Morphological differentiation in the Asian honey bees (Apis cerana) in China
2022-08-13ZHUXiangJieZHOUShuJingXUXinJianYUYingLongHUJunJunZHANGZhongYinQIWenZhongWANGBiaoYUANChunYingXIFangGuiZHOUBingFeng
ZHU Xiang-Jie, ZHOU Shu-Jing, XU Xin-Jian, YU Ying-Long,HU Jun-Jun, ZHANG Zhong-Yin, QI Wen-Zhong, WANG Biao,YUAN Chun-Ying, XI Fang-Gui, ZHOU Bing-Feng,*
(1. College of Animal Science (College of Bee Science), Fujian Agriculture and Forestry University, Fuzhou 350002, China;2. Guizhou Institute of Integrated Agriculture Development, Guizhou Academy of Agricultural Sciences, Guiyang 550006, China;3. Guangxi Apiculture Guidance Station, Nanning 530000, China; 4. Henan Institute of Science and Technology, Xinxiang,Henan 453003, China; 5. Gansu Apiculture Technology Extension Station, Tianshui, Gansu 741020, China;6. Guyuan Apiculture Experimental Station, Guyuan, Ningxia 756000, China;7. Liaoning Agricultural Development Service Center, Xingcheng, Liaoning 125100, China;8. Apicultural Research Institute of Jiangxi Province, Nanchang 330052, China)
Abstract: 【Aim】 The genetic differentiation research is an important link to understand the morphological diversity and adaptive evolution of honey bees. It is a prerequisite for the determination of the bioresource management unit and the protection unit and helps to protect the genetic resources of honey bees. This study aims to study the genetic differentiation and genetic resource distribution of the Asian honey bee, Apis cerana across the geographical environment in China by analyzing morphological differentiation. 【Methods】 A total of 6 147 worker bees of A. cerana were collected from 102 sampling sites across the complete distribution area of A. cerana in China. Sixty worker bees of each sampling site from 10-20 colonies were dissected and 33 morphological characteristics associated with the wings, individual size, hind leg, and body color were measured. A multivariate morphometric analysis was conducted and clusters with their morphological traits and distribution patterns were identified. 【Results】 According to the cluster results of discriminant analysis and principal component analysis, A. cerana in China can be divided into 14 morphological clusters. Five clusters with smaller body size were identified. Hainan cluster had the smallest body size, followed by South Yunnan cluster, Taiwan cluster, Southern cluster, and Northern cluster. These five clusters were significantly different in proboscis length, forewing length, the structure of the 3rd submarginal cell in the forewing, body color, and the length of the wax plate. Changbai cluster had the largest cubital index, wax plate size, and width of the stripe of tomentum on tergite 5. However, Bomi cluster of Tibet had the smallest width of the stripe of tomentum on tergite 5 in China. Northwest cluster had the longest hind legs. Five clusters in the West Sichuan Plateau were characterized by larger individuals and black body color. Batang cluster had the smallest cubital index (3.0169) and the largest individual size in China. The cubital index of the Aba cluster was inferior only to that of the Changbai cluster, and the wing lengths and the sizes of sternite 7 were the largest. Derong cluster was the darkest. Yajiang cluster was unique in wing vein angles (A4, N23, E9 and J10 were the smallest and B4 the largest). Chuandian cluster had the smallest body size on the Western Sichuan Plateau. 【Conclusion】 In this study, the morphometric analysis of A. cerana was conducted based on collection of samples across the complete distribution area of A. cerana in China, especially those from Bomi of Tibet, Taiwan Province, and the Western Sichuan Plateau. Fourteen clusters of A. cerana were obtained in China, including Hainan cluster, southern Yunnan cluster, Changbai cluster, Taiwan cluster, Bomi cluster, Aba cluster, Batang cluster, Derong cluster, Yajiang cluster, Chuandian cluster, Chuangui cluster, Northwest cluster, Southern cluster, and Northern cluster. The results of this study provide a theoretical basis for the protection and exploitation of genetic resources of A. cerana in China.
Key words: Apis cerana; morphology; genetic differentiation; population; multivariate analysis
1 INTRODUCTION
The honey bee plays an important role in promoting the increase of agriculture and husbandry, vegetation prosperity, and in maintaining a sustainable cycle of ecological environment (Burkleetal., 2013; Garibaldietal., 2013; Stanleyetal., 2017). The Asian honey bee (Apiscerana) is widely distributed throughout China. It is the only honey bee species raised before the introduction ofA.melliferaand has a long history of apiculture (Kuang and Kuang, 2003). During the long adaptation process, the Asian honey bee in China has formed special biological characteristics.A.ceranahas become the main beekeeping species in mountainous regions, and nectar collection from winter-flowering plants such asEriobotryajaponica,ScheffleraoctophyllaandEruyaemarginatais dependent on it (Gong and Zhang, 2000).
