Therapeutic effect of Qinghuayin (清化饮) against chronic atrophic gastritis through the inhibition of toll or interleukin-1 receptor domain-containing adaptor inducing interferon-β signaling pathway
2022-07-28HEYouchengYAOXiaolingLISihanZHOUHongjianXURuoyingZHENGRongXIAOWendiSUNingLINPingHUANGMinghan
HE Youcheng,YAO Xiaoling,LI Sihan,ZHOU Hongjian,XU Ruoying,ZHENG Rong,XIAO Wendi,SU Ning,LIN Ping,HUANG Minghan
HE Youcheng,ZHOU Hongjian,XU Ruoying,ZHENG Rong,XIAO Wendi,LIN Ping,HUANG Minghan,Department of Gastroenterology,the Second People's Hospital Affiliated to Fujian University of Traditional Chinese Medicine,Fuzhou 350001,China
YAO Xiaoling,LI Sihan,SU Ning,Basic Medical school of Guangzhou University of Chinese Medicine,Guangzhou 510006,China
Abstract OBJECTIVE:To examine the efficacy of Qinghuayin (清化饮,QHY) in rat chronic atrophic gastritis (CAG) models and explored the molecular mechanism of QHY in treating CAG.METHODS:In total,65 Wistar rats were randomly divided into the control (n =10) and CAG groups (n=55).CAG model rats were further divided into five groups:model (n=10),vitacoenzyme (n=10),low-dose QHY (n=10),medium-dose QHY (n=10),and high-dose QHY groups(n=10).We analyzed histopathological changes using hematoxylin and eosin staining and measured interleukin(IL)-6 and IL-8 levels in serum using enzyme-linked immunosorbent assay (ELISA) (Boster Bio,Pleasanton,USA).In addition,gastrin (GAS),pepsinogen I (PGI),and PGII expressions were evaluated using ELISA.The protein and mRNA expression of toll-like receptor 4(TLR4) and toll or interleukin-1 receptor domaincontaining adaptor inducing interferon-β (TRIF) was detected by Western blotting and quantitative reverse transcription-polymerase chain reaction,respectively.RESULTS:Our results revealed that histopathological changes in CAG model rates could be restored by low-,medium-,and high-dose QHY.The changes in GAS and PGI/II expression demonstrated that QHY improved CAG.Serum IL-6 and IL-levels were decreased by QHY administration.TLR4 and TRIF were upregulated at the mRNA and protein levels in the model group but downregulated by QHY administration.CONCLUSION:We concluded that QHY could effectively improve the histopathological changes of the gastric mucosa induced by CAG in rats.The therapeutic mechanism of QHY may be related to inhibition of the inflammatory factors IL-6 and IL-8 and suppression of TLR4/TRIF mRNA and protein expression.
Keywords:gastritis,atrophic;toll-like receptor 4;interleukin-6;interleukin-8;toll or interleukin-1 receptor domain-containing adaptor inducing interferon-β;Qinghuayin
1.INTRODUCTION
Chronic atrophic gastritis (CAG) is a common and refractory precancerous disease of gastric cancer.CAG is typified by the atrophy of glands caused by repeated damage to gastric mucosa epithelial cells,and it often accompanied by intestinal metaplasia and/or intraepithelial neoplasia.1CAG may lead to gastric carcinoma through a long,multi-stage,multi-gene mutation accumulation process.2Therefore,blocking or delaying CAG progression is of great significance for preventing the occurrence of gastric cancer.3
In modern medicine,precancerous lesions of CAG are usually monitoredviafollow-up observation.In recent years,it has been found that long-term supplementation with bioactive vitamins and minerals,including vitamin C and the trace element selenium,may reduce the risk of the transformation of CAG to gastric cancer.4Vitacoenzyme plays a crucial role in the redox process,which is important for preventing and blocking precancerous lesions of gastric cancer.The main components of vitacoenzyme are riboflavin and riboflavin derivatives,including vitamins and some trace elements.
QHY is a Traditional Chinese Medicine (TCM) prescription that can significantly alleviate the symptoms of epigastralgia,epigastric distention,and acid regurgitation in patients with CAG.5The effective pathological improvement rate was 82.9%,which suggests that the decoction can effectively reverse the atrophy of gastric mucosa glands.5However,the molecular mechanism of QHY in the treatment of CAG remains unclear.
