustion reaction performance of B.High F content of fluoropolymers can enhance combustion efficiency of B[29].BiF was employed to coat B,which could reduce the oxidation temperature and ignition delay time[30,31].Fluorinated graphene(GF)as fluoride-containing compound has been applied in advanced materials due to high heat stability(decomposition temperature>400C)and hydrophobicproperty(the contact angle is 158)[32—34].Furthermore,the excellent surface energy(~100 mJ/m)and thermal conductivity(~80 W/mK)of fluorinated graphene would enhance heat conduction and interfacial contact,which could improve mass diffusion and combustion reaction[35,36].
Herein,fluorinated graphene as surface activation was employed to coat B particles.Furthermore,in order to comparative study the effects on the ignition and combustion performance of B,B/KNOcoated with nano-Al and B/KNOcoated with GF have been prepared.Based on thermal analysis,ignition and combustion performance,the reaction process of boron coated with GF and nano-Al has been proposed to understand combustion mechanism.
2.Experimental section
2.1.Materials
B powder was purchased from ZhongNuo New Materials(purity:99.9%,particle size:325 mesh).Nano-Al was purchased from China New Metal Materials Technology Co.,Ltd(particle size:50—100 nm).Fluorinated graphene(GF)was purchased from XF Nano Company(sheet diameter:0.4—5 μm,fluorine content:47—58%).Purity KNOwas purchased from Xuzhou Jingke Reagent Instrument Co.,Ltd.Ethyl acetate was purchased from Aladdin Company,with an analytical purity of 99%.
2.2.Preparation process of B coated with nano-Al and GF composites
B coated with nano-Al or GF were prepared by acoustic resonance and solvent evaporation methods.In a typical experiment,40 mg of GF and 80 ml of ethyl acetate were added into the vibrating tank.Then,the ethyl acetate solution of GF was mixed uniformly to obtain stable GF dispersion through acoustic resonance technology for 20 min.Then,960 mg of boron powder was added into mixed liquids for ultrasonic treatment for 10 min,and then mixed by acoustic resonance technology for 6 min.The solvent was then removed from the solid precipitate.With the solvent removed,the coating materials can coat on the surface of boron through physical interaction.The solid mixture was washed with ethyl acetate.The products were dried in vacuum at 40C for 12 h to obtain B coated with GF.Nano-Al coated B composites were also prepared by the same process.The detailed information of different nano-Al coated B and GF coated B composites are shown in Table 1S.
2.3.Preparation of KNO3/B energetic composites
Nano-Al coated B/KNOand GF coated B/KNOenergetic composites were prepared by acoustic resonance mixing.500 mg of B coated with GF and 500 mg of KNOwere added into the vibrating tank,which was mixed by acoustic resonance platform for 6 min.The nano-Al coated B/KNOenergetic composites was also prepared by the same process.
2.4.Characterization
The morphologies of B coated with nano-Al and GF composites were characterized by field emission scanning electron microscopy(FE-SEM).X-ray photoelectron spectroscopy(XPS)and Energy dispersive spectroscopy(EDS)were used to analyze the uniformity of nano-Al and GF coated on B surface.The combustion products were further analyzed by X-ray diffraction(XRD)and FE-SEM.
The thermal properties of the energetic composites were obtained by thermal analysis techniques(TG-DSC).1.9—2.0 mg of the sample was added in crucible.The reaction tempearture was ranged from 50 to 800C with heating rate of 10 K/min under Natmosphere.The blank curve was obtained by running a blank crucible under the same experimental conditions.
The combustion process and flame structure were recorded by high-speed camera(Japan UX50)with rate of 500 FPS.The sample was added into aluminum alloy groove with size of 3 mm×3 mm×20 mm,and then ignited by a Ni—Cr wire(the diameter was 0.2 mm).The combustion reaction and test were carried out in air.The specific test situation is shown in Fig.1.
