Inhibition of glucose oxidase gene decreases the resistance of Mythimna separata (Lepidoptera: Noctuidae) larvae to Bacillus thuringiensis infection
2022-08-13YANGHangYANGHongJiaZHANGYaNanWANGXiaoXiFANDong
YANG Hang, YANG Hong-Jia, ZHANG Ya-Nan, WANG Xiao-Xi, FAN Dong
(College of Agronomy, Northeast Agricultural University, Harbin 150030, China)
Abstract: 【Aim】 The objective of this study is to explore the roles of glucose oxidase (GOX) in the development, digestion, and immune defense of Mythimna separata.【Methods】 The cDNA sequence of GOX gene from M. separata was cloned by transcriptome sequencing technology. The specific expression patterns of this GOX gene in different developmental stages (1st-6th instar larvae, pupae and 1-day-old adults) and tissues (foregut, midgut, hindgut, labial gland, Malpighian tubules, fat body, and integument) of the day-1 4th instar larvae, and the day-1 4th instar larvae of M. separata fed with maize leaves soaked in different concentrations (0.01%, 0.1%, 1%, and 10%) of glucose solution for 10 s and under starvation and refeeding conditions were detected by qRT-PCR. The role of this GOX gene in the resistance of M. separata to Bacillus thuringiensis was explored by RNAi and bioassay.【Results】 A 2 187 bp cDNA sequence of a novel GOX gene named MsGOX (GenBank accession no.: KY348779) was obtained from M. separata. It has the open reading frame of 1 821 bp in length, encoding a 606-amino acid polypeptide with the predicted molecular weight of 66.4 kD. Developmental expression profile revealed that MsGOX showed different expression levels in M. separata at different developmental stages, with the highest expression level at the 4th instar larval stage, and tissue expression profile showed that MsGOX was expressed in various tissues of the day-1 4th instar larvae,with the highest expression level in labial glands. MsGOX transcription could be induced differently by feeding larvae with different concentrations of glucose. The expression level of MsGOX reached the highest when the glucose concentration was 10%. The expression level of MsGOX in the day-1 4th instar larvae increased gradually with the starvation time increasing, and reached the peak at 24 h after starvation. When the larvae were refed with maize leaves after starvation, the expression level of MsGOX gradually increased. At 48 h after injection of dsMsGOX, the expression level of MsGOX was inhibited by 88.3% as compared to the control (injected with dsEGFP). The larval body weight, body length, and digestibility coefficient at 48 and 72 h after injection of dsMsGOX were significantly reduced, and the corrected mortality rates of larvae infected by B. thuringiensis at 48 and 72 h were enhanced, as compared to those in the control groups at the same time points. 【Conclusion】 MsGOX might participate in digestion and antibacterial processes in the midgut of M. separata. The results provide a basis for further studying the function of MsGOX and exploring novel strategy to control M. separata.
Key words: Mythimna separata; glucose oxidase; expression pattern; RNA interference; Bacillus thuringiensis
1 INTRODUCTION
Glucose oxidase (GOX) (EC: 1.1.3.4) is an important redox enzyme in living organisms. It specifically recognizes β-D-glucose and degrades it into gluconolactone and hydrogen peroxide. Gluconolactone is further converted to gluconic acid, while hydrogen peroxide will eventually turn into H2O and oxygen (Ramzan and Mehmood, 2009). GOX is widely distributed in animals, plants and microorganisms, especially in fungi. Researches of the characteristics and functions of insect GOX started relatively late and there is less information available. Eichenseeretal. (1999) discovered the presence of GOX activity inHelicoverpazea. The enzyme was secreted by the labial glands. GOX activity has been detected in the labial glands of hymenopteran and lepidopteran insects (Louisetal., 2013). By burning the silk slinger ofH.zeato block secretion of the labial gland enzyme, Musseretal. (2002, 2006) found that GOX could inhibit the nicotine content induced by insects feeding onNicotianatabacum. It is known thatSpodopteraexiguaGOX can act to suppress the transcript levels of genes involved inMedicagotruncatuladefense pathways (Bedeetal., 2006). GOX also has antibacterial effects. Studies have shown that GOX can produce hydrogen peroxide to prevent the growth and reproduction of aerobic bacteria (Liuetal., 2010). GOX can also be used as an antibacterial agent to inhibit the infection of insects by pathogens and increase insect immunity. Musseretal. (2005) found that GOX from the labial gland ofH.armigerahad antibacterial properties that inhibited the growth ofSerratiamarcescensandPseudomonasaeruginosa.GOXgenes have been cloned from insects includingApismellifera(GenBank accession no.: AB022907),H.armigera(GenBank accession no.: EU629216),H.zea(GenBank accession no.: FJ460711),Spodopteraexigua(GenBank accession no.: GU983912), andHeliothisviriplaca(GenBank accession no.: KT907054).