Genetic differentiation is an important link to understand the evolution of organisms (Mayr, 1947; Sobeletal., 2010). It is a prerequisite for the determination of the bioresource management unit (Matalaetal., 2014) and the protection unit (Schmidtetal., 2011), and helps to protect the genetic resources of honey bees. For a research scope that covers the complete geographical range, it is typically assumed that most of the encounteredA.ceranain China is part of the same subspecies (A.c.cerana) or belongs to one morphocluster. The exception is southern Tibet, where most scholars consider the subspeciesA.c.himalayato be prevalent. However, it can be further subdivided into several subclusters or populations (Ruttner, 1988; Hepburnetal., 2001, 2011; Radloffetal., 2005, 2010). Due to the lack of Chinese samples, Ruttner speculated that most of the Asian bees in China areA.c.cerana, while for the southwest of China they are assumed to beA.c.himalaya(Ruttner, 1988). The taxonomy ofA.ceranahas been revised based on raw databases of Germany, South Africa, Indonesia, and China, and raw databases of previously published results (Radloffetal., 2010). Except for Tibet, which was named the “Himalayancerana” and belongs to the Morphocluster II, the Chinese Asian bees that belong to the Morphocluster I, were named “Northerncerana” and further subdivided into three subclusters: the subcluster “Aba” including southern Gansu and central and northern Sichuan in northwestern China, a subcluster in central and eastern China, and a “southern”ceranasubcluster including southern Yunnan, Guangdong, Guangxi, and Hainan in China (Radloffetal., 2010).
Chinese researchers generally assume that subspecies or cluster exist in Chinese Asian bees. Yang suggested that the Asian bees in China could be divided into five subspecies or races according to their morphological and biological characteristics (Yang, 2001). These are the eastern Chinese bee (A.c.cerana), the Hainan Chinese bee (A.c.hainana), the south Tibet Chinese bee (A.c.skorikovi), the Aba Chinese bee (A.c.abansis), and the southern Yunnan Chinese bee (A.c.indica). In addition, due to their differences in ecological conditions in different areas, the Asian honey bee can be further divided into five types: Guangdong and Guangxi, Hunan, Yunnan-Kweichow Plateau, northern type, and Changbai Mountain type (Yang, 1981, 1982, 1984a, 1984b. 2001, 2009; Yang and Xu, 1982; Yangetal., 1986, 1995). Due to inconvenient traffic conditions and technical limitations of morphological methods at the time of this classification, the sample size and number of measurements were limited. However, currently available data suggest that the Chinese Asian bees are likely more differentiated. Multivariate statistical analysis was conducted onA.ceranafrom 68 sample sites throughout China. The Chinese Asian honey bee was divided into four clusters: bees from Jilin, Liaoning, and Beijing; larger bees from southern Gansu and Sichuan; smaller bees from Guangdong, Guangxi, Hong Kong, southern Yunnan, and Hainan; and the bees of the rest of China (Tanetal., 2008). According to both recent and historical research, China National Commission of Animal Genetic Resources preliminarily proposed thatA.ceranain China can be divided into nine local varieties: Northern Chinese bees, Southern Chinese bees, Central Chinese bees, Yungui Plateau Chinese bees, Changbai Mountain Chinese bees, Hainan Chinese bees, southern Yunnan Chinese bees, Aba Chinese bees, and Tibet Chinese bees (Ge and Shi, 2011). However, a basis for this classification was not provided. Furthermore, no clear reasons have been presented for the different views of the predecessors on the classification ofA.ceranain China. It has been suggested that the classification ofA.ceranarequires further study.
In summary, the study of the morphological differentiation ofA.ceranain China was gradually enhanced by improving the number of markers and utilized analytical methods, and by increasing the sample size. However, it must be noted that important regional data remain inadequate. For example, the topography of Sichuan is complex and changeable, and morphological differentiation of the established honey bee population was found (Zhuetal., 2017). However, these new data were not used for the classification ofA.ceranain China. Moreover, data from the Southeast of China are very limited where large populations ofA.ceranaexist. Only one sampling data point was used in Fujian, Jiangxi, and Anhui provinces, respectively, while no Zhejiang sample existed. Tibet and Taiwan samples were also missing (Tanetal., 2008; Radloffetal., 2010). These samples are very important and indispensable for a complete analysis of the infraspecific categories and geographical variation ofA.ceranain China.
In this study, these new and important samples from Tibet, Taiwan, the basin and highlands of southwestern China, and the Southeast of China were added, and with them, multivariate morphometric analyses ofA.ceranaacross China were conducted. This provides more information of morphological differentiation, especially with regard to the differentiation level of small populations on a large scale, and the population characteristics ofA.ceranain China, to further reveal the reasons behind population isolation.
2 MATERIALS AND METHODS
2.1 Experimental materials
Honey bee workers were collected from a total of 102 sampling sites throughout China, which covered different ecological areas and the complete distribution range of the Asian honey beeA.ceranain China (Table 1). The previously published raw databases of 25 samples of Sichuan (Zhuetal., 2017), eight samples of Guizhou (Luoetal., 2015), nine samples of Hainan (Zhou SJetal., 2018), and five samples of Fujian (Zhuetal., 2011a) were included in this study for a complete analysis of China. All other samples constitute unpublished new data. Sixty worker bees of each sampling site from 10-20 colonies were dissected (with the exception of 57 bees in Taipei and 90 bees in Antu, Jilin). The anatomical morphology features of a total of 6 147 honey bees were examined.