The ignition delay time was measured by COlaser(wave length was 10.6 μm,the focused spot was 4.5 mm)instrument(Fig.S1).50 mg of the sample was ignited by the laser with energy of 60 W and irradiation time of 1000 ms.The entire ignition and combustion process of the sample was recorded by high speed camera with rate of 1000 fps.The ignition delay time can be obtained based on experiment(Fig.S2).The average ignition delay time was calculated based on three tested results for each sample.The pressure output test was obtained through combustion experiment in a closed volume tank(330 ml).200 mg of sample was placed in an iron crucible and ignited by a Ni—Cr wire(the diameter was 0.2 mm).
3.Results and discussions
3.1.The morphology of B coated with nano-Al and GF
The morphology of sample was characterized by emission scanning electron microscope(FE-SEM).As shown in Fig.2(a),boron shows relatively smooth and irregular morphology with average size of~20 μm.Nano-Al appears to be spherical structure with particle size ranged from 50 to 100 nm(Fig.2(b)).The morphology of fluorinated graphene is shown in Fig.2(c),which shows layered structure.For B coated with nano-Al(Fig.2(d)),the diameter of the composited particles is about 20 μm.Comparedwith pure B,obvious nano-Al coated on the surface indicates that nano-Al has been successfully coated on the surface of B.The morphology of B coated with GF is shown in Fig.2(e).The surface of B coated with GF composite became relatively rough,combined with the morphology of GF,indicating that GF is coated on the surface of B.The nano-Al and GF coated on B would improve ignition and combustion performance.
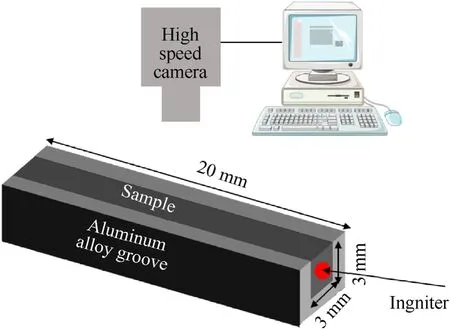
Fig.1.Schematic diagram of combustion experiment by high-speed photography.

Fig.2.(a—c)SEM images of boron,nano-Al and GF,(d—e)SEM images of B coated with nano-Al and GF.
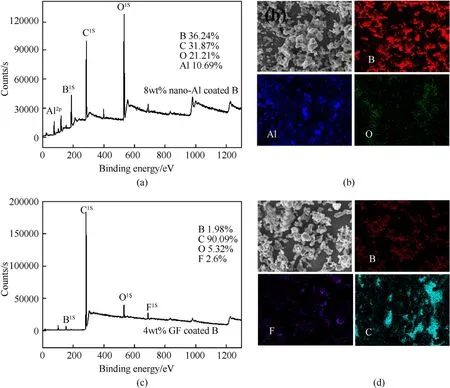
Fig.3.(a,c)XPS image of nano-Al and GF coated B composites,(b,d)EDS images of nano-Al and GF coated B composites.
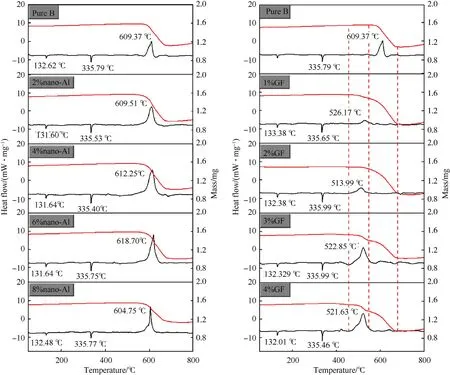
Fig.4.TG-DSC curves of KNO3/B coated with nano-Al and GF energetic composites.
In order to further illustrate the nano-Al and GF coated on the surface of B particles,XPS(Fig.3(a,c))analysis and EDS(Fig.3(b,d))analysis were performed.As shown in Fig.3(a)and Fig.3(b),Al element is observed on the surface of B.As shown in Fig.3(c)and Fig.3(d),C and F elements are obtained on the surface of B.For B coated with nano-Al,oxygen element has been observed on mapping and XPS results resulted from AlOshell.However,no oxygen element is observed in B coated with GF.The results further illustrate B has been completely coated by nano-Al and GF.For pure boron,some oxygen is observed due to the existence of BOlayer on the surface of B(Fig.S3).An XRD pattern of the sample is shown in Fig.S4.The diffraction peaks of(111),(200),(220)and(311)planes are observed for nano-Al.No peak of GF is observed may be contributed to amorphous state.Those results further indicate the B particles have been coated by nano-Al and GF.
