An international multicentric phase Ⅲ randomized controlled trial of time-acupoints-space acupuncture for the prevention of chemotherapy-induced fatigue in patients with early stage breast cancer:a study protocol
2022-07-28SaghachianMahastiBarryCarolineAsselainBernardLIYunfenNIEJianyunLIUDequanCHENDedianCHENChunxinLauraEstevezLIWenhuiJeanPierreArmandZHUMianshengJULiya
Saghachian Mahasti,Barry Caroline,Asselain Bernard,LI Yunfen,NIE Jianyun,LIU Dequan,CHEN Dedian,CHEN Chunxin,Laura Estevez,LI Wenhui,Jean-Pierre Armand,ZHU Miansheng,JU Liya
Saghachian Mahasti,Jean-Pierre Armand,Institute Gustave Roussy,Paris,France
Barry Caroline,Research Center of Epidemiology and public health,Paris,France
Asselain Bernard,Institute Curie of Paris,Paris,France
LI Yunfen,NIE Jianyun,LIU Dequan,CHEN Dedian,LI Wenhui,Yunnan Cancer Hospital,Third Affiliated Hospital of Kunming Medical University,Kunming,China
JU Liya,PreciMed Platform Europe,Paris,France
CHEN Chunxin,Laura Estevez,MD Anderson Cancer Hospital,Madrid,Spain
ZHU Miansheng,ARIATAS Association pour la Recherche et l’Information de l’ Acupuncture Time-Acupoints-Space,Paris,France;Yunnan Zhu's expert workstation,National famous traditional Chinese medicine expert of Mian-Sheng ZHU,Kunming,China
Abstract OBJECTIVE:To determine the difference in prevention of chemotherapy-induced fatigue between time-acupointsspace acupuncture (ATAS) administered weekly compared to sham acupuncture and non-acupuncture in patients with early breast cancer receiving adjuvant chemotherapy by epirubicine-cyclophosphamide (EC)followed by paclitaxel,as measured by the multidimensional fatigue inventory (MFI) over the previous week and Visual Analogue Scale measuring fatigue(VAS-F),and to evaluate the effects of ATAS on selfreported neuropathy pain,sleep,anxiety and depression.METHODS:In this multicenter clinical trial,we have randomized patients into 3 groups:ATAS,Sham and nonacupuncture with an unequal randomization of 2∶1∶1.A cloud related electronical clinical report form and smartphone platform was established for data entry.Patients with a history of stage Ⅰ-Ⅲ breast cancer scheduled to receive adjuvant chemotherapy.Acupuncture will be delivered once a week during chemotherapy with VAS-F evaluation.In order to qualify and quantify the mechanism of fatigue induced by chemotherapy with or without acupuncture,an evaluation of immune profiling was incorporate in this study.RESULTS:The presence and seriousness of chemotherapy-induced fatigue should be considered in therapeutic programs for early breast cancer treatment.Fatigue induced by adjuvant chemotherapy in patients with breast cancer remains a major concern affecting the quality of life significantly.Unfortunately,we do not have effective pharmacological interventions yet.And clinical trials of acupuncture in preventing chemotherapyinduced fatigue in patients with early breast cancer have not been reported.CONCLUSION:The findings of the trial will allow us to determine the effects of acupuncture treatment approach.We will also be able to confirm whether ATAS is better than sham acupuncture and non-acupuncture treatments.
Keywords:breast neoplasms;chemotherapy,adjuvant;fatigue;immunity;clinical protocols;time-acupoints-space acupuncture
1.INTRODUCTION
Breast cancer is the most common invasive cancer in women,affecting more than one million cases and over 411 000 deaths worldwide annually despite a decrease in overall mortality due to this disease.1There are around 50 000 new cases in France,more than 25 000 cases in Spain while near to 270 000 cases in China.2,3Today,breast cancer is viewed as a generalized systemic disease.Systemic therapy is consequently an integral part of the treatment of patients with early breast cancer.4The goal of this approach is to eliminate micro-metastases which are already present in the early stages of disease and thereby to prevent recurrence and increase the probability of cure.5The backbone of adjuvant chemotherapy treatment consists of a regimen which contains anthracyclines and taxanes.6However,chemotherapy carries severe side effects,such as bone marrow suppression,nausea,vomiting,depression,anxiety and particularly fatigue,that may worsen a patient's quality of life.