Table 1 Distribution of sampling sites with geographic coordinates of the Asian honey bee, Apis cerana in China
To avoid interference on morphological markers caused by the developmental environment, all worker bee samples were collected from normal colonies with large population. This helps to maintain a good developmental environment during the thriving period and ensures abundant nectar and pollen feed, thus providing good nutritional conditions.
To eliminate influences from artificial breeding colonies, the utilized samples ofA.ceranawere primarily collected from wild nests, which were good representation of the genetic characteristics of the local Asian honey bee. In areas such as Guangdong and Zhejiang provinces, where the beekeeping technology is highly developed, few colonies of the traditional type are available and samples can only be taken from beehives with movable frames. Therefore, samples were collected from multiple apiaries, and different maternal colony samples were selected in the same apiary. Workers were soaked in 100% ethanol for preservation.
2.2 Measurement of morphological characteristics
A total of 33 morphological characteristics were measured. These characteristics are: proboscis length, morphological markers associated with the wings (forewing length, forewing width, the cubital index, the distance of cubital vein a and cubital vein b, number of hind wing hamuli, and 11 wing vein angles A4, B4, D7, E9, G18, J10, J16, K19, L13, N23, and O26), and morphological markers associated with individual size (longitudinal length of tergite 3, longitudinal length of tergite 4, and longitudinal length of tergite 5, longitudinal length of sternite 4, length of wax plate on sternite 4, transverse length of wax plate on sternite 4, distance between wax plate on sternite 4, and longitudinal length of sternite 7, transverse length of sternite 7), morphological markers associated with hind leg (femur length, tibia length, metatarsus length, metatarsus width), and morphological markers associated with body color (the width of the stripe of tomentum on tergite 5, length of cover hair on tergite 6)(Rutteretal., 1978; Ruttnerr, 1988; Yang, 2001; Zhuetal., 2017).
2.3 Data analysis
To study the morphological differentiation of honey bees, discriminant analysis and principal component analysis were used. Stepwise discriminant analysis was performed using the 33 morphological characteristics from all sampling sites. Cluster analysis was conducted using the mean discriminant functions scores of each individual. The mean value and standard deviation of each morphological characteristic were calculated for each sampling site, and the principal component analysis was carried out using the mean value at each sampling site (Ruttner, 1988; Kandemiretal., 2005).
The morphological differentiation was determined via cluster analysis result and the Euclidean distance was used for cluster analysis (Zhuetal., 2011b). Then, all individual bees were assigned to the
corresponding cluster, and stepwise discriminant was performed. The accuracy of each individual that was part of the corresponding cluster was calculated via cross validation. The means of each discriminant function score of 14 clusters and the weight coefficients of each morphological characteristic on each discriminant function score were obtained. The differences of the mean scores of 14 clusters reflect the differences of morphological traits of each cluster (Kono and Kohn, 2015).
Statistica 20 software was used for all analyses.
3 RESULTS
3.1 Morphological differentiation of A. cerana in China
According to the result of cluster analysis based on the mean discriminant function scores of each individual from 102 sampling sites, the Asian honey bee was differentiated into 14 clusters, including: (1) Hainan cluster (Hainan Island); (2) Southern Yunnan cluster (Southwest of Yunnan); (3) Changbai cluster (from the Changbai Mountain of Northeast China); (4) Taiwan cluster (Taiwan); (5) Bomi cluster (South Tibet mountain area); (6) Aba cluster (Aba Prefecture of the Western Sichuan Plateau); three small populations of the Western Sichuan Plateau: (7) Batang cluster, (8) Derong cluster, and (9) Yajiang cluster; (10) Chuandian cluster (Liangshan, Sichuan Province, and northern Yunnan Province); (11) Chuangui cluster (Sichuan basin, Guizhou, Chongqing, and Hubei provinces); (12) Northwestern cluster (Shaanxi, Gansu, and Ningxia); (13) Southern cluster (Guangdong and Guangxi); and (14) Northern and Eastern cluster (Henan, Shanxi, Beijing, and Shandong; Anhui, Zhejiang, Fujian, and Jiangxi) (Fig. 1). The geographical distribution of these clusters is shown in Fig. 1. Through the cross validation, the percentages of individuals of 14 populations, that could be assigned into the corresponding population were 93.3%, 96.7%, 88.5%, 88.9%, 90.0%, 80.6%, 81.7%, 88.3%, 93.3%, 63.1%, 50.0%, 76.0%, 72.7%, and 69.8%, respectively, suggesting that most of the classification results were accurate.
And according to the result of principal component analysis (Fig. 2: A), the honey bee was differentiated into nine clusters: Hainan cluster, southern Yunnan cluster, Changbai cluster, Taiwan cluster, Aba cluster, Batang cluster, Chuandian cluster+Chuangui cluster, Northwest cluster+Bomi cluster+Yajiang cluster, and Southern cluster+Northern and Eastern cluster. Further, the Bomi cluster and Yajiang cluster can be separated from Northwest cluster, and Southern cluster can be separated from Northern and Eastern cluster (Fig. 2: B).