3.2.Thermal reaction of KNO3/B coated with nano-Al and GF
In order to study thermal reaction of B coated with nano-Al and GF,KNOas typical oxidizer was used to prepare KNO/B energetic composite.The thermal properties of KNO/B coated with nano-Al and GF were studied by TG-DSC.The TG-DSC curve of KNO/B coated with nano-Al and GF energetic composites is shown in Fig.4.There is a small endothermic peak at~132C caused by the transformation of KNOfrom the orthorhombic crystal to the triangular crystal.An endothermic peak also appeared at 334C owing to the melting of KNO[37].Furthermore,a significantly mass loss(~30%)is observed resulted from the thermal decomposition of nano-Al or GF coated B/KNOcomposites to form gaseous products.In fact,the gaseous products percentage of KNO53.42%)is larger than 30%,which indicates that oxidizers react with boron and generate less gaseous products.For KNO/B coated with nano-Al,the reaction heat shows a trend of first decreasing and then increasing with the increase of nano-Al ranged from 0 to 8 wt%.The maximum reaction heat(1116.83 J/g)is obtained for B coated with nano-Al of 6 wt%,which is larger than that of KNO/B(823.39 J/g).The results illustrate that nano-Al could enhance the energy output of B.
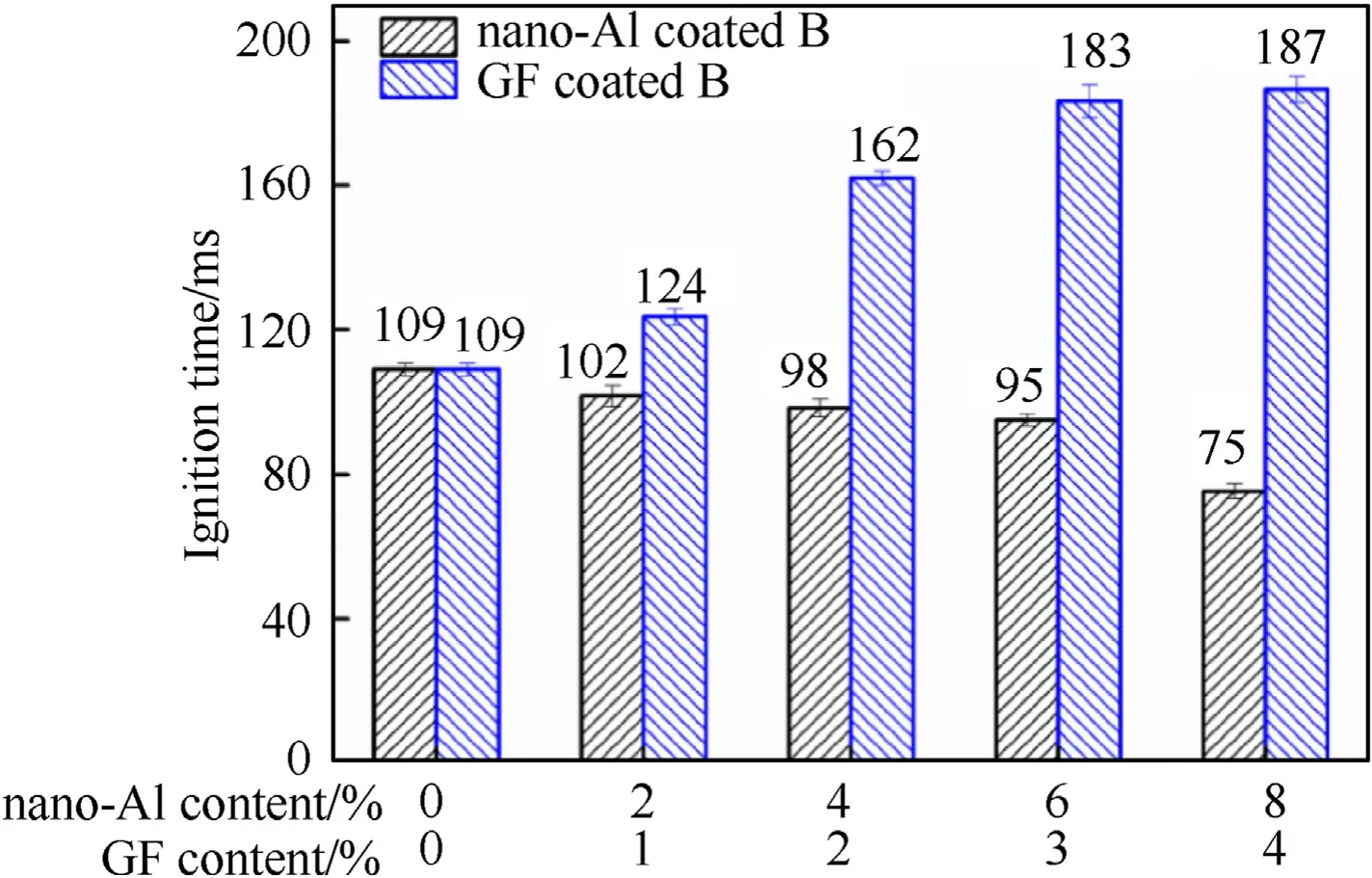
Fig.5.Ignition delay time of KNO3/B coated with nano-Al and GF energetic composite.
For KNO/B coated with GF of 3 and 4 wt%,the weight loss contains two stages from 510 to 780C.The first mass loss may be contributed to GF decomposition and the second mass loss due to KNOdecomposition.Compared with nano-Al,GF could reduce the exothermic peak of pure B.The lowest peak(513.99C)is observed for KNO/B coated with GF of 2 wt%,which is much lower than that of pure KNO/B(609.37C).Furthermore,the reaction heat also first decreases and then increases with the increase of GF.The maximum reaction heat(862.69 J/g)is obtained for KNO/B coated with GF of 4 wt%,which is higher than that of pure KNO/B and lower than that of KNO/B coated with nano-Al.The detailed information and results of DSC-TG have been listed in Table 2S.The results clearly illustrate that GF and nano-Al could improve the ignition and combustion performance of boron.
