COX-2、SFRP4和WBP2在子宫腺肌病病灶中的表达及与临床特征的关系
2022-07-18石路莹段静雅张继花李灿宇
石路莹 段静雅 张继花 李灿宇
【摘要】 目的:探究环氧化酶-2(cyclooxygenase-2,COX-2)、分泌型卷曲相关蛋白4(secreted frizzled-related protein 4,SFRP4)和WW域结合蛋白2(WW-domain binding protein 2,WBP2)在子宫腺肌病中的表达及其与子宫腺肌病临床特征的关系。方法:选取2019年1月-2020年6月在郑州大学第三附属医院确诊子宫腺肌病并进行手术切除子宫的患者40例,收集40例患者的异位内膜标本,统计患者的痛经、月经量及子宫大小等临床资料。采用免疫組化SP法分别检测COX-2、SFRP4和WBP2在子宫腺肌病异位病灶中的表达,分析其相互关系及其与子宫腺肌病临床特征的关系。结果:COX-2和SFRP4的表达呈正相关(rs=0.533,P<0.01),COX-2和WBP2的表达呈正相关(rs=0.544,P<0.01),WBP2和SFRP4的表达呈正相关(rs=0.574,P<0.01)。无或轻度痛经及中度痛经组与重度痛经组COX-2和SFRP4低表达与高表达分布情况比较,差异均有统计学意义(P<0.05),而两组WBP2低表达与高表达分布情况比较,差异无统计学意义(P>0.05)。子宫体积正常组与子宫体积增大组COX-2、SFRP4及WBP2低表达与高表达分布情况比较,差异均无统计学意义(P>0.05)。月经量正常组与月经量增大组COX-2、SFRP4及WBP2低表达与高表达分布情况比较,差异均无统计学意义(P>0.05)。
结论:COX-2、SFRP4和WBP2可能参与了子宫腺肌病的发生发展,COX-2可作为治疗子宫腺肌病潜在的分子靶点。
【关键词】 子宫腺肌病 COX-2 SFRP4 WBP2 免疫组化 痛经
Expressions of COX-2, SFRP4 and WBP2 in Adenomyosis and Their Correlations with Clinical Features/SHI Luying, DUAN Jingya, ZHANG Jihua, LI Canyu. //Medical Innovation of China, 2022, 19(13): 00-007
[Abstract] Objective: To explore the expressions of cyclooxygenase-2 (COX-2), secreted frizzled-related protein 4 (SFRP4) and WW-domain binding protein 2 (WBP2) in adenomyosis and their correlations with the clinical features of adenomyosis. Method: A total of 40 patients who were diagnosed with adenomyosis by pathology and underwent hysterectomy in the Third Affiliated Hospital of Zhengzhou University from January 2019 to June 2020 were selected. The ectopic endometrium specimens of the 40 patients were collected, and the clinical data of dysmenorrhea, menstrual volume and uterine size were collected. The expressions of COX-2,
SFRP4 and WBP2 in ectopic lesions of adenomyosis were detected by immunohistochemical SP method. The relationships between COX-2, SFRP4 and WBP2 and their relationships with the clinical characteristics of adenomyosis were analyzed. Result: The expression of COX-2 and SFRP4 was positively correlated (rs=0.533, P<0.01), the expression of COX-2 and WBP2 was positively correlated (rs=0.544, P<0.01), and the expression of WBP2 and SFRP4 was positively correlated (rs=0.574, P<0.01). There were significant differences in the distributions of low expression and high expression of COX-2 and SFRP4 between no or mild dysmenorrhea and moderate dysmenorrhea group and severe dysmenorrhea group (P<0.05), but there was no significant difference in the distribution of low expression and high expression of WBP2 between the two groups (P>0.05). There were no significant differences in the distributions of low expression and high expression of COX-2, SFRP4 and WBP2 between normal uterine volume group and increased uterine volume group (P>0.05). There were no significant differences in the distributions of low expression and high expression of COX-2, SFRP4 and WBP2 between normal menstrual volume group and increased menstrual volume group (P>0.05). Conclusion: COX-2, SFRP4 and WBP2 may be involved in the occurrence and development of adenomyosis, COX-2 can be used as a potential target for the treatment of adenomyosis.