In recent years,the roles of toll-like receptor 4 (TLR4)and the downstream toll or interleukin-1 receptor domain-containing adaptor inducing interferon-β (TRIF)signal transduction pathway in the pathogenesis of CAG have been revealed.6Therefore,we hypothesized that the mechanisms of QHY in treating CAG are associated with the regulation of TRIF signaling.To test this hypothesis,we established rat CAG models and analyzed the effects of QHY on gastric mucosa histology and the mRNA and protein expression of TLR4 and TRIF.
2.MATERIALS AND METHODS
2.1.Animals
Sixty-five male Wistar rats [4 weeks old;body weight,(110 ± 10) g] were purchased from Shanghai Slack Laboratory Animal Co.,Ltd.(Shanghai,China).All animal experiments followed the National Institute of Health Guidelines for the Care and Use of Laboratory Animals.All experiments were approved and supervised by the Animal Care and Use Committee of the Fujian University of TCM (approval No.3100101037).Rats were housed in a room at (22 ± 1) ℃ and 55% ± 5%humidity with a normal light/dark cycle (lights on at 8:00 AM to 8:00 PM).
2.2.QHY preparation
QHY was provided by the Pharmacy Department of the Second People’s Hospital affiliated to Fujian University of TCM,and it has been approved for use by the Fujian Provincial Food and Drug Administration (approval No.Z05104030).The composition of QHY is as follows:111.1 g of Yinchen (Herba Artemisia Capillaris),66.7 g of Cangzhu (Rhizoma Atractylodis Lanceae),33.3 g of Huanglian (Rhizoma Coptidis),66.7 g of Houpu (Cortex Magnoliae Officinalis),111.1 g of Chishao (Radix Paeoniae Rubra),100g of Huoxiang (Herba Agastaches Rugosa),33.3g of Doukou (Fructus Amomi Rotundus),and 222.2 g of Yiyiren (Semen Coicis).The drugs were soaked in an 8-fold excess of water for 0.5 h and then decocted three times (1.5,1,and 0.5 h).The decoctions were then combined and filtered.The concentration of the decoction was adjusted to 2.2 g/mL (raw material) at 60 ℃ and then bottled for sterilization.
2.3.Experimental design
2.3.1.Animal grouping
Rats were randomly divided into the control (C,n=10)and CAG groups (n=55).Two rats died during the 12-week modeling process.Moreover,three CAG rats were randomly sacrificed,and their gastric tissues were taken for pathological examination to confirm the success of modeling.Then,the remaining 50 rats were randomly divided into five groups after 1 week of adaptation as follows:model (M,n=10),low-dose QHY (LT,n=10),medium-dose QHY (MT,n=10),high-dose QHY (HT,n=10) and vitacoenzyme groups (V,n=10).
2.3.2.Establishment of the CAG rat model
Control rats were freely given clean water and fed a standard rat diet.The rats in other groups were subjected to irregular fasting and compulsive sporting.Then,M group rats were perfused with mixed 2% sodium salicylate and 60% alcohol for 12 weeks to stimulate gastric mucosa as described previously.7
2.3.3.Drug intervention
The doses of QHY were based on those in humans.The therapeutic doses in the LT,MT,and HT groups were 1.5,3.0,and 6.0 mL·kg−1·d−1,respectively.The rats in the V group were treated with 0.3 mL·kg−1·d−1vitacoenzyme.All the aforementioned agents were diluted with normal saline to 4 mL for gavage.Rats in the C and M groups received an equivalent volume of placebo (normal saline)viagavage.Treatments were administered for 30 d.
2.3.4.Specimen collection
After the drug intervention,rats were fasted for 23 h,anesthetizedviaan intraperitoneal injection of chloral hydrate (400 mg/kg),and subjected to laparotomy to collect abdominal aortic blood (3 mL).After resecting the gastric tissue,the gastric mucosa was cut along the large arc to open the stomach.The surface of the gastric mucosa was cleaned with normal saline.
Two blocks of tissues (the size of soybeans) at the gastric antrum were clipped and immediately frozen in liquid nitrogen.quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and Western blotting were then performed.The remaining gastric antrum tissues were fixed in 4% paraformaldehyde.