3.3.Ignition delay time of KNO3/B coated with nano-Al and GF
In order to study the effect of nano-Al and GF on the ignition performance of B,the ignition delay time of KNO/B was tested using COlaser.The ignition delay time of KNO/B coated with nano-Al and GF energetic composite are shown in Fig.5.For KNO/B coated with nano-Al,the ignition delay time significantly reduces with the increase of nano-Al.The decreased ignition delay time may be contributed to high reactivity,ignition and combustion performance of nano-Al,which could react with oxidizer to release energy and enhance B ignition.The reaction heat of nano-Al would increase with nano-Al increased and result in lower the ignition delay time.For the KNO/B coated with GF,the ignition delay time gradually increases with the increased of GF content.The increased ignition delay time may be contributed to endothermic effect of GF decomposition.
3.4.Combustion performance and pressure output of B/KNO3
The combustion reaction and pressure output are important characteristics of KNO/B energetic composites.The combustion process of KNO/B coated with nano-Al and KNO/B coated with GF are shown in Fig.6.The combustion process and flame area of KNO/B are enhanced through coating nano-Al.In addition,the flame areas are increased with the nano-Al ranged from 2 to 8 wt%.For KNO/B coated with GF energetic composite,the combustion and flame area have been increased.However,the combustion process and flame area are smaller than that of KNO/B coated with nano-Al.Furthermore,KNO/B coated with nano-Al and KNO/B coated with GF show different flame structure(Fig.S5).The flame of KNO/B coated with nano-Al is shape of the face.The flame of KNO/B coated with GF is slender structure.The difference of flame structure may be contributed to the excellent thermal conductivity of GF.In addition,no green flame is observed in B combustion process,which may be caused by the insufficient combustion of boron.In this experiment,the ratio of B/KNOare 50:50,which is much higher than that of stoichiometric ratio of chemical reaction(15/85).The results illustrate that B coated with nano-Al and GF could enhance combustion and reaction performance.