Mythimnaseparatais a very important agricultural pest of cereal crops in China and other Asian countries (Jiangetal., 2014). In this study, a full-length cDNA sequence ofMsGOXwas obtained fromM.separataby transcriptome sequencing technology. The expression patterns ofMsGOXin different developmental stages and different tissues of the day-1 4th instar larvae, and the day-1 4th instar larvae ofM.separatafed with maize leaves soaked in different concentrations of glucose solution, and subjected to starvation for different time and refeeding treatment were detected by qRT-PCR.MsGOXwas silenced by RNA interference (RNAi), and the morphological and physiological changes ofM.separataand the changes in the midgut microbiota were observed. After RNAi, the corrected mortality rate ofM.separatalarvae infected byBacillusthuringiensiswas examined. The results of this study can enrich our knowledge of biological functions of insect GOX and lay the foundation for future prevention and control ofM.separatainfestation by molecular methods.
2 MATERIALS AND METHODS
2.1 Test insects
M.separatalarvae were collected from maize fields at the Xiangyang Experimental Base of Northeast Agricultural University. The larvae were fed with fresh maize leaves in an artificial climate chamber. The temperature, relative humidity and photoperiod were (25+1)℃, 70%, and 14L∶10D, respectively. After emergence, adults were fed with 5% honeybee water. The females laid eggs in folded plastic ropes. Newly hatched larvae were cultured to different developmental stages for subsequent tests.
2.2 cDNA sequence cloning
The 1st-6th instar larvae, pupae, and 1-day-old adults were selected and sent to Annoroad Gene Technology Co., Ltd., Beijing, for transcriptome sequencing.GOXwas screened from the transcriptome database. To verify the accuracy of the sequences, RNA fromM.separatalarvae was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA). The extracted RNA was reversely transcribed using a reverse transcription kit [Toyobo (Shanghai) Biotechnological Co., Ltd., Shanghai, China]. To clone specific fragment ofGOX, PCR amplification was carried out in a solution containingTaqreaction buffer, 0.2 mmol/L of each dNTP,Taqpolymerase (1 U) (Promega), 1 μL cDNA templates, MsGOX-F/R primers (25 pmol of each) (Table 1), to a final volume of 25 μL. The following amplification protocols were used: 95℃ for 2 min; 35 cycles of 95℃ for 45 s, 52℃ for 50 s and 72℃ for 1 min; and a final extension step of 72℃ for 10 min. After PCR amplification, the product was sent to TransGen Biotech Co., Beijing, for sequencing. The results were compared with the original sequence to verify the accuracy of the sequence obtained from the transcriptome database.
2.3 Bioinformatics analysis
Nucleotide sequence ofGOXcloned in section 2.2 was translated into amino acid sequence using DNAMAN software. Isoelectric point and molecular weight of the amino acid sequence were predicted using the Compute PI/Mw tool (https:∥web.expasy.org/compute_pi/). The SignalP5.0 Server (http:∥www.cbs.dtu.dk/services/SignalP/) was used to predict signal peptides. Prosite (https:∥prosite.expasy.org/) was used to analyze domains and functional sites. The amino acid sequences of insect GOX and related glucose dehydrogenase (GLD) were downloaded from the NCBI database and compared (https:∥blast.ncbi.nlm.nih.gov/Blast.cgi). A phylogenetic tree was constructed using MEGA 6.0 and Clustal X software.
