Anti-inflammatory mechanism of rhein in treating asthma based on network pharmacology
2022-07-28FENGJunfangCHENOuWANGYibiao
FENG Junfang,CHEN Ou,WANG Yibiao
FENG Junfang,WANG Yibiao,Department of Pediatrics,the Second Hospital,Cheeloo College of Medicine,Shandong University,Jinan 250033,China
CHEN Ou,School of Nursing,Cheeloo College of Medicine,Shandong University,Jinan 250012,China
Abstract OBJECTIVE:To predict the anti-inflammatory targets and related pathways of rhein in the treatment of asthma by using network pharmacology,and to further explore its potential mechanism in asthma.METHODS:The corresponding targets of rhein were obtained from the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP),and the rhein-target network was constructed with Cytoscape 3.7.1 software.The Genbank and Drugbank databases were used to collect and screen asthma targets,and the rhein-target-disease interaction network was constructed.A target protein-protein interaction (PPI)network was constructed using the STRING database to screen key targets.Finally,Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis was used to identify biological processes and signaling pathways.The anti-asthmatic effects of rhein were tested in vitro,and the expression levels of proteins in the mitogen-activated protein kinase/nuclear factor kappa-B (MAPK/ NF-κB)signaling pathway were assessed by western blot analysis.RESULTS:Altogether,83 targets of rhein were screened in the relevant databases,989 targets of asthma were obtained in the National Center for Biotechnology Information (NCBI) GENE Database.PPI network analysis and KEGG pathway enrichment analysis predicted that rhein could regulate the epidermal active growth factor receptor (EGFR),mitogen-activated protein kinase 14 (MAPK14),tumour necrosis factor receptorsuperfamily member 1A (TNFRSF1A),receptor tyrosineprotein kinase erbB-2 (ERBB2),and other signaling pathways.Furthermore,we selected the MAPK signaling pathway to determine the anti-inflammatory effects of rhein.Consistently,further in vitro experiments demonstrated that rhein was shown to inhibit HBE cells inflammation.CONCLUSION:The anti-inflammatory mechanism of rhein in the treatment of asthma may be related to EGFR,MAPK14,TNFRSF1A and ERBB2 as well as their signaling pathways.To prevent the exacerbation of asthma,instead of targeting a single pathway or a single target,all these targets and their signaling pathways should be controlled holistically.Rhein may alleviate the inflammation of asthma by inhibiting the MAPK/NF-κB pathway.
Keywords:asthma; rhein; anti-inflammatory agents;pharmacology;drug development
1.INTRODUCTION
Asthma is an abbreviation of bronchial asthma.Asthma is a kind of chronic inflammatory disorder of the airways involving cells and corresponding components,and it is characterized by inflammation,hyperresponsiveness,stenosis and airway remodelling.Chronic inflammation is considered the hypostasis of asthma.1
Rhein is an effective monomer component,and it is separated and purified from traditional Chinese medicines,such as rhabarb.Rhein is a monanthraquinone 1,8-dihydroxyl anthraquinone derivative.Rhein has anti-inflammatory,antibacterial,antitumour and other effects.2,3However,there are relatively few reports on the anti-inflammatory efficacy of rhein in the treatment of asthma.
Network pharmacology has become an emerging research method in recent years.It is considered a new model for the next generation of drug research.The construction of biomolecule networks,such as the drugtarget-pathway,is the basis of network pharmacology.4,5Network pharmacology can explore the efficacy of drugs from the component-target-pathway,and the pathogenesis of diseases can also be reversed through drugs with known efficacy.6Hence,we used network pharmacology to explore the anti-inflammatory mechanism of rhein in the treatment of asthma and to provide new ideas for treating asthma.
2.MATERIALS AND METHODS
The Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform 2.3 (TCMSP),PubChem 1.2,Therapeutic Target Database 2020 (TTD),STITCH 5.0,DrugBank databases 5.1.7 were used to search for the chemical composition of the drug and its targets.Proteins related to diseases,and protein-protein interaction information can be found in the GenBank 12.21 and STRING Databases 11.0.We searched signaling pathways related to biomolecules in the Enrichr and Kyoto Encyclopedia of Genes and Genomes(KEGG).7Cytoscape 3.7.1 software and systemsdock 4.2.6 were used for network construction and molecular docking respectively.