Fig. 1 Tree diagram for the complete linkage ofthe Asian honey bee (Apis cerana) from102 sampling sites in ChinaThis graph is the result of cluster analysis with discriminant function values of the gravity center of each sampling site (based on the 33 anatomical morphology features of a total of 6 147 honey bees). The honey bee A. cerana in China was clustered into 14 clusters.
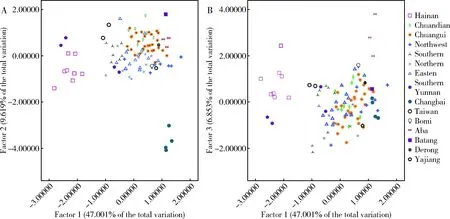
Fig. 2 Principal component plot of honey bee (Apis cerana) from 102 sampling sites in ChinaThis graph is the result of principal component analysis with mean factor 1 scores of 102 samples against factor 2 scores (A) and factor 1 scores against factor 3 scores (B).
3.2 Morphological traits of clusters of A. cerana in China
A total of 33 morphological characteristics were entered into the discriminant function and 13 discriminant functions were established via stepwise discriminant analysis. The first six discriminant functions explained 55.7%, 19.1%, 6.7%, 5.4%, 3.5%, and 2.7% of the overall variations, respectively, which totals 93.1%.
According to the distribution of 14 clusters based on discriminant function 1 score, five clusters with smaller body size were identified. Body sizes from small to large follow the order: Hainan cluster, Southern Yunnan cluster, Taiwan cluster, Southern cluster, and Northern cluster. Their mean scores were -4.912, -3.944, -2.072, -1.097, and 0.085, respectively (Fig. 3: A). The other nine clusters had larger individuals, and the average scores of function 1 were between 0.887 and 2.690. The major discriminant factors of function 1 were: proboscis length, the wing vein angle B4, forewing length, the wing vein angle A4, the distance of the cubital vein b, the length of hairs on tergite 6, the cubital index, and the length of the wax plate on sternite 4. The results indicate that the five clusters were significantly different in proboscis length, forewing length, the structure of the 3rd submarginal cell in the forewing, body color, and the length of the wax plate.
According to the distribution of nine clusters with larger body size for the discriminant function 2 score, two clusters could be identified: Changbai cluster and Northwest cluster. The mean scores of them were 3.335 and 1.348, respectively, and the average scores of function 2 of the remaining seven clusters from the West of China ranged from -0.602 to -1.594 (Fig.3: B). The major discriminant factors of function 2 were cubital index, length of wax plate, distance of cubital vein a, the wing vein angle A4 and B4, the length of hairs on tergite 6, proboscis length, tibia length, forewing length, and longitudinal length of tergite 5. The results showed that there were significant differences in these factors among the Changbai cluster, Northwest cluster, and other samples of western China. The cubital index of the Changbai cluster was the largest, with an average value of 5.3547, which exceeded that of the honey bees in the other areas in China by more than 1. In addition, the wax plate size and the width of the stripe of tomentum on tergite 5 were the biggest. The Northwest cluster had the longest hind legs and hairs on tergite 6. The proboscis length of the Changbai cluster and the Northwest cluster was shorter than that of the nine clusters with larger individuals (Table 2).
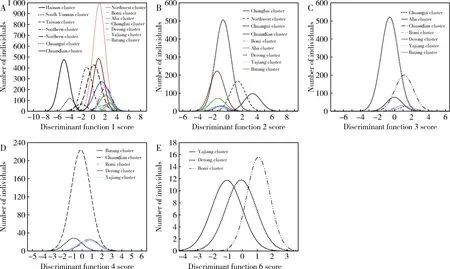
Fig. 3 Distribution curves of scores of canonical form discriminant functions of all 14 clusters of Apis cerana in ChinaIndividual bees were assigned to the corresponding clusters, and stepwise discriminant analysis was performed. A-E: All individual bees of the 14 clusters distributed on discriminant function 1, 2, 3, 4, and 6 scores, respectively. The differences in mean scores of these 14 clusters reflect the differences in the morphological trait of each cluster. Based on the discriminant function scores of each morphological characteristic contributing to the major discriminant factors of function, the morphological trait of each cluster was found.
According to the distribution of seven clusters from western China with regard to discriminant function 3 score, two clusters could be identified: Aba and Chuangui. Their mean scores were -0.593 and -0.055, respectively, and the average scores of function 3 of the remaining five clusters ranged from 0.686 to 1.432 (Fig. 3: C). The major discriminant factors of function 3 were cubital index, width of the stripe of tomentum on tergite 5, distance of the cubital vein b, transverse length of sternite 7, and forewing length. The cubital index of the Aba cluster was inferior only to that of the Changbai cluster, and the wing lengths and the sizes of sternite 7 were the largest. Among the seven clusters, the Chuangui cluster had the shortest proboscis length, smallest forewing size, and the shortest width of the stripe of the tomentum on tergite 5.