[Key words] Adenomyosis COX-2 SFRP4 WBP2 Immunohistochemistry Dysmenorrhea
First-author’s address: The Third Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, China
doi:10.3969/j.issn.1674-4985.2022.13.001
子宫腺肌病(adenomyosis,AM)是指子宫内膜腺体及间质侵入子宫肌层,异位子宫内膜组织引起周围肌纤维增生及肥大[1],引起弥漫性或局限性病变的疾病。绝大多数患者会出现继发性痛经、慢性盆腔痛、月经量过大及继发性不孕等症状[2],严重影响患者的身心健康。子宫腺肌病的病因和发病机制尚不十分清楚,组织损伤及子宫内膜内陷及上皮间质转化可能是子宫腺肌病的重要发病机制[3]。文献[4]表明,与无子宫腺肌病的正常子宫内膜相比,子宫腺肌病在位和异位内膜细胞中β-catenin的蛋白水平更高,Wnt/β-catenin通路参与了子宫腺肌病发生发展过程[5-6],而Wnt/β-catenin信号通路的失调对促进上皮间质转化的发生发挥重要作用[7-8]。在癌症组织中,抑制环氧化酶-2(COX-2)的表达后Wnt/β-catenin信号通路的激活受到抑制,抑制COX-2后癌症细胞的侵袭和转移也可受到显著抑制[9]。COX-2在子宫腺肌病异位内膜中呈高表达[10],COX-2的表达可显著影响子宫腺肌病的发生发展及痛经的发生[11-12],但COX-2參与子宫腺肌病发展的具体机制尚不清楚。WW域结合蛋白2(WBP2)是一种新兴的肿瘤相关因子,在癌症组织中,WBP2的高表达被证实是激活Wnt/β-catenin通路的潜在因子[13-14]。分泌型卷曲相关蛋白4(SFRP4)是Wnt/β-catenin经典信号通路的抑制剂[15],SFRP4的高表达可明显抑制Wnt/β-catenin通路的激活。SFRP4与WBP2在子宫腺肌病组织中的表达尚未见文献报道。本研究检测了COX-2、SFRP4与WBP2在子宫腺肌病异位病灶中的表达及其相关性,进而探究各因子参与子宫腺肌病发生发展的可能机制,为子宫腺肌病的诊断及早期预测寻找分子靶点。