2.3.5.Determination of biochemical indices
Regarding the biochemical indices related to the stomach status,gastrin (Gas) levels were measured using a Human Gastrin enzyme-linked immunosorbent assay(ELISA) kit (Shanghai Yuan Mu Biotechnology Co.,Ltd.,Shanghai,China) following the manufacturer’s procedure.Serum pepsinogen Ⅰ(PGI) and Ⅱ (PGII)levels were measured using ELISA kits (Shanghai Yuan Mu Biotechnology).The color reaction was measured using a microplate ELISA reader (MR 700 Microplate Reader,Thermo Fisher Scientific,Waltham,MA,USA).
2.3.6.Concentrations of interleukin-6 (IL-6) and IL-8
Serum IL-6 and IL-8 concentrations were measuredviaELISA.After anticoagulation of rat blood samples (3 mL)with heparin sodium,rat blood was cryopreserved and centrifuged at 4 ℃ for 15 min at 3500 rpm.The supernatant was obtained and used according to the instruction manual.
2.3.7.Hematoxylin and eosin (HE) staining
Gastric antrum tissues were fixed in 4% paraformaldehyde for 24 h,embedded in paraffin,cut into five 4-μm-thick pieces,and stained with HE.The histopathological changes of gastric mucosa were observed under a microscope.
2.3.8.Detection of TLR4 and TRIF mRNA expression
Total RNA was extracted according to the procedure of the TRIzol total RNA extraction kit (Tiangen,Beijing,China).According to the instructions of the MMLV reverse transcription kit (Promega,CA,USA),mRNA levels were detected using the SYBR Green Ⅰ qPCR method.
The PCR reaction:pre-denaturation at 95 ℃ for 2 min,denaturation at 95 ℃ for 30 s,annealing at 60 ℃ for 40 s,extension at 72 ℃ for 45 s,40 cycles at the above temperature,final extension at 72 ℃ for 5 min.RNA levels were determined using an ND2000 C ultramicro spectrophotometer (Thermo Fisher Scientific,Waltham,MA,USA).Using the Ct value as target gene expression and GAPDH as an internal control,the 2−ΔΔCtmethod was used to calculate the relative expression of target genes.The primers used in this study are listed in Table 1.
2.3.9.Detection of TLR4 and TRIF protein expression
TLR4 and TRIF protein expression was detected by western blotting using the conventional method.Sodium dodecyl sulfate polyacrylamide gel electrophoresis was conducted,followed by protein transfer to a nitrocellulose membrane. Enhanced chemical fluorescein imaging was performed,and radiographs were taken.Image analysis was performed using Quantity One software (Bio-Rad,Hercules,USA).The ratio of target bands to gray levels of β-actin was calculated.
2.4.Statistical analysis
The data were analyzed using SPSS version 22.0 (IBM,Armonk,NY,USA).Data are expressed as the mean ±standard deviation ().Multiple comparisons were performed by one-way analysis of variance and the least significant difference test.The classification data were analyzed using the rank sum test.P<0.05 indicated statistical significance.
3.RESULTS
3.1.Rat mortality
In total,three rats died during the experiment while exhibiting extreme lethargy and emaciation.In particular,two rats in the CAG group died in the late period of the modeling process.In addition,one rat in the M group died during treatment,but no deaths occurred in other groups.After dissecting tissues from CAG rats,we found that the gastric mucosa was shallow and thinner.The arrangement of the lamina propria was disordered,and its abundance was decreased,accompanied by atypical hyperplasia or mild intestinal metaplasia.
3.2.Efficacy of QHY
3.2.1.Pathological examination
Compared with the M group findings,improvement was observed in the four experimental groups,with the best results noted in the HT group.Microscopically,HT rats exhibited a substantially higher number of glands in the gastric mucosa,and they were arranged neatly.Meanwhile,the mononuclear cells in the lamina propria were fewest in this group (Figure 1).