2.4 Spatiotemporal expression profiling
The total RNA was extracted from different developmental stages (1st-6th instar larvae, pupae and 1-day-old adults) ofM.separata, and tissues including foreguts, midguts, hindguts, labial glands, Malpighian tubules, fat bodies, and integuments of the day-1 4th instar larvae. Three biological replicates were set up, with three individuals sampled as one replicate for each developmental stage and 15 individuals dissected as one replicate for each tissue. The extraction of total RNA and reverse transcription reaction were conducted according to the instructions of RNA TRIzol®Reagent Kit (Invitrogen, Carlsbad, CA, USA) and Reverse Transcription Kit (Toyobo, Shanghai), respectively. qRT-PCR was carried out with 1 μL of the reverse-transcription product and 0.4 mmol/L of each primer in a total volume of 20 μL using the THUNDERBIRD SYBR qPCR Mix Kit (Toyobo Life Science, Osaka, Japan) according to the kit’s instructions. qRT-PCR was performed using an iCycler iQ5 real-time PCR detection system (Bio-Rad Laboratories, Hercules, CA, USA). qRT-PCR primers MsGOXq-F/R were designed by using Primer Premier 5.0 software based on the obtained nucleotide sequence (Table 1). The qRT-PCR reaction protocols were as follows: after an initial denaturation at 95℃ for 3 min, the reaction used 40 cycles of denaturation at 95℃ for 30 s, annealing at 59℃ for 30 s, and elongation at 72℃ for 30 s. Then, for dissolution curve analysis, the temperature was increased from 65℃ to 95℃ at 1℃/s. The relative expression level ofMsGOXwas calculated by the 2-ΔΔCtmethod (Pfaffl, 2001). Three technical replicates were set up for the sample of each biological replicate. The expression levels ofMsGOXwere normalized using the geometric mean of the expression levels of two reference genes (β-actinandGAPDH) (Livak and Schmittgen, 2001; Vandesompeleetal., 2002).
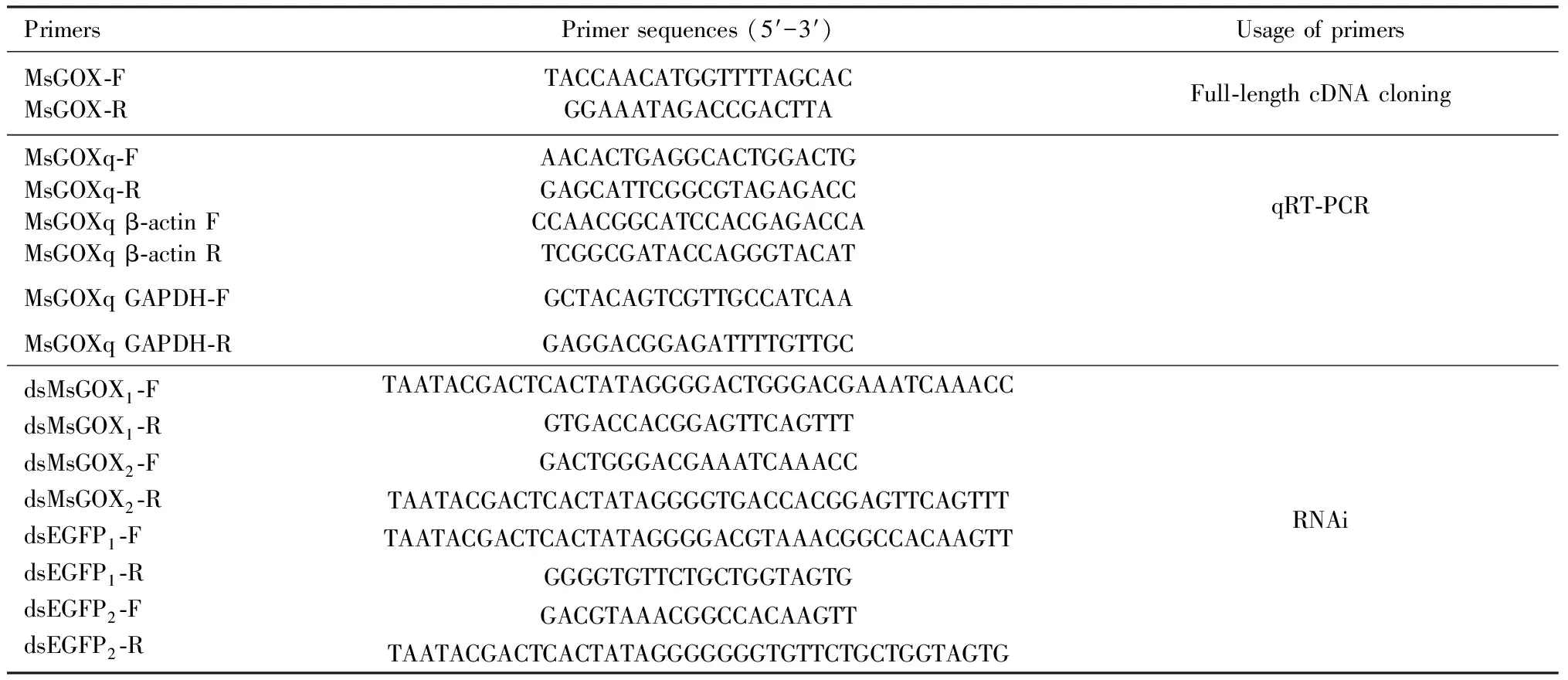
Table 1 Primer sequences used in the study
2.5 Glucose induction
The day-1 4th instar larvae ofM.separatawere divided into five groups (15 larvae for each group), which were fed with maize leaves soaked in distilled water (as a blank control) and different concentrations (0.01%, 0.1%, 1%, and 10%) of glucose solution, respectively, for 10 s. After 3 consecutive days of feeding, RNA was extracted and qRT-PCR (see section 2.4) was used to determine the expression levels ofMsGOX. Three biological replicates for each group and three technical replicates for each biological replicate were set up.