The pressure output and pressurization rate of KNO/B coated with nano-Al and GF energetic composites are shown in Fig.7.Compared with pure KNO/B,nano-Al and GF coated B show a significantly influence on the pressure output.The highest pressure and pressurization rate are decreased and then increased with nano-Al or GF increased in KNO/B.For KNO/B,the highest pressure and pressurization rate are 92.66 kPa and 386 kPa/s for 8 wt%nano-Al.The result is similar to B coated with titanium[38].However,GF shows relatively weak effect on the pressure output.For B coated with FG of 1 wt% and 2 wt%,it is hard to enhance pressure output due to less GF.The highest pressure and pressurization rate are improved by coated 3 wt% and 4 wt%.

Fig.6.Combustion reaction and flame structure of KNO3/B coated with nano-Al and GF.
3.5.The effect of B/O ratio on ignition and pressure output performance
For KNO/B,the ratio of O and B play a key role in ignition and combustion reaction.Therefore,the effect of B/O ratio on the ignition delay time was further studied.Based on the above experimental results,B coated with nano-Al of 6 wt%and GF of 4 wt%,B/O ratios of KNO/B were 4:6,5:5,6:4 and 7:3,which were selected in the experiment.
As shown in Fig.8,the pressure output and pressurization rate of KNO/B coated with nano-Al and GF are decreased with B/O ratio ranged from 4:6 to 7:3.The main reason contains two aspects.On the one hand,more B could not be reacted due to lack of oxygen with B/O ratio increased.On the other hand,gaseous product would decrease resulted from KNOdecomposition.Therefore,the pressure output is reduced with B/O ratio ranged from 4:6 to 7:3.
The ignition delay time is shown in Fig.9.As the B/O ratio increases,the ignition delay decreases.The main reason is that the less KNOrequires less energy to decompose,and the rate of oxygen generation faster,which resulted in lower the ignition delay.In summary,there is a dynamic equilibrium point between pressure output and ignition delay,that is,a higher-pressure output and a lower ignition delay time can only be achieved with an optimal B/O ratio.