1 资料与方法
1.1 一般资料 选取2019年1月-2020年6月在郑州大学第三附属医院经病理证实为子宫腺肌病且行手术治疗的患者40例。纳入标准:绝经前女性;所有病例均经病理诊断为子宫腺肌病;所有患者临床资料完整。排除标准:术前3个月使用激素类药物;合并内分泌疾病、恶性肿瘤、免疫系统疾病等;合并卵巢囊肿、子宫内膜异位症等雌激素依赖性子宫内膜相关性疾病。40例患者年龄39~55岁,平均(47.15±4.19)岁。将40例子宫腺肌病异位内膜组织分为增生期25例,分泌期15例;无或轻度痛经及中度痛经31例,重度痛经9例;月经量正常12例,月经量增大28例;子宫体积正常27例,子宫体积增大13例。本研究经医学伦理委员会批准,患者及家属知情同意。
1.2 方法
1.2.1 组织标本 术中取AM 患者异位内膜组织立即放入10%的福尔马林中固定,脱水、石蜡包埋,蜡块常温保存。蜡块切片待测,切片厚度4 mm。
1.2.2 免疫组化检测 采用免疫组织化学SP法测定AM异位内膜组织中COX-2、SFRP4和WBP2的 表 达 情 况。实验过程严格按照免疫组化试剂盒中提供的说明书进行,使用0.01 mmol/L的枸橼酸钠(pH=6.0)缓冲液进行抗原修复,使用一抗稀释液将一抗进行稀释,COX-2、SFRP4和WBP2稀释浓度分别为1︰50、1︰400和1︰100。PBS代替一抗孵育作为阴性对照,阳性对照由武汉三鹰抗体公司提供。COX-2、SFRP4和WBP2一抗均来自武汉三鹰公司。