2.6 Starvation induction
The day-1 4th instar larvae were divided into three groups (15 larvae for each group), which were processed for five time periods. In the first group, the larvae were fed with maize leaves for 3, 6, 12, 24 and 48 h as the control groups. In the second group, the larvae were starved for 3, 6, 12, 24 and 48 h. In the 3rd group, the larvae were refed with maize leaves for 3 h after 3-h starvation, refed with maize leaves for 6 h after 6-h starvation, refed with maize leaves for 12 h after 12-h starvation, refed with maize leaves for 24 h after 24-h starvation, and refed with maize leaves for 48 h after 48-h starvation. RNA was extracted and qRT-PCR (see section 2.4) was used to determine the expression levels ofMsGOX. Three biological replicates for each group and three technical replicates for each biological replicate were set up.
2.7 Functional analysis by RNAi
RNAi was used to silenceMsGOXin the day-1 4th instar larvae. RNAi primers were designed according to instructions from E-RNAi (http:∥www.dkfz.de/signaling/e-rnai3/). For dsRNA synthesis, fragments corresponding toMsGOX, andEGFP, which are 288 bp in length, were generated by PCR using the primers dsMsGOX1-F/R, dsMsGOX2-F/R, dsEGFP1-F/R, and dsEGFP2-F/R as shown in Table 1.EGFPwas used as a negative control for the non-specific effects of dsRNA. The templates were synthesized using the following PCR protocols: 94℃ for 5 min; 10 cycles of 94℃ for 30 s, 52℃ for 30 s, and 72℃ for 30 s; 35 cycles of 94℃ for 30 s, 52℃ for 30 s and 72℃ for 30 s; followed by a final extension of 72℃ for 10 min. The PCR products ofMsGOXandEGFPwere separated on agarose gels, purified using a purification kit (TaKaRa), and used forinvivotranscription in the T7 RiboMAXTMExpress RNAi System (Promega, Madison, WI, USA). dsRNA (2 μL, 1 μg/μL) was injected into the abdomen of the day-1 4th instar larvae using a microsyringe. After injection, the larvae were put into an artificial climate cabinet and fed with maize leaves. The larvae were collected 24, 48 and 72 h after injection for detecting the expression level ofMsGOX. RNA was extracted and qRT-PCR (see section 2.4) was used to determine the expression level ofMsGOX. Three biological replicates (15 larvae for one biological replicate) for each collecting time after injection and three technical replicates for each biological replicate were set up.
To examine the effect of RNAi on larval morphology and physiology, the larval body length, body weight, and digestibility coefficient were examined 3, 6, 12, 24, 48, and 72 h after injection.
Digestibility coefficient=
The insecticidal activity ofB.thuringiensiswas tested against the 4th instar larvae ofM.separatafollowing the procedure described previously (Navon and Klein, 1990).B.thuringiensisisolate 25 provided by the Institute of Biological Control, Northeast Agricultural University was used. Previous screening tests showed that the LC50ofB.thuringiensisagainstM.separatalarvae was 2×109cfu/mL. Fresh maize leaves were soaked in 2×109cfu/mLB.thuringiensissuspension for 10 s. After injection with dsRNA, 20 larvae were starved immediately for 24 h and then fed with maize leaves soaked inB.thuringiensissuspension for 24 h. After feeding, theB.thuringiensis-soaked leaves were replaced withB.thuringiensis-free leaves. The number of dead larvae was determined 24, 48 and 72 h after treatment. Three replicates (15 larvae for one biological replicate) were performed for each treatment.