According to the distribution of five clusters from the Qinghai-Tibet Platean with regard to discriminant function 4 score, two clusters were identified: Batang and Chuandian. Their mean scores were -0.870 and -0.161, respectively (Fig. 3: D). The major discriminant factors of function 4 were cubital index, forewing width, length of cover hair on tergite 6, wing vein angles B4 and A4, distance of cubital vein a, forewing length, and tergite 4 longitudinal length. The cubital index of the Batang cluster was the smallest in China, being only 3.0169, while those of the other clusters exceeded 3.6. In addition, the largest individual cluster was in the Batang cluster in China. The individuals of the Chuandian cluster were the smallest on the Western Sichuan Plateau, which manifested in the shortest longitudinal length of tergites 3, 4 and 5, and longitudinal length of sternites 4 and 7, and the smallest size of the wax plate on sternite 4 and hind leg. Furthermore, their body color was black.
The means of discriminant function 6 score of Bomi, Derong, and Yajiang cluster were 1.063, -0.102, and -1.052, respectively (Fig.3: E). The major discriminant factors of function 6 were wing vein angle L13, longitudinal length of sternite 7, transverse length of wax plate on sternite 4, length of cover hairs on tergite 6, the longitudinal length of tergite 5, the transverse length of sternite 7, wing vein angles G18, metatarsus length, wing vein angle A4, longitudinal length of tergite 4, distance of cubital vein a, and the wing vein angles O26, J10, and B4. The cubital index of the Bomi cluster exceeded 4, and the width of the stripe of tomentum on tergite 5 was the smallest in China. The Derong and Yajiang clusters were similar to Aba and Batang clusters: the bees were large and dark, with bees of the Derong cluster being the darkest. Except for the difference in body color, the Yajiang cluster was unique in wing vein angles (A4, N23, E9 and J10 were the smallest and B4 the largest) (Table 2).
4 DISCUSSION AND CONCLUSIONS
4.1 Genetic differentiation of A. cerana in China
A systematic genetic resources research of the Asian honey bee in China was conducted during the 12th Five-Year Plan period with the support of the China agriculture research system. In this study, important samples that were insufficient or lacking in previous studies, especially those from Bomi, Taiwan,


and the Western Sichuan Plateau were collected. Based on this enlarged sample size, the morphological differentiation of the whole distribution area of the Asian honey bee in China was analyzed. The results of morphological classification and distribution of Hainan, South Yunnan, Bomi, Changbai, and Southern clusters are basically consistent with those reported by previous studies (Yang, 2001; Ge and Shietal., 2011).
The Asian honey bees in Bomi, also known as southern Tibet honey bees, are distributed in the southern foot of the Himalaya Mountains. Few reports are available on the honey bees of Tibet. Only Yang reported the morphological data of honey bees in southern Tibet, and identified them as a subspecies (Yang, 2001). The particular religious and cultural environment in Tibet causes great difficulties for sample collection in the Tibet area. Therefore, no reports about honey bees in Tibet have been published since that of Yang. The morphology of the Bomi honey bee was measured in this study and was compared to that of honey bees in other areas of China. Apparent morphological differentiation was found between the Bomi cluster and the other clusters in China. Honey bees in Bomi had the second largest body size only surpassed by the clusters of the Western Sichuan Plateau. In addition, it has a specific wing vein structure. With regard to body color, the Bomi honey bee is distinctive, because the width of the stripe of tomentum on tergite 5 is short (1.0683 mm); however, the length of the hairs on tergite 6 is long (0.4057 mm). This may be related to the unique ecological conditions of Bomi.
In previous studies, the Taiwan Asian honey bee was considered to belong to the South China type based on morphological method (Yang, 2001; Ge and Shi, 2011); however, molecular data indicated that compared to the mainland Asian honey bee, the Taiwan honey bee has distinct genetic characteristics. The mitochondrial DNA ofA.ceranain Taiwan is characterized by the absence of most of the non-coding sequences and deleted short sequences (Smith and Hagen, 1996). The COI, COII, and 16S ribosomal RNA gene fragments of mitochondrial DNA from two Taiwan individuals showed that the Taiwan honey bee may be an independent lineage (Tanakaetal., 2001; Zhaoetal., 2014). However, the small sample size limits the reliability of this suggestion. In this study, a large sample size of Taiwan bees was used, and the results showed obvious morphological differentiation betweenA.ceranaon Taiwan Island and those of the Chinese Mainland. Furthermore, the obtained microsatellite and mitochondrial DNA molecular genetic analysis (unpublished data) results also support this suggestion. This is mainly because the Taiwan Strait has blocked gene flow between the honey bees of Taiwan and the honey bees on the adjacent mainland, resulting in differentiation.
At the whole Chinese scale, this study further clarified that the core areas of honey bee distribution in Aba are located in Maerkang, Rangtang, and Xiaojin. At the same time, three small populations (Batang, Derong, and Yajiang) in the valley of the Western Sichuan Plateau were identified, which was consistent with the results of our study regarding the morphology ofA.ceranain Sichuan. The formation mechanism of these small populations and the definition of the Aba population have been presented in detail in this paper (Zhuetal., 2017).