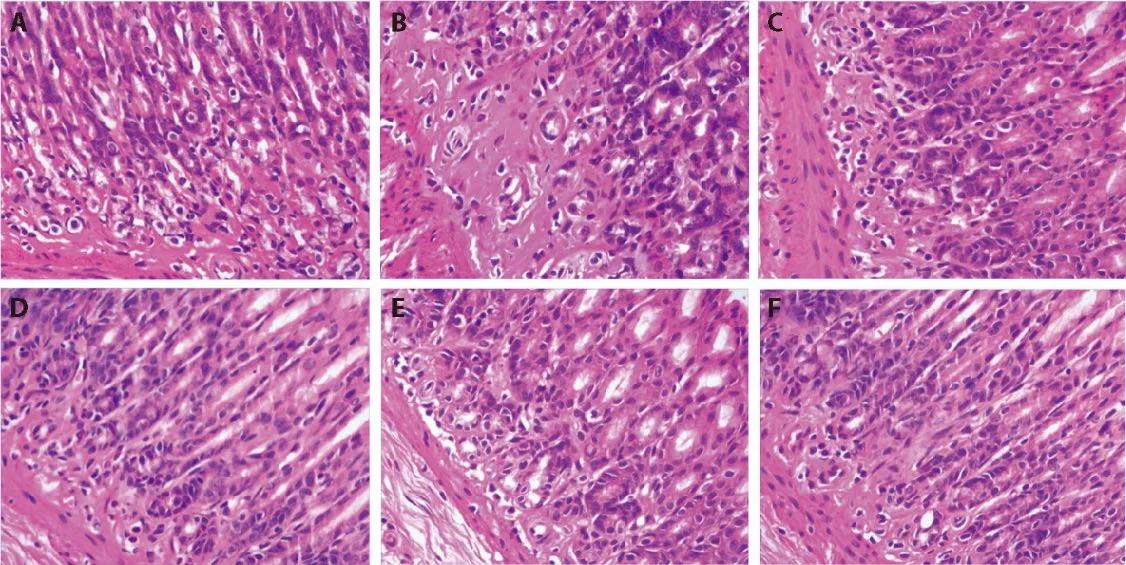
Figure 1 Representative images of the gastric mucosa in the groups
3.2.2.Biochemical indices
Both GAS levels and the PGI/PGII ratio,which are typical indicators of CAG,were significantly lower in the M group than in the C group.GAS levels were significantly lower in the M and LT groups than in the C group.Compared with the M group,GAS expression was significantly increased in the MT and HT groups (Table 2).The PGI/PGII ratio was significantly increased after high-dose QHY treatment (Table 2).These findings demonstrated that QHY treatment improved CAG.
3.2.3.QHY suppressed IL-6 and IL-8 expression
ELISA revealed that serum IL-6 and IL-8 expression was significantly higher in the M group than in the C group(P <0.01).Compared with the M group findings,serum IL-6 and IL-8 levels were significantly decreased in all four experimental groups (P <0.01),and the reduction was most obvious in the HT group.This may be attributable to the inhibition of various inflammatory signaling pathways,resulting in the suppression of serum inflammatory factor levels (Table 3).
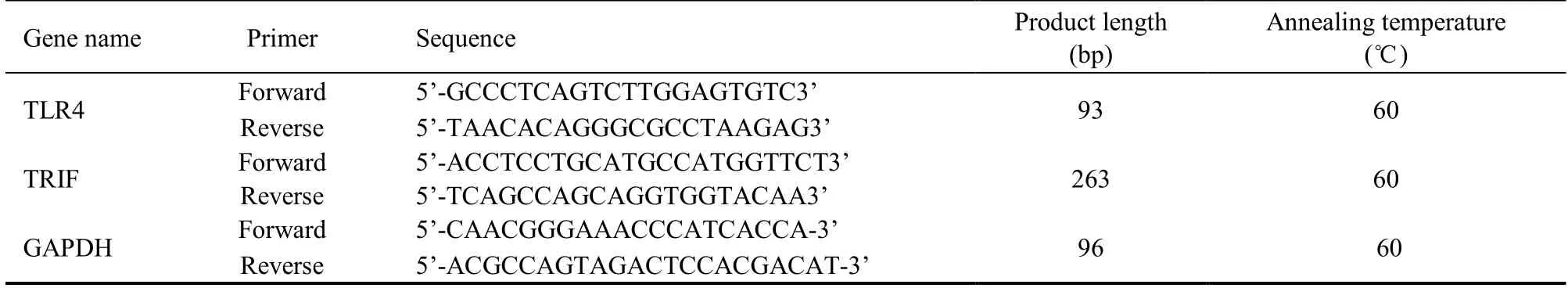
Table 1 Polymerase chain reaction primers information
Table 2 Serum biochemical indexes ()
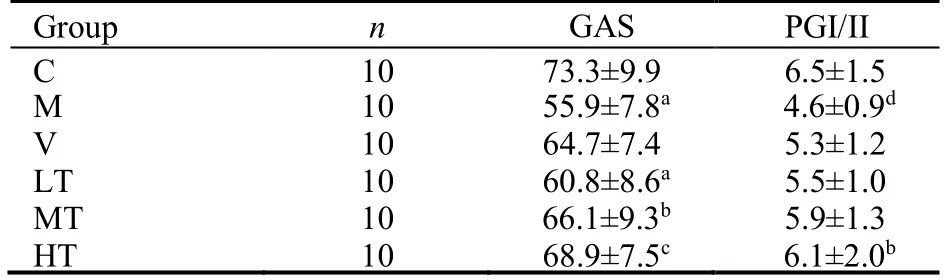
Table 2 Serum biochemical indexes ()
Notes:the therapeutic doses in the LT,MT,and HT groups were 1.5,3.0,and 6.0 mL·kg−1·d−1,respectively.The rats in the V group were treated with 0.3 mL·kg−1·d−1 vitacoenzyme.Rats in the C and M groups received normal saline via gavage.Treatments were administered for 30 d.C:control group;M:model group;HT:high-dose Qinghuayin (QHY) group;MT:medium-dose QHY group;LT:low-dose QHY group;V:vitacoenzyme group.aP <0.01,dP <0.05,vs C group;bP <0.05,cP <0.01 vs M group.