1.2.3 免疫组化结果判定 用低倍和高倍镜观察切片,结果均由两位高年资病理医师在双盲条件下独立判定。随机选取10个 高倍视野,计数阳性细胞占总细胞的百分比:<1%计为0分;1%~25%计为1分;26%~50%计为2分;51%~75%计为3分;>75%计为4分。染色强度判断:0分为无着色,1分为浅棕黄色,2分为棕黄色,3分为棕褐色。将阳性细胞百分比评分与染色强度评分相乘即为免疫组化最终评分。最终根据两者的乘积将染色情况分为4级,0分为阴性(-);1~4分为弱阳性(+);5~8分为阳性(++);9~12分为强阳性(+++),阴性及弱阳性为低表达,阳性及强阳性为高表达。
1.2.4 痛经、月经量及子宫大小评估 使用视觉模拟评分(VAS)评估患者痛经程度,VAS评分分为四个级别:无痛经(0分)、轻度痛经(1~3分)、中度痛经(4~6分)和重度痛经(7~10分)。月经量使用月经失血图(PBAC)进行评估,收集患者使用后的卫生巾,根据每张卫生巾的血染程度进行评分:轻度,血染面积占卫生巾的1/3以下,计1分;中度,血染面积占卫生巾的1/3~3/5,计5分;重度,血染面积基本为整个卫生巾,计20分。遗失血块<1元硬币,计1分;≥1元硬币,计5分。评估整个经期的月经量,评分>100分为月经量≥80 mL,可诊断为月经失血量增大[16]。由本院高年资超声科医生使用经阴道三维超声进行子宫体积的测量,测出子宫长、宽和高。子宫体积=长度(cm)×宽度(cm)×高度(cm)×0.523,大于350 cm3为子宫增大[17]。
1.3 统计学处理 使用SPSS 23.0统计软件分析数 据,计量资料采用M(P25,P75)进行统计描述,采用Man-Whitney秩和检验。采用Spearman相关性检验分析COX-2、SFRP4和WBP2表达的相关性。采用字2检验分别分析COX-2、SFRP4、WBP2表达与患者的临床资料的关系。P<0.05为差异有统计学意义。
2 结果
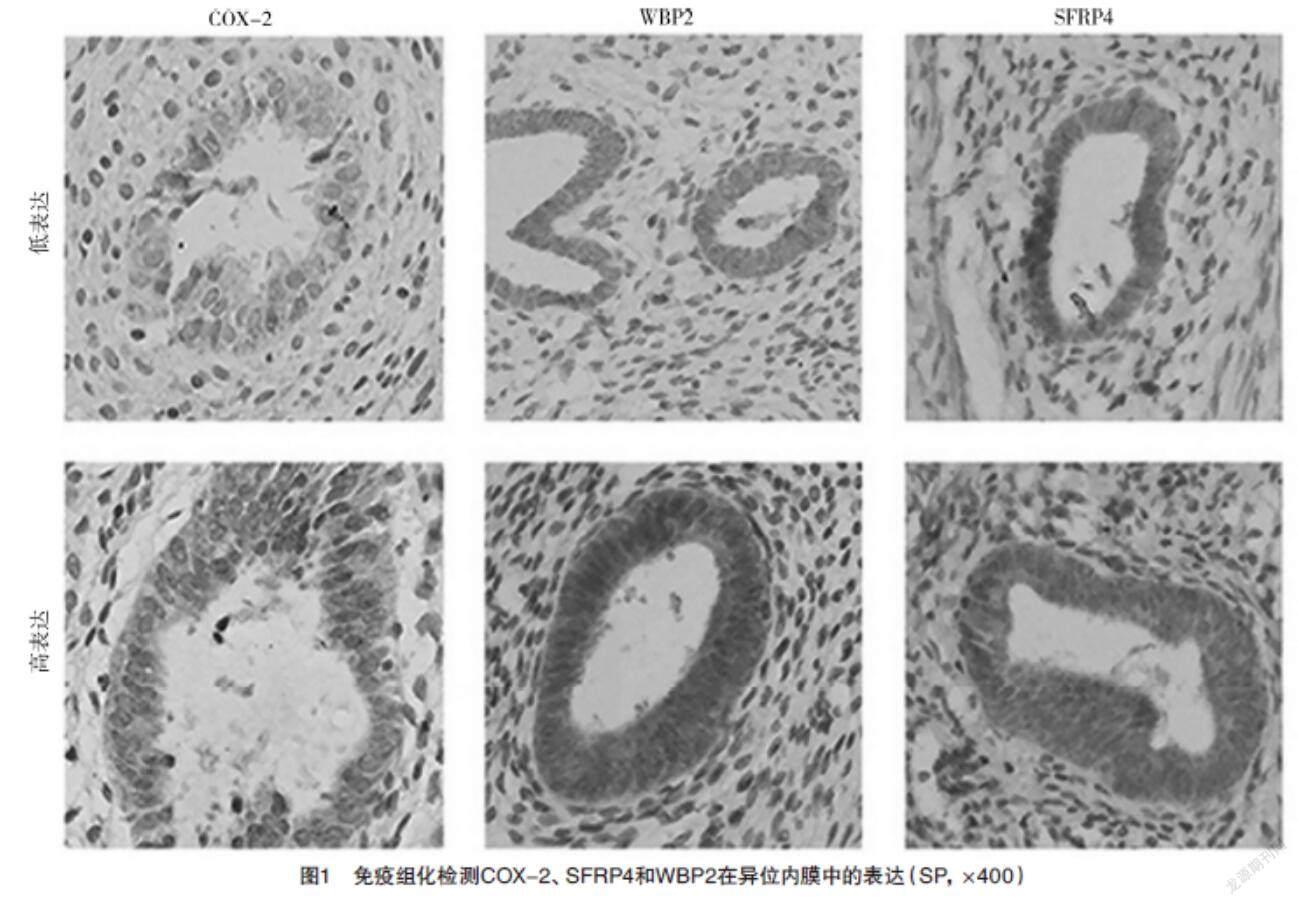
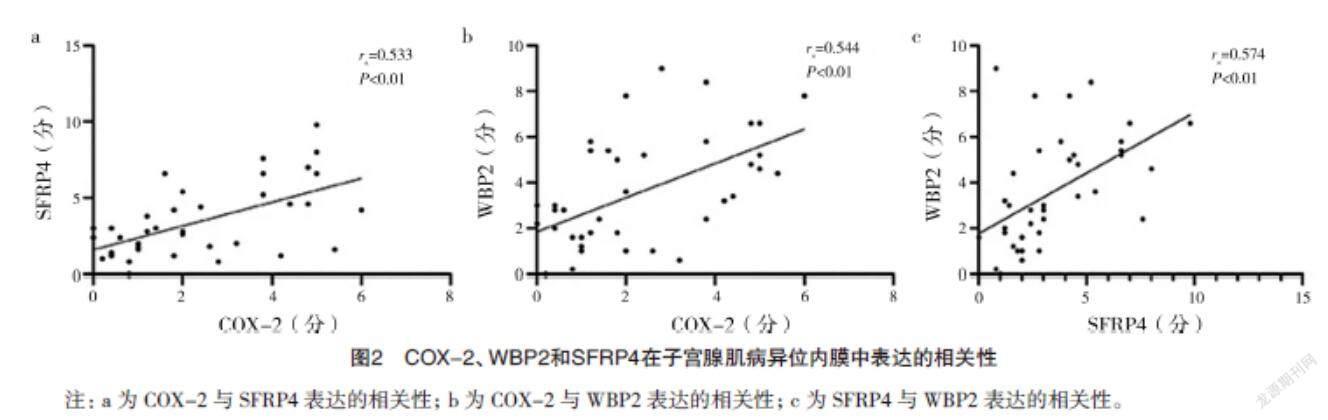