2.1.Prediction of the targets of rhein
The data about the three-dimensional chemical structure of rhein was searched and exported from the TSCM 2.3 and PubChem 1.2.Then the data was imported into the Swiss target prediction database 2019,and reverse molecular docking was performed.Predictive targets were obtained by using Homo sapiens as the target set.The results were used in further studies.
2.2.Molecular docking studies of rhein and its target proteins
The Protein Data Bank-ID (PDB-ID) of the rhein-target proteins was queried in the PDB database 1.1.The data was imported into systemsDock for molecular docking studies.The docking score was used to determine the matching degree between rhein and the target.The score ranged from 0-10,and the greater the value was,the stronger the interaction was.8
2.3.Construction of the rhein-target network
A drug-target interaction network of rhein and its potential targets were constructed by using Cytoscape 3.7.1software.
2.4.Construction of the rhein-target protein interaction network
By using STRING Database,the rhein-target protein interaction network was constructed by setting the protein type to Homo sapiens,setting the minimum interaction threshold to medium confidence,and keeping the remaining parameters silent.
2.5.Anti-inflammatory target protein screening
In the TTD 2020,information on the anti-inflammatory target proteins was searched by using “anti-inflammatory”as the keyword,nine anti-inflammatory target proteins were selected for subsequent use,and these target proteins were put into Cytoscape 3.7.1 software to construct an anti-inflammatory target protein-protein interaction (PPI) network.9
2.6.Screening of the anti-inflammatory targets of the rhein effect and construction of an anti-inflammatory target network
By choosing Multiple proteins,setting organism to Homo sapiens,the rhein-target proteins and the antiinflammatory proteins were imported into the STRING Database to construct the network.Screening values above 0.7 indicated a high confidence of protein interactions.
2.7.Search for asthma-related proteins in humans
The GenBank database of the National Center for Biotechnology Information (NCBI) was used to search for asthma-related targets by using “asthma and Homo sapiens” as the keywords,and the asthma-related proteins of a human being can be acquired.
2.8.Network of the anti-inflammatory targets of rhein in the treatment of asthma
The anti-inflammatory target proteins of rhein and human asthma-related proteins were imported into the Cytoscape 3.7.1 software to build the “disease-target”network,and to screen the anti-inflammatory targets related to the incidence of asthma.
2.9.KEGG pathway enrichment of the target genes
The Enrichr was used to analyse the KEGG biological pathway enrichment of the target genes to predict the anti-inflammatory targets of rhein.
2.10.Drug and reagents
Rhein (purity ≥ 99%) was provided by MedChem-Express Company (Shanghai China).The human bronchial epithelial (HBE) cell line imported from the United States was obtained from the American type culture collection (Beijing,China).Cell culture reagents including fetal bovine serum (FBS),Roswell Park Memorial Institute-1640 (RPMI-1640) medium,and antibiotic+amphiphilic solution,dimethyl sulfoxide(DMSO),ovalbumin (OVA,grade V) were supplied by Sigma-Aldrich Company (Missouri,USA).Lipopolysaccharide (LPS) was purchased from Solarbio Technology Company (Beijing,China),NF-kBp65(A2547),p38MAPK (A1401),p-p38MAPK (AP0297),β-Tubulin (AS014) were provided by ABclonal Technology Company (Wuhan,China).
2.11.Exploration of safe doses of rhein in HBE cells
Cell viability was evaluated by the cell counting kit-8(CCK-8) assay.The drug dose was selected within a range nontoxic to cells,and this dose ensured comprehensive accurate results of the subsequent experiments.
2.12.Culture and treatment of HBE cells
The HBE cell lines were previously obtained from the American type culture collection.The cells were cultured in appropriate bottles with sterile RPMI-1640 medium supplemented with 1% antibiotic+amphiphilic solution and 10% foetal bovine serum and incubated at 37 °C in a humidified atmosphere containing 5% CO2and 95%atmospheric air for 24 h.The culture medium was changed every 2 d.Cells were grown to 80% confluence prior to treatment.Rhein was dissolved in culture grade dimethyl sulfoxide (final concentration <0.1%) in serum-free media.To investigate the protective effect of rhein,primary cultured HBE cells were treated with 0.1,0.5,and 1.0 μM of rhein for 2 h and then treated with 100 μg/mL LPS+1 μg/mL OVA for 24 h while the OVA+LPS group received 100 μg/mL LPS+1 μg/mL OVA treatment alone.The inflammation model refers to the reference.10
2.13.Cell viability assay
Cell viability was evaluated by using the CCK-8 assay.HBE cells (2 × 105cells in 200 μL of RPMI medium +2% FBS per well in a 96-well plate) were treated with rhein at concentrations of 0.1,0.5 and 1.0 µM.After 24 h,the supernatant was removed,and 100 μL of the medium was added with CCK-8 solution to form crystals of formazan in the viable cells.The plate was incubated for 4 h.The wells were immediately analysed in a spectrophotometer at a wavelength of 450 nm.Cell viability (%) was expressed as a percentage relative to the untreated control cells.