The Northern cluster is distributed in Shandong, Henan, Shanxi, and Beijing provinces. Previous studies also included the Asian honey bees from Shaanxi, Ningxia, and Gansu (Ge and Shi, 2011). Judged by the morphological data, the honey bees of Shanxi and Henan are of the most typical Northern cluster. The number of Asian honey bee colonies in Beijing and Shandong is small, and according to their geographical proximity, the honey bees in Beijing and Shandong are classified as this cluster. However, the honey bees of Shaanxi, Gansu, and Ningxia have unique morphological traits, thus forming a new cluster: the northwestern cluster.
The Chuandian cluster, is similar to the previous Yunnan-Kweichow Plateau Asian honey bee (Yang, 2001; Ge and Shi, 2011), differs mainly with regard to whether the samples from western Guizhou belong to this cluster. According to morphological analysis, Panxian and Jinping clusters of western Guizhou are the transitional type between Chuandian and Chuangui clusters. Therefore, the honey bees of western Guizhou cannot be identified without further data. Because this cluster is mainly distributed in Liangshan Prefecture, Sichuan Province, and Northern Yunnan Province, the name has been changed to the “Chuandian cluster”.
The Chuangui cluster is mainly distributed in the Sichuan Basin and the adjacent Guizhou, Chongqing and Hubei provinces. The honey bees of this area have been classified as central China Asian honey bees in previous studies (Ge and Shi, 2011). According to its geographical distribution, this cluster received the name Chuangui cluster. In addition, the central China Chinese bee also includes populations of the honey bee from Jiangxi, Anhui, West of Zhejiang, and South of Jiangsu (Ge and Shi, 2011). However, morphological differentiation was found between the Chuangui cluster and the cluster of eastern China. The results of morphological analysis showed that the samples from Fujian, Zhejiang, Anhui, and Jiangxi provinces in eastern China were dispersed in the northern cluster in a cluster diagram. The following reasons were found: (1) Beekeepers in this region generally have high management skills, and can artificially breed queens, resulting in directional genetic changes of natural populations. (2) A subset of the Asian honey bees in this area were reared via migratory beekeeping, which enhanced the inter-regional gene flow. (3) The commercialization of bee queens has also accelerated the gene flow in developed honey bee breeding areas, resulting in instability of the genetic structure. Therefore, the samples from Fujian, Zhejiang, Anhui, and Jiangxi in eastern China are proposed to be divided into one cluster: the Eastern cluster.
4.2 Morphological traits and distribution pattern of A. cerana in China
The individual size of the Asian honey bee in China was larger at high altitudes than at low altitudes, and the same result was found for latitudes. The size regional variation conforms to Bergman’s law. In the high-altitude areas of the Western Sichuan Plateau, individuals of the Asian honey bees in Aba, Derong, Yajiang, and Batang cluster were the largest, followed by the Bomi cluster, in turn, the Chuangui and Chuandian clusters. Medium-sized honey bees were distributed in Changbai cluster, Northwest cluster, and Northern cluster. The bees of Hainan, southern Yunnan, Taiwan, and Southern clusters were small.
The Asian honey bees at high altitude and high latitudes have a deep body color. The morphological characteristics associated with body color were the width of the stripe of the tomentum on tergite 5 and the length of hairs on tergite 6. Using both markers, Changbai cluster was identified as the darkest, followed by Derong and Northwest clusters, then Aba, Batang, Yajiang, and Chuandian clusters. In the low altitude clusters of both Chuangui and North, and in the low latitude clusters of South Yunnan, Hainan, Taiwan, and South China, the body color was light.
The environments of Bomi, Batang, Derong, and Yajiang are similar, which leads to a similarity in morphological characteristics due to selection, and convergent evolution may occur. However, in the neutral wing vein index (which is less affected by selection) (Zhuetal., 2011c), these clusters have their own unique wing vein structural characteristics.
The cubital index of the bees of Changbai cluster was significantly higher than that of other clusters. The cubital index is the ratio of the distance of cubital vein a to that of cubital vein b. Since the distance of cubital vein a of the bees in Changbai cluster was longer and the distance of cubital vein b was shorter (Table 2), the cubital index was significantly larger. At the same time, protrusion abnormalities occurred in the 3rs-m position of the fore wing and the proportion of individuals with vein mutation was 45.56% (41/90). Both of these phenomena resulted in clear changes in the wing structure of the third submarginal cell. This vein variation pattern was also found in adult worker honey bees that were exposed to low temperature during the sealed brood stage (Zhuetal., 2018). Whether the changes of these veins are adaptable to a local low-temperature environment remains unclear. If it is hereditable, this variation could be a specific genetic marker of Changbai cluster. In that case, it would be interesting what kind of biological significance this variation has for the fitness of theA.cerana.
4.3 Genetic differentiation in the Qinghai-Tibet Plateau
The Qinghai-Tibet Plateau provides ideal natural conditions for studying the genetic differentiation of species. Especially the Hengduan Mountains in the eastern part of the Qinghai-Tibet Plateau, with their unique plateau-canyon landscape, area hot spot for the study of biodiversity and population genetics (Xingetal., 2017). It has been speculated that the center of origin of the western bee (A.mellifera) may be found in the Himalayan-Hengduan Mountains of southwestern China (Hanetal., 2012; Wallbergetal., 2014).