Fatigue is a major concern in cancer patients,it was estimated 50%-90% of cancer patients experience fatigue.7Although fatigue is most prevalent in patients undergoing chemotherapy or those with advanced disease,it is also a significant problem among cancer survivors.8Previous studies showed that 30%-40% of breast cancer survivors continue to experience chronic fatigue after the completion of chemotherapy,higher than comparable populations without prior chemotherapy.9Recent study showed that 31% of breast cancer patients report fatigue at the end of treatment,with post-treatment fatigue continuing for 11% and 6%of patients at 6 and 12 months,respectively.10Postchemotherapy chronic fatigue has a major impact on the quality of life and health care utilization of cancer survivors.1The National Comprehensive Cancer Network reported that fatigue affects 70% to 100% of patients with cancer.Therefore,management of cancer related fatigue is an urgent need.Unfortunately,exact and effective pharmacological strategies for the management of fatigue are lacking.
Current management of cancer-related chronic fatigue includes correction of underlying medical conditions,non-pharmacologic treatment such as cognitive–behavioral interventions,life style changes and exercise,and pharmacologic agents and dexmethylphenidate,modafinil,or corticosteroids.11In response,an increasing number of studies of complementary and alternative medicine (CAM) have been performed.As an indispensable part of CAM,Traditional Chinese Medicine (TCM) has been gradually acknowledged worldwide for the management of fatigue.12In TCM,fatigue is divided into the category of consumptive disease due to non-restoring imbalance betweenYinandYangand insufficient viscera’s vitalQiand blood in the long term caused by anti-cancer treatments,and other factors,such as depression and pain.13Consequently,TCM holds that regulating the viscera and tonifyingQiand blood are keys to the successful treatment of fatigue.The time-acupoints-space acupuncture (ATAS) is one the school of acupuncture methods.As pronounced in the name,the main technique of ATAS consists the selection of the points according to two main rules.Firstly,the selection of the points according to the time of practice,because of the ancient theory on chronological circulation of the energy along the meridians.By adjusting the acupuncture stimulation in the right place at the right time is aimed to enhance the sensitivity and efficacy of the needle therapy.Secondly,the selection of the points according to the patient's statement after a TCM diagnosis.According to the principle of TCM,the fatigue induced by chemotherapy is a complex of deficiency ofQi(energy) and of blocking of blood fluid.The intervention by acupuncture is aimed to a holistic regulation.
In this study,one main schedule of acupuncture points is established and associated with six other sub-groups of point composition,with a light difference with each other according to the clinical aspect of the patients.Such complex and flexible treatment is not an obstacle to pragmatic randomized trials.“Indeed,in pragmatic trials,the idea is to interfere as little as possible with usual care,therefore a standardized treatment protocol is not required if it is not the actual practice”,described in the guideline for complementary medicine.14
2.MATERIALS AND METHODS
2.1.Trial design overview
Trial registration:ChiCTR-IPR-17013652,registered for the Protocol version 3.2 dated from 2018/04/20.The study was approved by the ethics committee of Yunnan cancer hospital (YJZ 201705).This protocol of the randomized sham controlled pilot trial of ATAS for the prevention of chemotherapy-induced fatigue in women with early stage breast cancer receiving adjuvant chemotherapy was initiated in Gustave Roussy Cancer Institute France and the support of the expert methodologists in complementary medicine from the Research Center of Epidemiology and public health(INSERM) France.
A randomized controlled trial is necessary to determine the effectiveness of ATAS on the prevention of the fatigue in patients with early breast cancer receiving adjuvant chemotherapy by EC followed by paclitaxel (T).Good trial design requires the magnitude of the clinically important effect size to be stated in advance.Thus,some knowledge of the population variation of the outcome is necessary.However,reliable data for the proposed trial populations under investigation do not exist so far.
Thus the randomized clinical trial (RCT) will have two phases:
(a) A pilot phase to estimate the parameters needed for sample size calculation of the main trial,assess the feasibility of conducting the main trial (in terms of recruitment,acceptability of the intervention,true and sham acupuncture practice,follow-up and data collection methods) and allow for minor modifications before further large-scale recruitment.If we succeed in recruiting at least an average of two patients per month per arm and per country,the study would progress to the main trial and patients recruited during the pilot phase will be included in the analysis.
(b) The full trial phase will continue to recruit the target sample (sample size calculation estimated using the parameters established in the internal pilot).