2.14.Western blot analysis
To observe the inflammation prevention effects of rhein on inflammation induced HBE cells,we treated cell samples with rhein (0.1 to 1 μM) and induced inflammation by applying LPS (1 μg/mL)+OVA(0.1 mg/mL).MAPK/NF-κB activation,which plays an important role in inflammatory responses,was analysed by western blot analysis to evaluate the effects of rhein in treating HBE cells.Cells were lysed with Radio immunoprecipitation (RIPA) lysis buffer including protease inhibitor (Solarbio,Beijing,China).Total protein concentration of obtained extract was quantified with a bicinchoninic acid (BCA) protein assay kit (Pierce,Appleton,WI,USA).After separation by sodium dodecyl sulphate polyacrylamide gel electrophoresis(SDS-PAGE),the protein was transferred onto a polyvinylidene difluoride (PVDF) membrane (Millipore,Temecula,CA,USA).Afterwards,the membrane was blocked with 5% milk for 1 h,and then incubated with primary antibodies against NF-kB p65 (A2547),p38MAPK (A1401),p-p38MAPK (AP0297),β-Tubulin(AS014) (all purchased from ABclonal) overnight at 4 ℃.
The primary antibodies were diluted with 5% milk at a dilution of 1 ∶1000.Subsequently,the primary antibodies were probed with the secondary antibody HRP Goat Anti-Rabbit IgG (H+L) (AS014) (1∶3000;ABclonal Technology Company,Wuhan China) for 1h at room temperature.Subsequently,the signals of protein bands were captured with Image LabTMSoftware ((Bio-Rad,Hercules,CA,USA).
2.15.Statistical analysis
Descriptive statistics were performed using Graphpad Prism 7.0 (Graphpad Software Company,the United States of America).The measurement data is expressed as the mean ± standard deviation.Comparisons among groups were performed by one-way analysis of variance.Comparisons of two groups were performed byt-test.P<0.05 was considered statistically significant.
3.RESULTS
3.1.Rhein and its predicted targets
Eighty-three predicted targets of rhein were searched in STITCH,DrugBank databases.A drug-target interaction network of rhein and its potential targets were constructed by using Cytoscape 3.7.1 software in Figure 1.
3.2.Rhein-target network results
The rhein-target protein interaction networks were constructed by using STRING Database in Figure 2.
3.3.Network of rhein anti-inflammatory targets during treatment of asthma
The merge function of Cytoscape 3.7.1 software(National Resource for Network Biology) was used to combine the rhein-predicted target network with the antiinflammatory target PPI network in the TTD database,considering the overlapping results and a high confidence interval above 0.7,and the results showed that EGFR,MAPK14,TNFRSF1A and ERBB2,related to asthma,were anti-inflammatory targets of rhein in Figure 3.
3.4.KEGG pathway enrichment of the target genes
According to the KEGG pathway enrichment of the target genes,the anti-inflammatory target proteins of rhein associated with asthma were involved in 87 signaling pathways.The Enrichr analysis results were sorted in descending order of the combined score.The top ten pathways included the MAPK signaling pathway,hepatitis C signaling pathway,epithelial cell signaling during Helicobacter pylori infection,immune signaling pathway,and proteoglycans in cancer signaling pathway andet alin Figure 4.