Table 3 Serum interleukin (IL)-6 and IL-8 levels in the different groups (pg/mL,)
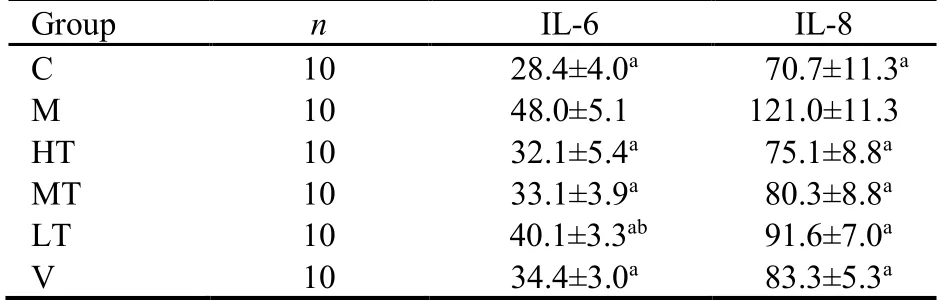
Table 3 Serum interleukin (IL)-6 and IL-8 levels in the different groups (pg/mL,)
Notes:the therapeutic doses in the LT,MT,and HT groups were 1.5,3.0,and 6.0 mL·kg−1·d−1,respectively.The rats in the V group were treated with 0.3 mL·kg−1·d−1 vitacoenzyme.Rats in the C and M groups received normal saline via gavage.C:control group;M:model group;HT:high-dose Qinghuayin (QHY) group;MT:medium-dose QHY group;LT:low-dose QHY group;V:vitacoenzyme group.Treatments were administered for 30 d.aP <0.001 vs M group;bP <0.05 vs M group.
On the basis of pathological findings and changes in serum IL-6 and IL-8 levels,we confirmed the efficacy of QHY against CAG in animal models.
3.2.4.Preliminary observations of the effects of QHY on TRIF-dependent signaling pathways
In the present study,we preliminarily examined the mRNA and protein expression of TLR4 and TRIF.
3.3.Comparison of TLR4 and TRIF mRNA expression among the groups
qRT-PCR revealed that TLR4 and TRIF mRNA expression was significantly higher in the M group than in the C group (P <0.01).Compared with the M group data,TLR4 and TRIF mRNA expression was decreased by treatment with QHY or vitacoenzyme (P <0.01),with the strongest effects observed in the HT group (Table 4).