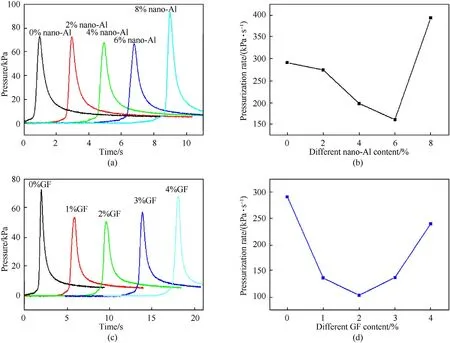
Fig.7.Pressure output curves and pressurization rate of KNO3/B coated with nano-Al and GF.
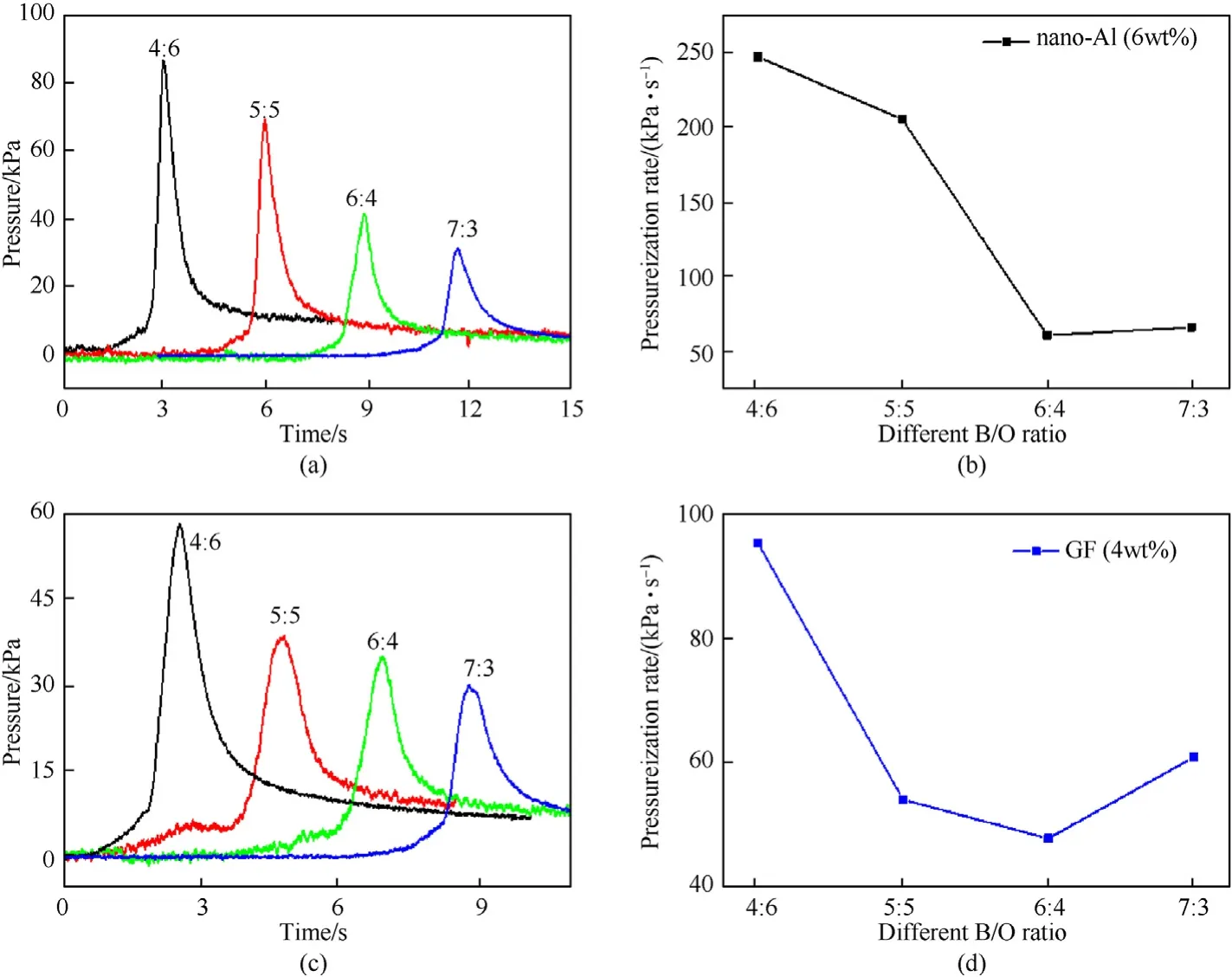
Fig.8.Pressure output curve and pressurization rate of KNO3/B coated with nano-Al and GF composites with different B/O ratio.

Fig.9.Ignition delay time of KNO3/B coated with nano-Al and GF composites with different B/O ratio.
Nano-Al and GF as surface activated materials can enhance ignition and combustion of B.KNO/B coated with nano-Al and GF energetic composites shows different thermal reaction,ignition and combustion performance.In order understand the reaction process of B coated with nano-Al and GF,a diagram(Fig.10)is proposed based on the above results.The surface reaction of B coated with nano-Al is shown in Fig.10(a).Nano-Al shows more reactivity and ignition performance than that of B[16,21].Thus,nano-Al would be reacted with oxidizer during ignition of KNO/B energetic material.The oxidation reaction of nano-Al generates lots of heat,which provide more heat to induce ignition of B.Therefore,nano-Al as second energy could enhance ignite and combustion performance of B particles.
Fig.10(b)shows the reaction process of KNO/B coated with GF energetic composites.GF would be decomposed to form some F radicals and fragments.The oxide layer BOreacts with fluorine(F)to form OBF and BFgases[39,40],which can eliminate the inert BOshell at the relatively low temperature.Some pore would be formed in BOshell,which can promote oxidizer diffusion and contact with boron core.Therefore,two different pathway may be obtained by the surface activation of nano-Al and GF for the reaction of KNO/B that improves the ignition and combustion reaction performance.