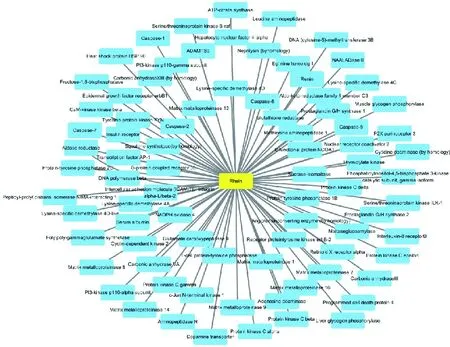
Figure 1 Rhein and its predicted targets eighty-three predicted targets of rhein were found in the search tool for interactions of chemicals(STITCH) database,Drugbank database
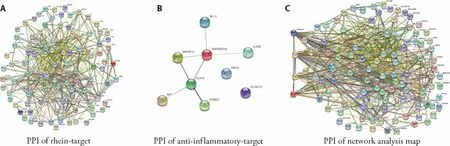
Figure 2 Rhein-target network results
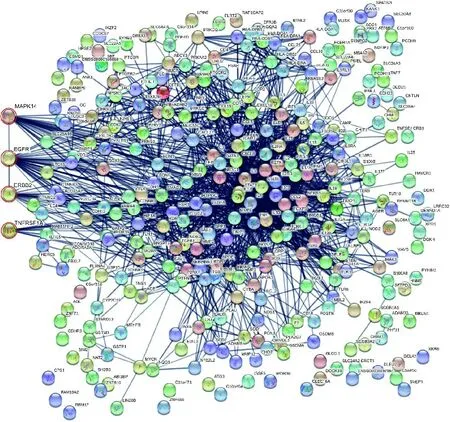
Figure 3 Network of rhein anti-inflammatory targets during treatment of asthma
3.5.Reverse docking scores of rhein and its related targets
The PDB-IDs were imported into systemsDock for molecular docking studies.The targets of rhein included CREB1,ERK1,SRC,RasH,PAK1,HRas,PDK1,Grb2,MP2K1,Myc,EGFR,MEK1,and their docking scores are 8.785,8.476,8.254,8.015,8.012,7.506,7.408,7.085,6.972,6.446,6.032 and 5.643 respectively.The docking score was above 5,verified the reliability of the predicted targets.
3.6.Cytotoxicity of rhein on HBE cells
To examine the cytotoxicity of rhein on HBE cells,the cells were treated with various concentrations of rhein (0 to 200 μM) in RPMI medium and fetal bovine serum(FBS) for 24h,and the cell viability were evaluated by CKK-8 assay.Data represent the mean ± standard deviation of three independent experiments,each experiment was performed in triplicate.Cell viability of each group:0 μM (100% ± 0%);10 μM (105.2% ± 4.21%)vs0 μM,P=0.2972;20 μM (102.3% ± 3.43%)vs0 μM,P=0.5744;40 μM (96.82% ± 1.25%)vs0 μMP=0.056;100 μM (85.77% ± 3.1%)vs0 μM,P=0.0096;200 μM(64.09% ± 4.72%)vs0 μM,P=0.0016.P <0.05 was considered statistically significant.There was no observed cytotoxicity of rhein in the HBE cells when the final concentration of rhein was less than 40 μM.For rhein,all concentrations (below 1.0 mg/mL) used in this research did not affect with the survival of HBE cells.
3.7.Inflammation prevention effects of rhein on HBE cells via MAPK/NF-κB pathway
The results showed that rhein treatment dose dependently inhibited phosphorylation of MAPKp38 and significantly reduced the expression of NF-κB of p65 in the HBE-cell activated MAPK/NF-κB pathway by LPS +OVA recombinant.Rhein treatment is associated with the MAPK/NF-κB pathway,and rhein would prevent inflammation in Figure 5.