Most parts of the Qinghai-Tibet Plateau have such high altitudes that the Oriental bees (A.cerana) cannot survive here. Few are distributed naturally throughout canyons. It has been found that these narrow and long canyons easily interrupt gene flow between populations, resulting in many small populations. A morphological characterization of the Asian honey bee (A.cerana) on the Western Sichuan Plateau has been conducted, and its unique differentiation mechanism has been preliminarily discussed (Zhuetal., 2017). The mechanism of population differentiation in this region can be summarized as follows: (1) Divergent evolution caused by differences in the ecological environment in response to altitude,e.g., differentiation between Aba and Chuandian clusters at high altitude and Chuangui cluster at low altitude. (2) Geographical isolation as a result of the plateau barrier,e.g., the differentiation of Bomi cluster, Aba and Yajiang clusters, Chuandian cluster and three small clusters, and Batang and Yajiang clusters, due to the existence of a plateau where honey bees cannot survive and span (altitude not less than 4 000 m). This result is also supported by molecular data (Yuetal., 2019). The Tibetan honey bees are mainly distributed throughout low-altitude valleys, surrounded by snowy mountains above 5 000 m. The sampling site in Bomi is located in low-lying areas along the banks of the Yigong Zangbo and Palong Zangbo valleys. This area belongs to a relatively closed natural environment, which blocks the genetic exchange between Bomi and the outside honey bee populations and results in morphological differentiation. (3) The presence of bee-free areas in narrow and long plateau canyons likely causes blockages in gene flow,e.g., Batang and Derong clusters in the Jinsha River Valley. There are many river valleys in this area and it is likely that other small populations exist.
4.4 The survival crisis of A. cerana in China
The distribution ofA.ceranain China is shrinking in the northern fringe. Prior to the introduction of western honey bees (A.mellifera), Asian honey bee (A.cerana) were widely distributed throughout China except in Xinjiang, western Gansu and Qinghai, and most of Tibet (Yang and Xu, 1982). In the 1980s, Asian honey bees in China were distributed in the South of the region of three northeastern provinces, Hebei, Shanxi, Shaanxi, Ningxia and eastern Gansu, and the number of honey bees in northeastern China and the middle and lower reaches of the Yangtze River decreased (Yang, 1982). Over the past 100 years, the number of Asian honey bees in China has decreased by more than 80% and their distribution area has shrunk by more than 75% (Yang, 2009). Currently, the distribution area of the Asian honey bee in China is shrinking from their northern distribution boundary line to South. Today, there are few Asian honey bees in Northeast China except at Changbai Mountain and its surrounding areas (Zhouetal., 2018).
Habitat destruction, interspecific competition as a result of the introduction of a large number of western honey bees (A.mellifera), and other factors, resulted in a decrease in the number of local honey bee populations, which caused the loss of genetic diversity of the Asian honey bee. This study found that the level of genetic diversity of the Asian honey bee (A.cerana) in Changbai Mountain was low (Yuetal., 2013), indicating that their ability to cope with environmental change was reduced. Further deterioration of the ecological environment or unexpected negative events will cause a survival crisis in this marginal population ofA.cerana. Therefore, it is necessary to protect this marginal population and to take effective measures to restore the genetic diversity of the honey bee, to suppress the continued reduction of their distribution (Zhouetal., 2018).
In central and eastern China, the distribution of Asian honey bees is fragmented. Especially in plain and shallow mountainous areas, with their reduction of the number of honey bees,A.ceranacannot be found in many places. In recent years, due to the improvement of the ecological environment and governmental attention to the beekeeping industry, the decreasing trend ofA.ceranapopulation has slowed down.
For the populations of the Western Sichuan Plateau and Bomi, living space, nectar, and pollen plant sources are limited; consequently, population size is limited (Yuetal., 2019). Furthermore, these become limiting ecological factors for honey bee distribution and the survival risk of these small populations is high. EspeciallyA.ceranain Bomi has become more vulnerable, due to the high price of local honey bee products leads to the introduction of colonies of otherA.ceranapopulations as well asA.melliferafor economic benefit. Here, the local government is called to intensify supervision efforts and to focus on the protection of localA.ceranaresources.
4.5 Conclusions
In this study, the morphometric divergence results of honey bee,A.ceranaacross China were reported, based on supplementary samples that were insufficient or lacking in previous studies, especially those from Bomi, Taiwan, and the Western Sichuan Plateau, and based on enlarged sample size: a total of 6 147 worker bees ofA.ceranafrom 102 sampling sites across the complete distribution area ofA.ceranain China.
At the whole Chinese scale, the first five clusters were detected with the most obvious morphological differentiation and with the most unique morphological characteristics. They were Hainan cluster (Hainan Island), Southern Yunnan cluster (Southwest of Yunnan), Changbai cluster (from the Changbai Mountain of Northeast China), Taiwan cluster (Taiwan) and Bomi cluster (South Tibet mountain area).