This study concerns a 6-month,3-arm trial using a 2∶1 ∶1 ratio to true ATAS acupuncturevssham acupuncturevsnon acupuncture control (Figure 1).The patient randomization list has to be generated by France Inserm unit before sending to each specific clinical center.Patients,study staff and study clinicians are blinded to randomization condition for patients randomized to the true and sham acupuncture conditions;only the study acupuncturists,the study expert committee,and one lead site (PreciMed) study coordinator are aware of the randomization.
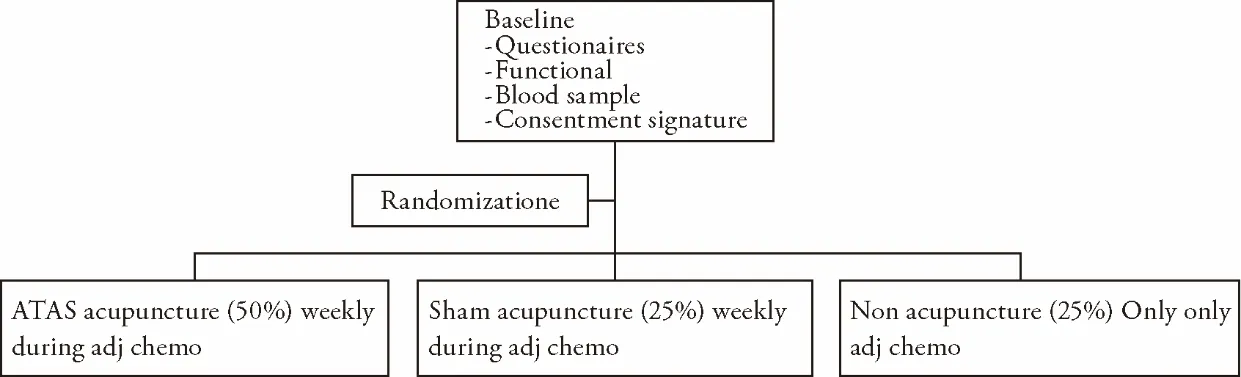
Figure 1 Study design
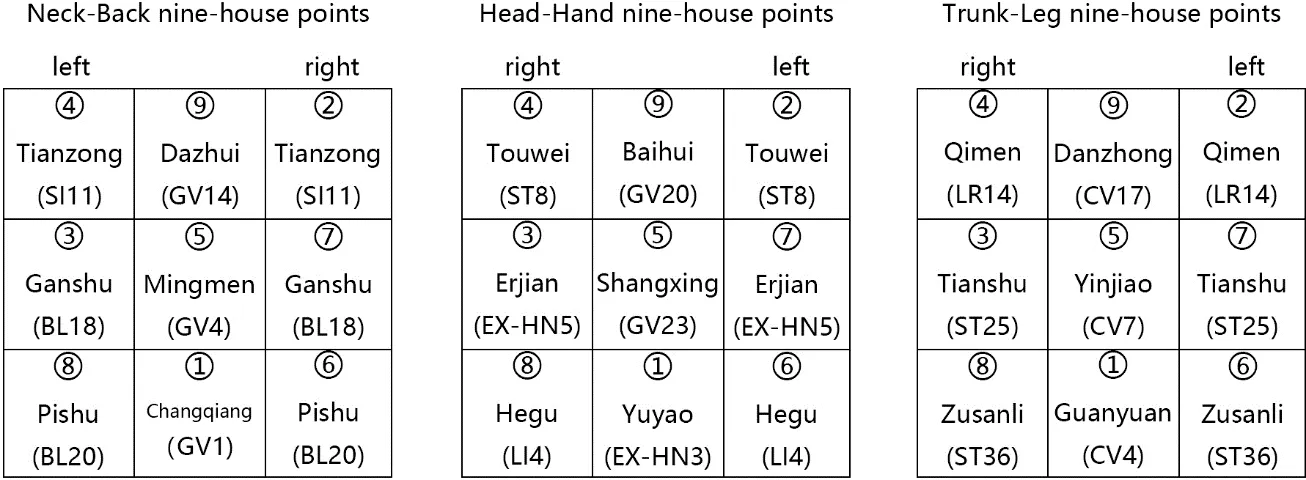
Figure 2 Basic protocol of time-acupoints-space acupuncture
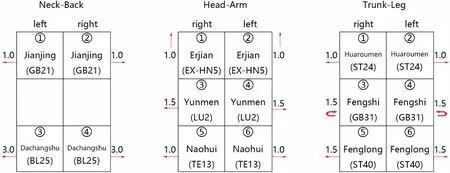
Figure 3 Schema of Sham acupuncture
2.2.Primary endpoint
The primary objective of the trial is the difference in fatigue between groups,as measured by the total score of the Multidimensional Fatigue Inventory (MFI).The MFI is a 20-item self-report instrument designed to measure multiple fatigue characteristics and the impact on function through five subscales (general fatigue,physical fatigue,reduced activity,reduced motivation and mental fatigue).15The Chinese,French and Spanish versions of all the above questionnaires were selected for proven and validated versions.16-18
Fatigue is assessed with the MFI 20 at baseline (T1),after 3-4 cycles of epirubicine-cyclophosphamide (EC) (T2),at the end of 3-4 cycles of paclitaxel (T3) and 1-month post-chemotherapy (T4).Longitudinal assessments of fatigue through VAS-F scale weekly during the whole course of chemotherapy and until 1 month after completion of chemotherapy.