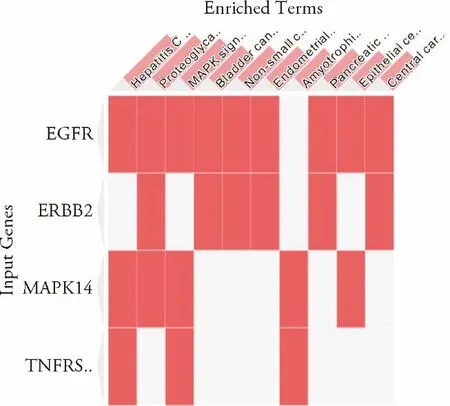
Figure 4 KEGG pathway enrichment of the target genes
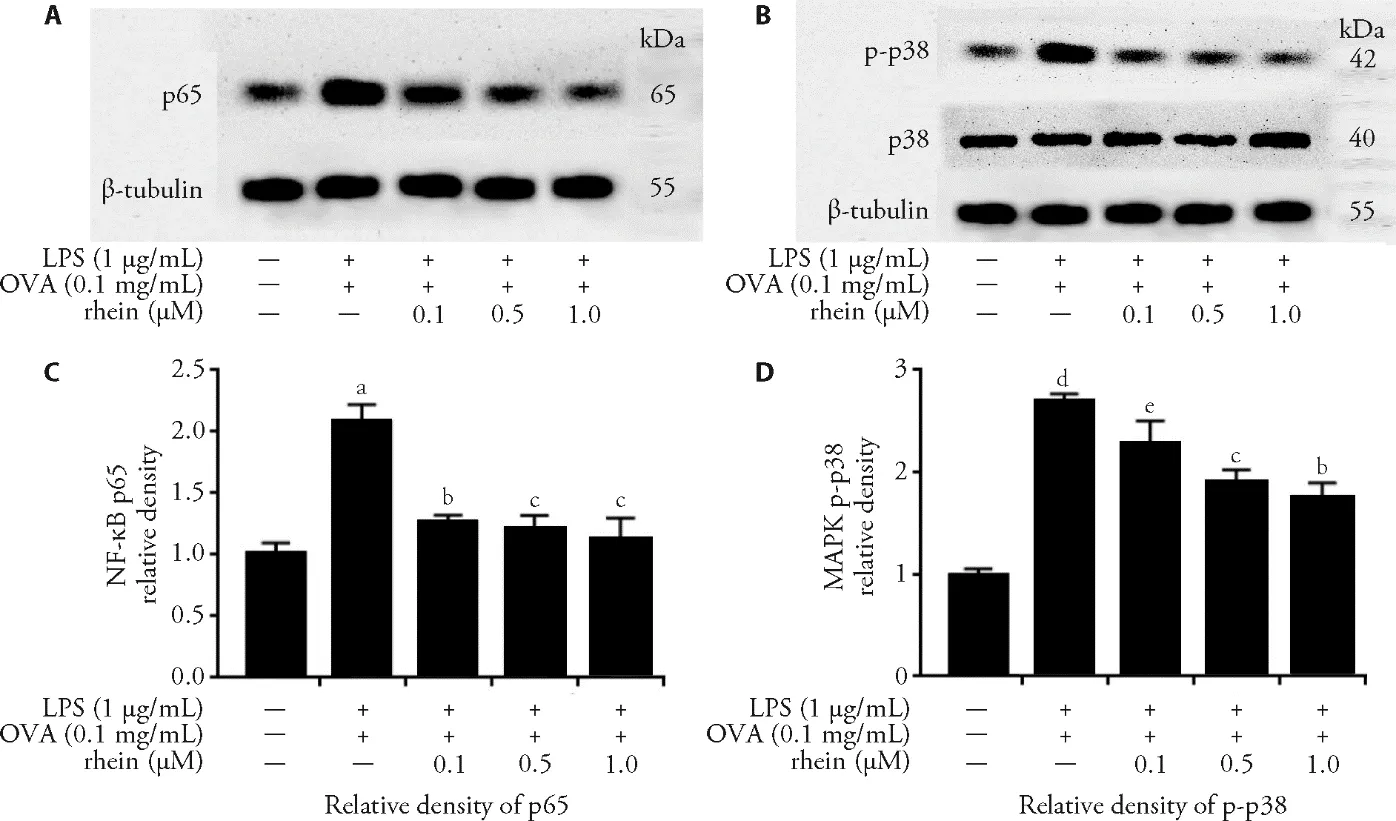
Figure 5 Inflammation prevention effects of rhein on human bronchial epithelial (HBE) cells via mitogen-activated protein kinase/ nuclear factor-kappa B (MAPK/NF-κB) pathway
4.DISCUSSION
It has been reported that rhein has anti-inflammatory activities.11The results of this study show the antiinflammatory effects of rhein.Xu Youdong explained the anti-inflammatory effects of rhein based on its molecular mechanism.12EGFR,MAPK14,TNFRSF1A,ERBB-2 are the main targets of the anti-inflammatory effects of rhein.Rhein can directly act on EGFR,MAPK14,TNFRSF1A and ERBB2 and can also indirectly act on other targets to exert its antiinflammatory effects.13,14
EGFR is an epidermal growth factor receptor that is widely distributed in epithelial tissues and plays an important regulatory role in the development of respiratory inflammation.15,16EGFR inhibitors can effectively inhibit acute inflammation of the rat respiratory tract caused by exogenous zinc ions.17EGFR inhibitors reduce the symptoms of allergic asthma caused by dust mites by reducing the production of proinflammatory factors,such as IL-6 and IL-8.18Thus,it is speculated that rhein interacts with EGFR,blocking the binding of EGFR to pro-inflammatory cytokines,to exert an anti-inflammatory effect.