4.Conclusion
GF(fluorinated graphene)and nano-Al as surface activated materials are employed to coat B particles to improve ignition and combustion performance.GF and nano-Al coated B is beneficial for increasing the reaction heat of B/KNO.The largest reaction heat is about 1116.83 J/g of KNO/B coated nano-Al of 6 wt%.Compared with nano-Al,GF could significantly reduce the exothermic peak.For B coated with nano-Al of 8 wt%,the ignition delay time is only 75 ms,which is much shorter than that of pure B(109 ms).Furthermore,nano-Al and GF as coating could improve pressure output and combustion performance.The highest pressure(92.66 KPa)and pressurization rate(386 kPa/s)are obtained for KNO/B coated with nano-Al of 8 wt%.B/O ratios plays important role in pressure output and ignition delay time of B/KNO,which could tune combustion performance and energy output.Based on experimental results,combustion reaction process of B coated with nano-Al and GF is proposed to understand the mechanism.Thiswork may offer a practical approach to improve ignition and combustion performance of B for applications.
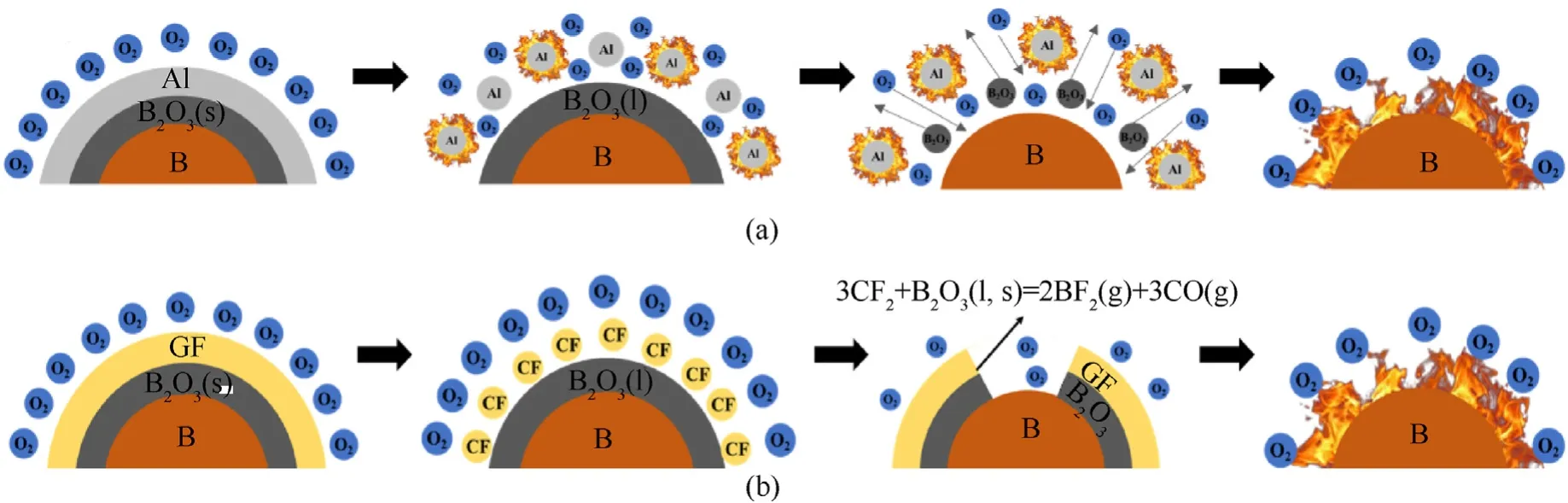
Fig.10.Combustion reaction process of KNO3/B coated with nano-Al and GF.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
This work is supported by the National Natural Science Foundation of China(11872341 and 22075261).
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dt.2021.09.012.
杂志排行
Defence Technology的其它文章
- 3D laser scanning strategy based on cascaded deep neural network
- Damage analysis of POZD coated square reinforced concrete slab under contact blast
- Autonomous maneuver decision-making for a UCAV in short-range aerial combat based on an MS-DDQN algorithm
- The properties of Sn—Zn—Al—La fusible alloy for mitigation devices of solid propellant rocket motors
- Numerical investigation on free air blast loads generated from centerinitiated cylindrical charges with varied aspect ratio in arbitrary orientation
- Natural convection effects on TNT solidification inside a shaped charge mold