Table 4 mRNA expression of TLR4 and TRIF in the different groups ()
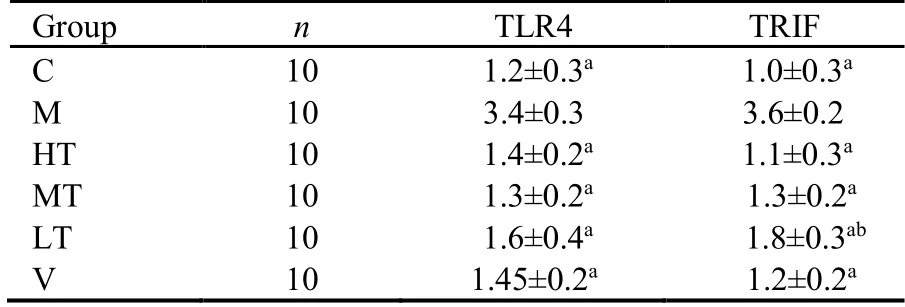
Table 4 mRNA expression of TLR4 and TRIF in the different groups ()
Notes:A and B present the gene expression of TLR4 and TRIF,respectively.The rats in the V group were treated with 0.3 mL·kg−1·d−1 vitacoenzyme.Rats in the C and M groups received normal saline via gavage.Treatments were administered for 30 d.C:control group;M:model group;HT:high-dose Qinghuayin (QHY) group;MT:medium-dose QHY group;LT:low-dose QHY group;V:vitacoenzyme group.The therapeutic doses in the LT,MT,and HT groups were 1.5,3.0,and 6.0 mL·kg−1·d−1,respectively.TLR4:toll-like receptor 4;TRIF:toll or interleukin-1 receptor domain-containing adaptor inducing interferon-β.aP <0.001 vs M group;bP <0.01 vs V group.
3.4.Comparison of TLR4 and TRIF protein expression among the in each group after intervention
Western blotting revealed that TLR4 and TRIF levels were lowest in the C group.Conversely,TLR4 and TRIF protein expression was elevated in the M group and decreased by treatment with QHY or vitacoenzyme.Compared with the M group,the largest decreases in TLR4 and TRIF protein levels were recorded in the HT group (Table 5).
Table 5 Protein expression of TLR4 and TRIF in the different groups ()
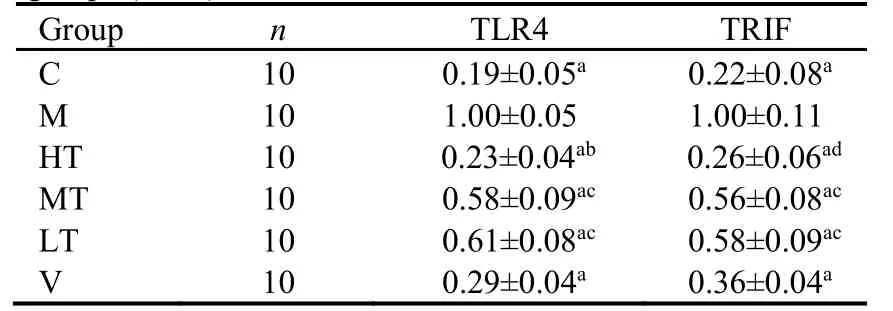
Table 5 Protein expression of TLR4 and TRIF in the different groups ()
Notes:the therapeutic doses in the LT,MT,and HT groups were 1.5,3.0,and 6.0 mL·kg−1·d−1,respectively.The rats in the V group were treated with 0.3 mL·kg−1·d−1 vitacoenzyme.Rats in the C and M groups received normal saline via gavage.Treatments were administered for 30 d.C:control group;M:model group;HT:high-dose Qinghuayin (QHY) group;MT:medium-dose QHY group;LT:low-dose QHY group;V:vitacoenzyme group.TLR4:toll-like receptor 4;TRIF:toll or interleukin-1 receptor domaincontaining adaptor inducing interferon-β.aP <0.001 vs M group;bP <0.05,cP <0.001,dP <0.01,vs V group.
4.DISCUSSION
CAG may present with symptoms of dyspepsia,vitamin B12 and/ or iron deficiency anemia,and autoimmune diseases,especially autoimmune thyroid diseases,8-10characterized by intestinal metaplasia,pseudopyloric metaplasia and/ or fibrosis instead of primitive gastric glands.11Its close relationship with gastric cancer has led to its classification as a precancerous disease.1The diagnosis rate has increased over time because ofchanges in diet,lifestyle,medical concepts,electronic gastroscopy,and other factors.At present,there is no definite intervention with curative effects.