2.8 Data analysis
Statistical analyses were performed by SPSS 18.0 software (IBM Corp., Armonk, New York, USA). Differences between the means of groups were examined by a one-way analysis of variance followed by Duncan’s multiple range test.P<0.05 was considered significant. Column charts were generated using Excel 2007 software.
3 RESULTS
3.1 cDNA and deduced amino acid sequences
Through transcriptome sequencing, a novel cDNA sequence ofGOX, namedMsGOX(GenBank accession no.: KY348779) was obtained fromM.separata.It is 2 187 bp in length, and has a 1 821-bp open reading frame, encoding a 606-amino acid sequence with the predicted molecular weight of 66.4 kD, and theoretical isoelectric of 4.79. MsGOX belongs to the glucose-methanol-choline (GMC) reductase family. The amino acid sequence contains two conserved protein domains: GMC oxidoreductase signature 1, and GMC oxidoreductase signature 2.
3.2 Phylogeny
The sequence alignment results showed that MsGOX shows 65%-75% amino acid sequence identity with GOXs fromS.exigua(GenBank accession no.: ADL38963),H.armigera(GenBank accession no.: ACC94296),H.zea(GenBank accession no.: ACJ71598), andHeliothisviriplaca(GenBank accession no.: AMR44226). MsGOX clustered with other GLDs from Lepidoptera, such as those fromGalleriamellonella(GenBank accession no.: XP_026754993),Bombyxmori(GenBank accession no.: XP_012548096), andPapilioxuthus(GenBank accession no.: KPI98901) (Fig. 1).
3.3 Spatiotemporal expression patterns
MsGOXwas expressed in all the tested developmental stages ofM.separata. The expression level ofMsGOXinM.separataat the 4th instar larval stage was the highest, which was 18.1-, 4.7-, 3.6-, 1.7-, 4.1-, 12.6- and 2.6-fold as high as those at the 1st, 2nd, 3rd, 5th and 6th instar larval, pupal, and adult stages, respectively (Fig. 2: A).MsGOXwas expressed in various tissues of the day-1 4th instar larvae. The expression level ofMsGOXin labial gland was the highest, which was 44.5-, 11.6-, 22.8-, 4.9-, 6.9- and 11.0-fold as high as those in the foregut, midgut, hindgut, Malpighian tubules, fat body, and integument, respectively (Fig. 2: B).

Fig. 1 Phylogenetic tree of glucose oxidase (GOX) and related glucose dehydrogenase (GLD) from insectsby neighbor-joining method based on amino acid sequences (1 000 replicates)

Fig. 2 Relative expression levels of MsGOX in different developmental stages (A) and tissuesof the day-1 4th instar larvae (B) of Mythimna separataData in the figure are mean±SE. Different letters above bars indicate significant difference in the gene expression level between different developmental stages and different tissues (P<0.05, Duncan’s multiple range test).
3.4 Expression under starvation and refeeding induction
There was no significant difference in the expression levels ofMsGOXbetween larvae fed with maize leaves soaked in distilled water (CK), and 0.01% and 0.1% glucose solutions. The expression levels ofMsGOXin the day-1 4th instar larvae fed with maize leaves soaked in 1% and 10% glucose solutions were significantly increased and that in larvae fed with maize leaves soaked in 10% glucose solution was the highest, being 22.3-, 16.8-, 13.1- and 5.7-fold as high as those in larvae fed with maize leaves soaked in 0, 0.01%, 0.1%, and 1% glucose solutions, respectively (Fig. 3: A). The expression level ofMsGOXin the starvation treatment group increased significantly at 12, 24 and 48 h as compared to that in the control group (fed with maize leaves)(P<0.05), and reached the peak at 24 h which was about 8.3-fold as high as that in the control group. The expression level ofMsGOXin the refeeding groups at 12, 24 and 48 h increased significantly as compared to that in the control group (fed with maize leaves)(P<0.05)(Fig. 3: B).