Taking Sichuan as the center and its surrounding areas, the complex terrain has created abundant resources ofA.cerana. On the Western Sichuan Plateau, honey bee always lives in canyons due to a limitation of suitable habitats, which were surrounded by plateau where honey bees cannot survive and span, while in a narrow and long canyon, it is easy to form many bee-free areas because of the lack of nectar and pollen plants. So gene flow between canyons can be interrupted. Many clusters of the Western Sichuan Plateau, including Aba, Batang, Derong and Yajiang clusters, were detected. The differences in the ecological environment in response to altitude in a small area leads to divergent evolution, such as differentiation between Aba and Chuandian clusters (Liangshan, Sichuan Province, and northern Yunnan Province) at high altitude and Chuangui cluster (Sichuan basin, Guizhou, Chongqing and Hubei provinces) at low altitude.
The honey bees of Shaanxi, Gansu, and Ningxia have unique morphological traits, thus differentiated from Northern cluster (Henan, Shanxi, Beijing, and Shandong), forming a new cluster: Northwest cluster. The most typical Southern cluster in China isA.ceranafrom Guangdong and Guangxi. Eastern cluster (Anhui, Zhejiang, Fujian, and Jiangxi), did not form stable morphological characteristics. It may be due to the commercialization of colonies or queens, and migratory beekeeping, which have enhanced the inter-regional gene flow.
ACKNOWLEDGEMENTSWe are grateful to master’s degree candidates ZHENG Xiu-Juan, LUO Qun, YING Pin-Fang, GUO Hui-Ping, YANG Kai-Chieh, and CHEN Dao-Yin of our laboratory for their honeybee morphometric analysis and laboratory assistance. We are also thankful to the following persons for assistance in making the collections: AN Jian-Dong, and PENG Wen-Jun, from Institute of Apicultural Research, CAAS; LIU Jin-Zu from Beijing Apiculture Company; ZHANG Ying-Sheng, TENG Yue-Zhong, and GUO Cheng-Jun, from Shanxi Jinzhong Honey Bee Breeding Center; YUAN Xiao-Bo, from Liaoning Agricultural Development Service Center; XUE Yun-Bo, NIU Qing-Sheng, and CHEN Dong-Hai, from Jilin Province Institute of Apicultural Science; YANG Ming-Fu, beekeeper from Jilin; HUA Qi-Yun, Jinhua Academy of Agricultural Sciences; JIN Tang-Dong and LUO Jian-Neng, from Cixi Bureau of Animal Husbandry and Veterinary Medicine; WANG Li-Guo, from Jiangshan Bureau of Animal Husbandry and Veterinary Medicine; CHEN Xue-Lang, from Wenzhou Taichang Beekeeping Professional Cooperative; QIU Ru-Min, from Changxing Italian Honeybee Technology Co., Ltd.; MENG Xiang-Jin, from Department of Agriculture and Rural Affairs of Anhui Province; ZHU Guo-Jun, WANG You-Sheng, and ZHOU Qi-Sheng, beekeepers from Anhui; WANG Guang-Qi from Longyan Bureau of Animal Husbandry and Veterinary Medicine, and WU Ke-Qing, GONG Jian-Jun, YAN Fu-Zhi, ZENG Qing-Quan, and LIAO Wen-Xin, beekeepers from Fujian; ZENG Zhi-Jiang from Jiangxi Agricultural University; XU Bao-Hua from Shandong Agricultural University; YU Shi-Ning from Bee Breeding and Extension Center of Shandong Province; LIU Xin-Ying, and LOU De-Long from the Center of Bee Industry on Seed-breeding and Popularization in Shandong Province; CAI Zhao-Long from Hubei Fengzhibao Apiculture Ltd.; WU Yu-Ping from Hubei Tongcheng Agricultural Radio and Television College; MAI Hong-Zhang, beekeeper from Guangdong; GAO Jing-Lin from Environment and Plant Protection Institute, Chinese Academy of Tropical Agriculture Science; DAI Rong-Guo and JI Cong-Hui, from Chongqing Academy of Animal Sciences; WANG Jian-Wen, LI Nian-Zhou, ZHANG Yi-Qiang, WANG Shun-Hai, and WANG Zhi-Min, from Sichuan Province Apiculture Management Station; XU Zu-Yin, from Guizhou Academy of Agricultural Sciences; FAN Ying from Department of Agriculture and Rural Affairs of Guizhou Province; HAN Jia-Ming, from Tongren Agricultural and Rural Bureau; LIN Zun-Cheng, from Department of Agricultural and Rural Affairs of Yunnan Province; MIAO Si-Wei, WANG Kun, XIONG Jian, YU Yu-Sheng, and FAN Ru-Jun, entrepreneurs related to honey bee industry of Yunnan Province; ZHANG Shi-Wen and MIAO Zheng-Ying from Gansu Apiculture Technology Extension Station; MEI Xun from Animal Husbandry Bureau of Min County, Gansu Province; LI Jing-Yun, beekeeper from Gansu; WANG Qing, SHI Jin-Hu, YE Shan-Bin, LAI Kang, XIA Xiao-Cui, WU Xian-Da, WANG Yuan, ZHENG Xiu-Juan, YING Pin-Fang, LUO Qun, YANG Kai-Chieh, HAN Wei-Chao, and XIA Feng-Zhi, master’s degree candidates in our laboratory. Our gratitude should also go to friends and beekeepers that may not be listed here.