2.3.Secondary endpoints
(a) Self-rated anxiety and depression (at T1,T2,T3 and T4).Hospital Anxiety and Depression Scale (HADS,Annex 3) consists of 14 items,seven items of the depression subscale (HADS-D) and seven items of the anxiety subscale (HADS-A);19(b) self-reported neuropathic pain (at T1,T2,T3 and T4) using the FACT/GOG-NTX (VERSION 4) (http://www.facit.org/FACITOrg/Questionnaires);(c) sleep quality (at T1,T2,T3,T4) assessed by the Insomnia Severity Index (ISI);14(d) immune biomarkers variations during treatment:mRNA sequencing.
2.4.Sample size determination
The primary hypothesis of the study is that true acupuncture will decrease the fatigue associated with the disease and the treatment for breast cancer compared to sham acupuncture or waitlist controls,assessed one month after the end of adjuvant chemotherapy.
Westipulate a 2.5% two-sided type I error,accounting for two comparisons,true acupuncture vs sham acupuncture and true acupuncturevswait-list control,in order to maintain an overall type I error of 5%.
According to the first data from the pilot study,the standard deviation of the MFI score at baseline is close to 25 points.A conservative approach is to consider that the standard deviation of the difference between T4 and baseline will be also of 25 points.
To ensure the study a 90% power to detect a 10 points difference in the change of MFI score between T4 and baseline,468 evaluable patients are to be included in the trial,according to a 2∶1 ∶1 allocation.
Considering a 5% drop out rate,492 patients are to be included:246 in the ATAS arm,123 in the SHAM arm,and 123 in the control arm.As three countries are expected to equally participate to the trial (China,France and Spain),164 patients per country are planned to be randomized.As there is no change in the inclusion criterion in the treatment,the patients randomized in the pilot study will be included in the final analysis.
2.5.Eligibility criteria,recruitment and consent
All participants provide written consent.Institutional.Eligibility criteria were selected to identify the patients aged >18 years with a history of stage Ⅰ-Ⅲ breast cancer scheduled to receive adjuvant chemotherapy by 3-4 cycles of EC followed by 3-4 cycles of T as per each center’s standard protocol.Trial participants are identified and recruited at each clinical site by clinical and research staff.
2.6.Inclusion criteria
Voluntary written consent is mandatory before any study related procedure not part of standard medical care,with the understanding that the patient may withdraw consent at any time without prejudice to future medical care.
(a) Patients aged >18 years;
(b) Recently diagnosed and operated for primary breast cancer,no evidence of distant metastases.
(c) Patients eligible for adjuvant chemotherapy.
(d) Karnofsky physical status >60.
(e) Patients must be able to adhere to the study visit schedule and other protocol requirements.
(f) Patients affiliated with an appropriate social security system.
2.7.Exclusion criteria
(a) Known severe inflammatory or metabolic disease or any other uncontrolled medical condition or comorbidity that might interfere with subject’s participation.
(b) Prior treatment with acupuncture in the previous 4 months.
(c) Treated for cancer in the five years preceding recruitment (except basal skin cancer).
(d) Mental disorders,no capacity for civil conduct or with limited capacity for civil conduct.
(e) Needle phobic patients.
(f) Dermatological disease within acupuncture needling area.
2.8.Pilot study
As highlighted previously,the pilot trial objective is not to prove superiority of the treatment but to test trial procedures and processes and get estimates of parameters for the main trial sample-size calculation.Therefore,the sample size formulae based on formal power considerations are not usually applicable to pilot trials.Whiteheadet al30developed a method of calculating the optimal solution to the required pilot trial sample according to the likely standardised effect size for the main trial.We consider a medium standardized effect size (between 0.3 and 0.7) of ATAS on fatigue to be likely.In this case,for a main trial designed with 80%power and two-sided 5% significance,they recommend pilot-trial sample sizes per treatment arm of 10.