2.1 免疫组化检测COX-2、WBP2和SFRP4蛋白的表达 COX-2、SFRP4和WBP2在增生期、分泌期子宫内膜中的表达比较,差异均无统计学意义(P>0.05)。见表1。COX-2、WBP2和SFRP4均在子宫腺肌病异位内膜组织中的腺体细胞的胞膜和胞质中特异性表达,子宫肌层无明显表达,见图1。在子宫腺肌病异位内膜病灶中,COX-2和SFRP4的表达呈正相关(rs=0.533,P<0.01),COX-2和WBP2的表达呈正相关(rs=0.544,P<0.01),WBP2和SFRP4的表达呈正相关(rs=0.574,P<0.01),见图2。
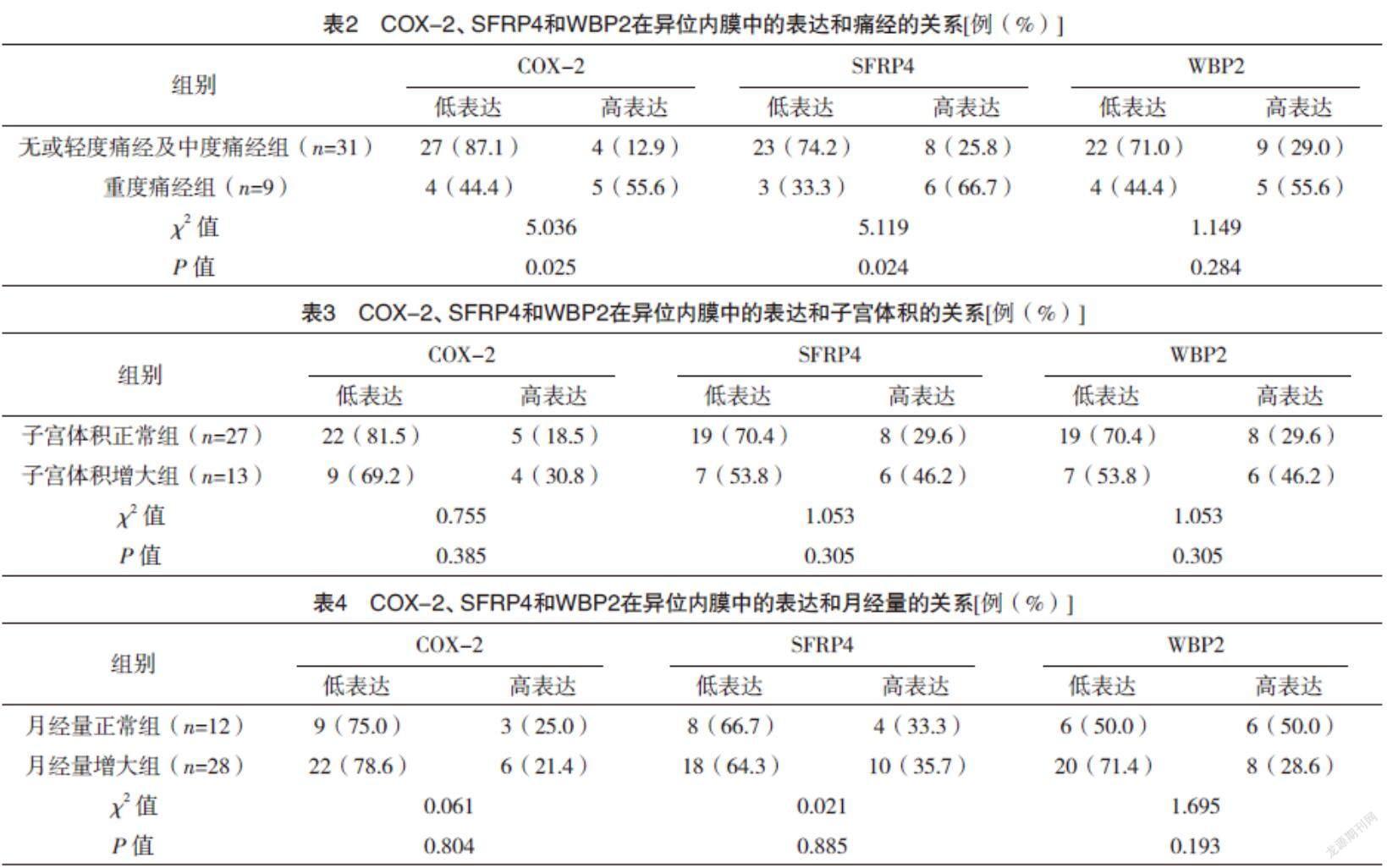
2.2 COX-2、SFRP4和WBP2在异位内膜中的表达与痛经程度、月经量和子宫体积的关系 无或轻度痛经及中度痛经组与重度痛经组COX-2和SFRP4低表达与高表达分布情况比较,差异均有统计学意义(P<0.05),而两组WBP2低表达与高表达分布情况比较,差异无统计学意义(P>0.05)。子宫体积正常组与子宫体积增大组COX-2、SFRP4及WBP2低表达与高表达分布情况比较,差异均无统计学意义(P>0.05)。月经量正常组与月经量增大组COX-2、SFRP4及WBP2低表达与高表达分布情况比较,差异均无统计学意义(P>0.05)。
3 讨论
子宫腺肌病的发病机制尚不明确,组织损伤和子宫内膜内陷及上皮间质转化为两种重要的发病机制假说。类固醇激素表达异常、慢性炎症、神经血管生成、细胞异常增殖等也参与了子宫腺肌病的发生发展[18]。COX-2在正常组织中表达极少,但当组织受到损伤或炎癥刺激时,COX-2在组织中的表达可明显升高。COX-2在癌症组织中参与了慢性炎症的发生及细胞的侵袭[19-20],且与癌症的预后显著相关。COX-2在子宫腺肌病异位子宫内膜中的表达较正常子宫内膜明显升高[21],且COX-2在子宫腺肌病中的过度表达影响了血管的生成、炎症的浸润及神经元的分布[22-23],本课题组前期研究表明,抑制COX-2的表达可明显减少子宫腺肌病异位内膜间质细胞的侵袭和浸润[24],表明COX-2可能参与了子宫腺肌病的发生发展。