3.5 Biological functions revealed by RNAi
The expression levels ofMsGOXin the day-1 4th instar larvae at 24, 48 and 72 h after injection of dsMsGOXdecreased 62.5%, 88.3% and 35.8%, respectively, as compared to those in the control groups injected with dsEGFPat the same time points (Fig. 4: A), indicating a significant silencing effect of RNAi onMsGOX. The corrected mortality rates of the day-1 4th instar larvae infected byB.thuringiensisfor 48 and 72 h in the dsMsGOXinjection group were significantly increased as compared to that the control group injected with dsEGFP(P<0.05), being 0.72- and 1.38-fold as high as that in the control group injected with dsEGFP, respectively (Fig. 4: B).
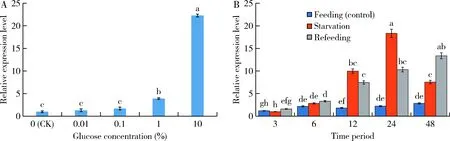
Fig. 3 Relative expression levels of MsGOX in the day-1 4th instar larvae fed with maize leaves soakedin different concentrations of glucose solution (A) and under starvation and refeeding conditions (B)CK: Distilled water; Feeding: Fed with maize leaves; Refeeding: Refed with maize leaves for 3 h after 3-h starvation, refed with maize leaves for 6 h after 6-h starvation, refed with maize leaves for 12 h after 12-h starvation, refed with maize leaves 24 h after 24-h starvation, and refed with maize leaves for 48 h after 48-h starvation. In the induction experiment of glucose, larvae were fed with maize leaves soaked in distilled water (as a blank control) and different concentrations (0.01%, 0.1%, 1%, and 10%) of glucose solution for 10 s. After 3 consecutive days of feeding, the expression level of MsGOX was detected. Data in the figure are mean±SE. Different letters above bars indicate significant difference in the gene expression level between different treatments at P<0.05 level (Duncan’s multiple range test).

Fig. 4 Relative expression levels of MsGOX in the day-1 4th instar larvae (A) and the corrected mortality rateof Mythimna separata infected by Bacillus thuringiensis (B) after RNAiIn the RNAi experiment, 2 μL of dsRNA (1 μg/μL) was injected into the abdomen of the day-1 4th instar larvae using a microsyringe. After dsRNA injection, the larvae were put into an artificial climate cabinet and fed with maize leaves. The larvae were collected at 24, 48 and 72 h after dsRNA injection for detecting the expression level of MsGOX. In the induction experiment of B. thuringiensis, fresh maize leaves were soaked in 2×109 cfu/mL B. thuringiensis suspension for 10 s, the day-1 4th instar larvae starved for 24 h after RNAi were fed with maize leaves soaked in B. thuringiensis solution for 24 h, and finally fed with Bt-free maize leaves. Data in the figure are mean±SE. Different letters above bars indicate significant difference in the gene expression level and corrected mortality rate between different treatment time (P<0.05, Duncan’s multiple range test).
Morphological and physiological examination showed that the larvae injected withdsMsGOXshowed slow movement, and their feeding amount was significantly reduced, but no death occurred. The body weight and body length of larvae at 24, 48, and 72 h after injection of dsMsGOXshowed a continuously significant decrease as compared with those in the control groups at the same time point (P<0.05)(Fig. 5: A, B). The calculated digestion coefficients of larvae at 48 and 72 h after injection of dsMsGOXdecreased significantly as compared with those of the control groups at the same time points (P<0.05)(Fig. 5: C). It can be speculated that MsGOX may be important for digestion ofM.separata.
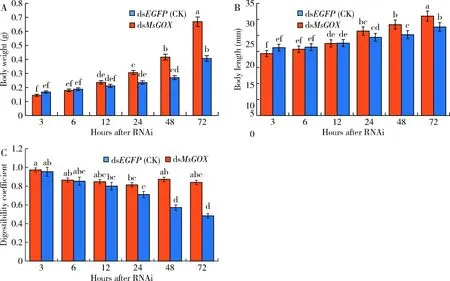
Fig. 5 Body weight (A), body length (B) and digestibility coefficient (C) of the day-1 4th instar larvaeof Mythimna separata after RNAiData in the figure are mean±SE. Different letters above bars indicate significant difference between different treatment time(P<0.05, Duncan’s multiple range test).
4 DISCUSSION
In this study, a novel cDNA sequence ofMsGOXwas identified fromM.separata. Phylogenetic tree indicated that MsGOX has relative close relationship with other GOXs from Noctuidae (Fig. 1).