In this study,we confirmed the effectiveness of QHY by examining pathological sections and measuring IL-6 and IL-8 levels in a rat model of CAG.We also performed a preliminary observation to determine the effects of QHY on TRIF signaling pathways and found that the downregulation of inflammatory signaling pathways may play a key role in the mechanisms of QHY,which requires further verification.
Pathological examination revealed the pathological changes of CAG in a rat model,such as gastric gland atrophy,intestinal metaplasia,and low-grade intraepithelial neoplasia,which indicates the successful establishment of the model.Then,high,medium,and low doses of QHY and vitacoenzyme were used as interventions,and the results demonstrated the effects of each modality on pathological changes associated with CAG in rats.Notably,high-dose QHY more substantially improved the observed pathological changes than the other treatments.In addition,the treatment also reversed pathological changes,characterized by low-grade intraepithelial neoplasia,the disappearance of intestinal metaplasia,and gastric gland thickening.
Conversely,more researchers are paying attention to the roles of the TLR signaling pathway and downstream factor TRIF in the mechanism of CAG progression.TLRs,a class of type I transmembrane proteins,received their name because their extracellular domains are homologous to the toll protein inDrosophila.TLRs can recognize exogenous ligands and certain endogenous ligands,12such as lipopolysaccharide in the outer membrane of gram-negative bacteria and its derivatives with a conserved lipid A structure.Among the TLRs,TLR4 is the most closely associated with the occurrence and development of CAG.6,13When activated,TLR4 activates two downstream signaling pathways:MyD88-dependent and MyD88-independent (TRIF-dependent)signal pathways.14
In the TRIF-dependent signaling pathway,the TIR domain of TLR4 binds to the ligand through TRAM and TRIF,and the N-terminal TRAF-binding domain binds to TRAF-3.TRAF-3 is an E3 ubiquitin ligase that can activate TBK1 and IKK-i complexesviaself-ubiquitin at K63.TBK1/IKK-i phosphorylation leads to the phosphorylation of downstream IRF3 and dimerization in the nucleus,which results in the induction of IFN-β production and expression of the interferon (IFN) gene.This case eventually leads to the overexpression of tumor necrosis factor-α,IFN-β,IL-2,IL-6,and IL-8 and a series of inflammatory factors.15
This study demonstrated that TLR4 and TRIF mRNA and protein levels were significantly higher in the M group than in the C group (P<0.01).Meanwhile,IL-6 and IL-8 levels were significantly increased in animals with CAG (P<0.01),which suggests that gastric mucosa atrophy might be accompanied by persistent TLR4 and TRIF activation and elevated IL-6 and IL-8 expression.TRIFs may play an essential role in the MyD88-independent signaling pathway and provide new targets for the clinical treatment of CAG.In this study,we observed a significant increase in TLR4/TRIF levels in the gastric mucosa in the M group,which suggests the existence of this therapeutic target.The data also suggested that QHY may induce its effects by suppressing the upregulation of inflammatory signaling pathways,but this requires further validation.
In conclusion,these results indicated that QHY can effectively improve the pathological changes of the gastric mucosa in rats with CAG.Compared with the M group findings,TLR4/TRIF mRNA and protein levels were significantly decreased by all doses of QHY,and IL-6 and IL-8 concentrations were decreased,especially in the HT group.Therefore,we conclude that the therapeutic effect of QHY on CAG in rats may be achieved through the downregulation of TLR4/TRIF in tissues.
5.ACKNOWLEDGMENTS
We thank Joe Barber Jr.,from Liwen Bianji,Edanz Editing China (www.liwenbianji.cn/ac),for editing the English text of a draft of this manuscript.
猜你喜欢
——四种敦煌写本别字异文所见
杂志排行
Journal of Traditional Chinese Medicine的其它文章
- Mechanisms of immune regulation for acupuncture on chronic respiratory diseases
- Mechanism of Huashi Xingyu Qingre recipe (化湿行淤清热方) in treating oral lichen planus based on network pharmacology and clinical trial verification
- Anti-inflammatory mechanism of rhein in treating asthma based on network pharmacology
- An international multicentric phase Ⅲ randomized controlled trial of time-acupoints-space acupuncture for the prevention of chemotherapy-induced fatigue in patients with early stage breast cancer:a study protocol
- Mining intrinsic information of convalescent patients after suffering coronavirus disease 2019 in Wuhan
- Menstrual cycle characteristics as an indicator of fertility outcomes:evidence from prospective birth cohort study in China