Recruitment per centre is planned as follows:
Pairs (Gustave Roussy Cancer Institute France):ATAS group (n=20)+No acupuncture group (n=10)+Sham acupuncture group (n=10).
Madrid (MD Anderson Cancer Hospital,Madrid):ATAS group (n=20)+No acupuncture group (n=10)+Sham acupuncture group (n=10).
Kunming (Yunnan cancer hospital):ATAS group (n=20)+No acupuncture group (n=10)+Sham acupuncture group (n=10).
3.RESULTS
3.1.ATAS acupuncture protocols
The patients included in this study will receive one acupuncture treatment (ATAS or sham acupuncture)weekly,during the whole duration of adjuvant chemotherapy. When applicable,acupuncture is performed on the planned day of chemotherapy for weekly chemotherapy cycles or on day of chemotherapy and in between intervals (days 8,15,and 21 for monthly cycles).When acupuncture and chemotherapy at same day,the acupuncture should be performed before chemotherapy.
Both the true and sham acupuncture treatments consist of 60-minute sessions administered weekly including the steps of TCM diagnosis and ATAS schedule,needling and needle remaining for 30 min.ATAS acupuncture is semi-standardized.
Stainless steel,single-use,sterile and disposable needles are used for the intervention and are inserted at traditional depths and angles.The full body acupuncture needles used are 0.25 × 25 mm,0.25 × 40 mm,and 0.30× 40 mm (manufactured by Suzhou Huanqiu Acupuncture Medical Appliance Co.,Ltd.,N.8 Chuangxing Industrial Zone,Weitang Town,Xiangcheng District,Suzhou City,Jiangsu Province 215134,China;distributed Novasan S.A.,Medical &Health Products,C/Churruca,18 Bajo,28004 Madrid,Spain).
Subjects of the intention to treat population are defined as those who signed consent and completed the baseline questionnaire.The effect of acupuncture on fatigue will be evaluated according to the intention to treat principle(primary analyses) and on a per-protocol basis excluding participants of the acupuncture groups who completed less than 75% of acupuncture sessions.
ATAS acupuncture,different from other classical regiment,concerns a personalized therapeutical intervention according to the time,the disease and the TCM diagnostic of patients.This study is aimed to evaluate the effect of ATAS:a group of 7 compositions of acupoints (1 principle plus 6 variables) in the fatigue induced by adjuvant chemotherapy in patients with breast cancer.Figure 2 illustrates the basic points of ATAS acupuncture point protocol.
3.2.Sham acupuncture protocol
Sham acupuncture is used as a control in scientific studies that test the efficacy of acupuncture in the treatment of various illness or disorders.It consists the use of non-meridian-non-acupoint acupuncture,designed by referring protocols used in randomized controlled clinical studies with acupuncture.The duration,and frequency of the sessions in the sham acupuncture group are the same as for the ATAS group.The sham protocol consists of a core-standardized prescription of minimally invasive,shallow needle insertion utilizing thin and short needles at nonacupuncture points.In each session,sixteen standardized points are needled bilaterally and superficially using fine needles (0.25 × 25 mm,superficial acupuncture at nonmeridian points,Figure 3).DeQiand manual stimulation of the needles are avoided.
Patients in the arm of no acupuncture participate the evaluations for the full duration of their chemotherapy.Basic space-points:Pattern of Liver and SpleenQideficiency,to tonifying Liver and SpleenQi:
3.3.Prior training of acupuncturists
The specific training session has to be organized before clinical trial for ATAS and sham acupuncture method.Only licensed medical doctor acupuncturists after training and examination could be exercise the acupuncture interventions.At the time of study initiation,the acupuncturists are already on staff at the clinical sites,or on staff at the community-based clinics affiliated with the cancer center or academic medical center.
3.4.Randomization and blinding of acupuncture status
Once a participant is enrolled in the trial,they are randomized by the electronical clinical report form(eCRF) system in a 2∶1∶1 ratio to ATAS acupuncture vs sham acupuncturevscontrol.If a patient is randomized to the true or sham acupuncture condition,the acupuncturists could notice that a patient has been assigned.Next,the acupuncturist logs into the eCRF system to retrieve the assignment.The acupuncturist is the only member of the site study team that is unblinded to the sham and true acupuncture assignment.