MAPK14,which is also called p38α,is one of the four p38 MAP kinases in mammals.It plays an important role in the cellular cascade triggered by pro-inflammatory cytokines or extracellular stimuli and is a key signaling molecule in the lung inflammation induced byS.pneumonia.19Inflammatory factors,such as IL-1β,IL-6 and TNF-α,can positively regulate signaling pathways,like ERK and nuclear factor-κB (NF-κB),by activating the p38 MAPK signaling pathway and cascading to amplify the inflammatory response.20The transcriptional cascade regulated by P38 MAPKs leads to the production of pro-inflammatory factors,such as TNF-α and IL-1β,which in turn leads to the activation of enzymes involved in inflammation.21It is speculated that rhein exerts its anti-inflammatory effect by inhibiting the release of proinflammatory factors,such as TNF-α and IL-1β,by inhibiting MAPK14.
TNFRSF1A is a type 1 TNF receptor that mediates inflammatory responses mainly by activating NF-κB,p38,and ERK1/2 to induce IL-6 and IL-8 synthesis and apoptosis.22It is thus concluded that rhein interacts with TNFRSF1A to reduce the secretion of anti-inflammatory factors,such as IL-6,and exert an anti-inflammatory effect.
ERBB2,which is called HER2,NEU and CD340,is a 185-kDa cell membrane receptor encoded by the protooncog-ene erbB2 and is a member of the EGFR family.ERBB2 can induce IL-6 autocrine activity,which in turn affects t-he JAK-STAT pathway or NF-κB pathwaymediated inflammatory responses.23It is speculated that rhein interacts with EERB2 and may exert antiinflammatory effects by regulating the secretion of IL-6.24,25
In this study,the anti-inflammatory mechanism of rhein was confirmed by network pharmacology.The main targets of the anti-inflammatory effect of rhein and the related signaling pathways were predicted.Rhein exerted an anti-inflammatory effect by acting on multiple targets.However,network pharmacology research is based on network modelling,database resource development and software application.The network model has certain differences from the in vivo environment.
The results from our present study clearly displayed that a signaling nexus involving rhein,MAPK/NFκB pathway and inflammation in the HBE cells.We have demonstrated that rhein can reduce the expression of phosphorylation-p38 and p65 induced by LPS+OVAviathe MAPK/NF-κB pathway in HBE cells,and further reduce the inflammatory responses.26However,further investigation is still needed as there are some limitations in our study.The main limitation of our study is that we did not examine the anti-asthmatic role of rhein in vivo.Based on network pharmacology analysis,rhein is likely to alleviate asthma through different mechanisms,such as anti-inflammatory activity.Therefore,further study should be performed to verify the role of rhein in asthmatic animal models.
Our findings indicate that rhein may not only have the anti-inflammatory function by regulating MAPK14,EGFR,EERB2 and TNFRSF1A,but also have the effect of treating asthma by regulating the MAPK/NF-κB signaling pathway.Additionally,the expression of MAPK/NF-κB pathway proteins was validated in HBE cell lines by Western blot.This study reinforces the idea that rhein can be considered a relevant candidate for the treatment of inflammatory allergic airway disease and,at least in HBE cells,rhein did not induce toxic effects.
杂志排行
Journal of Traditional Chinese Medicine的其它文章
- Mechanisms of immune regulation for acupuncture on chronic respiratory diseases
- Mechanism of Huashi Xingyu Qingre recipe (化湿行淤清热方) in treating oral lichen planus based on network pharmacology and clinical trial verification
- An international multicentric phase Ⅲ randomized controlled trial of time-acupoints-space acupuncture for the prevention of chemotherapy-induced fatigue in patients with early stage breast cancer:a study protocol
- Mining intrinsic information of convalescent patients after suffering coronavirus disease 2019 in Wuhan
- Menstrual cycle characteristics as an indicator of fertility outcomes:evidence from prospective birth cohort study in China
- Effectiveness and safety of red yeast rice predominated by monacolin K β-hydroxy acid form for hyperlipidemia treatment and management