MsGOXwas expressed in different developmental stages ofM.separata, and showed the highest expression level in the 4th instar larva (Fig. 2: A). WhenM.separatalarvae enter the period of overfeeding at the 4th instar stage, their intake increased obviously. The quantity of food consumed required more GOX for digestion in the midgut. It can be inferred that MsGOX is related to feeding byM.separata. This was also apparent in study of other insects. For example, inHelicoverpaarmigera, it was found that the GOX activity was the highest at the most active feeding stage (Zong and Wang, 2004). The GOX activity inH.zeashowed a similar pattern (Eichenseeretal., 1999).
MsGOXwas expressed in different tissues of the day-1 4th instar larvae ofM.separata, and the relative expression level in labial glands was significantly higher than those in other tested tissues (Fig. 2: B). This result was similar to that in other lepidopteran insects. For example, inH.armigera, the GOX activity in the labial glands was significantly higher than those in the foregut, midgut, Malpighian tubules, hemolymph, and other tissues (Tangetal., 2012). Similarly, the expression level ofBmGOX72 in silk glands, a homologous organ of silkworm mandibular glands, was significantly higher than those in other tissues (Cheng, 2011). It can be speculated that the GOX in the midgut may come from the labial glands and it enters the intestine with insect feeding (Eichenseeretal., 1999; Macauley-Patricketal., 2005). These results indicate that GOX plays a role in insect feeding.
Previous studies found that the expression ofGOXin insects was affected by sugar intake. The expression level ofGOXin labial glands ofH.armigerafeeding on tobacco was significantly higher than that in the control group fed with an artificial diet (Gogetal., 2014). Larvae reared on different host plants produce varying amounts of GOX in their labial glands (Peiffer and Felton, 2005). These results indicated that insects feeding on different foods had a great influence on the expression ofGOX. In addition, different concentrations of glucose solution had different effects on the expression ofMsGOX. It was found that GOX activity in the 5th instar larvae ofH.armigeraincreased significantly as the concentration of glucose solution fed to the larvae increased (Tangetal., 2012). In our experiments of glucose induction, the higher concentration of glucose solution resulted in the higher expression level ofMsGOX(Fig. 3: A), indicating that glucose content might be the main factor affecting the expression ofGOX.
MsGOXtranscripts in starved larvae ofM.separatafirst increased, and decreased significantly after a period of time (Fig. 3: B). These results indicated that, when there was not enough energy such as glucose to support normal physiological activities in starved larvae, they would degrade fat in the body into energy substances such as glucose. When the glucose concentration increased, the expression level ofMsGOXalso increased (Fig. 3: A). However, only a small portion of the fat in the insect could be converted into glucose through gluconeogenesis. When the limitation point was reached, the expression level of GOX gradually decreased. Here, the expression level ofMsGOXwas significantly upregulated on refeeding after starvation as compared to that in the control group (Fig. 3: B). This upregulation may be related to a biocompensated growth phenomenon. Compensatory growth research in aquatic animals including more than 30 species of fish and crustaceans is more extensive than that in insects (Wu and Dong, 2000; Jiangetal., 2002). Studies showed that beef cattle and edible lambs with growth restriction for a period had a significantly greater growth rate than those without growth restriction (Berge, 1991; Yangetal., 2014). The above results indicated thatM.separatalarvae that were fed after starvation might show a compensatory growth phenomenon. It can be speculated that GOX plays an important role in regulating the body metabolism and maintaining normal physiological activities ofinsects.
After RNAi ofMsGOX, the expression level ofMsGOXinM.separatalarvae decreased (Fig. 4: A). The larvae injected withdsMsGOXshowed slow movement, but no death occurred. The body weight, body length and digestibility coefficient ofM.separatalarvae at 48 and 72 h after injection of dsMsGOXwere significantly reduced as compared with those in the control groups (injected with dsEGFP) at the same time points (Fig. 5: A, B, C). It can be speculated that GOX may be related to digestion inM.separata.
Bioassay usingB.thuringiensisagainstM.separataafter RNAi showed that the corrected mortality rate of the larvae increased whenMsGOXwas inhibited (Fig. 4: B). The reason may be that inhibition ofMsGOXcauses less production of hydrogen peroxide which has the ability of antibacteria. The reduction of antibacterial factors in the midgut ofM.separataleads to the increase of the insecticidal activity ofB.thurieningsis. This experiment provides a basis for future research using GOX and biocontrol agents for insect pest control.