To maintain blinding,the data on the true or sham point prescription administered at each visit will be entered into a separate locked database,which will only be accessible to the study acupuncturist(s) and an unblinded CRA at France who does not have contact with patient
3.5.Adjuvant chemotherapy
The adjuvant chemotherapy performed in our three centers is quite similar,e.g.a regiment of EC followed by T.However,each center has established its own chemotherapy protocols in daily clinical practice,among currently validated regimens (Table 1).
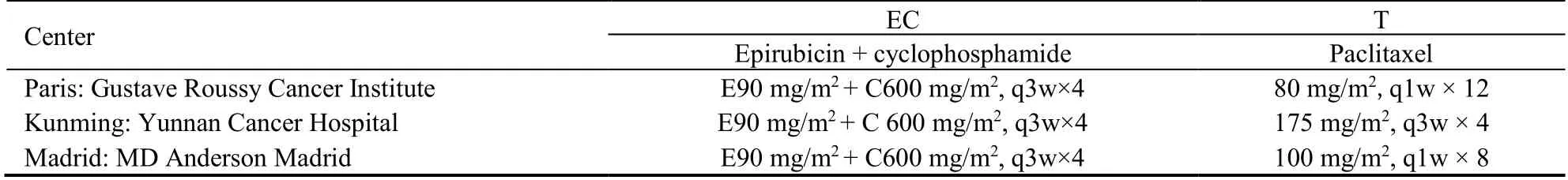
Table 1 Chemotherapy regimens in three centers
3.6.Immune biomarker profiling
Cancer-related fatigue (CRF) is defined as a distressing,persistent,subjective sense of physical,emotional,and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recentactivity and differs from the normal fatigue that accompanies everyday life,which is usually temporary and relieved by rest.
The mechanisms responsible for this condition are poorly understood.Major obstacles to defining the relevant pathophysiology of this symptom include the inherent subjectivity of fatigue,the difficulty in establishing objective behavioral correlates and the wide variety of conditions unrelated to cancer or its treatment that contribute to fatigue.In order to explore the mechanism of fatigue induced by chemotherapy with or without acupuncture,an investigation of Immune-Inflammatory biomarkers of CRF in patients with breast cancer were summarized,an evaluation of immune profiling using mRNA sequencing was incorporate in this study.
3.7.Assessments
The schedule of intervention and assessments is summarized in Table 2.

Table 2 Schedule of intervention and assessments
Four time points of assessments were defined,before chemotherapy as T1,end of EC as T2,end of T(paclitaxel) as T3,one month after end of chemotherapy as T4.
All self-evaluation questionnaires,such as MFI,HDAS,ISI,FACT/GOG-NTX (VERSION 4),have to be evaluated at T1-T4.
The VAS-F has to be evaluated weekly.3-4 cycles of EC every 3 weeks (total=9-12 weeks).3-4 cycles of paclitaxel (weekly or monthly).
The blood samples for immune profiling study have to be collected at T1-T4.
3.8.Data collection
For each patient included in the trial,a paper informed consent has to be completed and signed by the investigator or the person designated by the investigator.The eCRF will gather both clinical data and party of the data collected during the analysis of samples.
4.DISCUSSION
Different than other acupuncture RCT,we introduced the strict and rigorous methodology in the evaluation of a semi-standardized and lightly variable personalized acupuncture protocol,ATAS acupuncture,in order to reply the real quotidian life of patients with breast cancer under adjuvant chemotherapy.The detailed assessments and quality control are applied for the study.
The fatigue related to cancer or induced by chemotherapy could be a biological phenomenon of immune or inflammation level.Therefore,it is important to evaluate potential biomarkers of chemotherapy induced fatigue in patients with breast cancer.
杂志排行
Journal of Traditional Chinese Medicine的其它文章
- Mechanisms of immune regulation for acupuncture on chronic respiratory diseases
- Mechanism of Huashi Xingyu Qingre recipe (化湿行淤清热方) in treating oral lichen planus based on network pharmacology and clinical trial verification
- Anti-inflammatory mechanism of rhein in treating asthma based on network pharmacology
- Mining intrinsic information of convalescent patients after suffering coronavirus disease 2019 in Wuhan
- Menstrual cycle characteristics as an indicator of fertility outcomes:evidence from prospective birth cohort study in China
- Effectiveness and safety of red yeast rice predominated by monacolin K β-hydroxy acid form for hyperlipidemia treatment and management