本研究表明,在子宫腺肌病异位内膜组织中,COX-2和WBP2的表达呈正相关。WBP2为激活Wnt/β-catenin信号通路的潜在因子[13-14],而Wnt/β-catenin信号通路是促进上皮间质转化发生的重要通路[7-8],且COX-2的表达也可影响Wnt/β-catenin信号通路的表达[9]。因此,在子宫腺肌病异位内膜中,COX-2可能与WBP2相互作用进而参与子宫腺肌病的上皮间质转化过程。COX-2为花生四烯酸转化为前列腺素的限速酶,前列腺素F2α(prostaglandin F2α,PGF2α)是重要的致痛因子,前列腺素的过度表达可促进痛经症状的发生,有研究表明,COX-2在子宫腺肌病异位内膜的腺体细胞中显著高表达,且COX-2的过度表达与子宫腺肌病继发性痛经症状有显著相关性[25]。本研究也显示,COX-2在异位内膜中的表达与痛经的严重程度之间存在明显相关性。COX-2可能通过产生前列腺素进而影响子宫腺肌病痛经症状的发生,并通过影响Wnt/β-catenin信号通路的激活参与子宫腺肌病的发生发展。
SFRP4为Wnt信号通路的拮抗剂[26-27],SFRP4的表达可抑制Wnt/β-catenin信号通路的异常激活。Wnt/β-catenin信号通路的异常激活与子宫腺肌病的发生密切相关[4,28]。SFRP4因子的高表达在多种癌症组织中被验证,在肝癌细胞中,SFRP4的表达可明显抑制癌症细胞的Wnt/β-catenin信号通路的激活,且SFRP4的甲基化可使SFRP4失去原有功能[29]。在结直肠癌组织和食管癌组织中,SFPR4的甲基化明显增高[30-31],SFRP4的甲基化减少了对Wnt/β-catenin信号通路的抑制,进而促进了癌症的发生。但文献[32]认为,在癌症组织中SFRP4可能没有参与抑制Wnt/β-catenin信号通路的激活,而是由癌症间质细胞生成并参与了癌症细胞的侵袭和转移过程。因而SFRP4在癌症组织中的表达不止通过调节Wnt信号通路,还可能参与其他机制调节癌症的发生发展。本研究结果显示,SFRP4在子宫腺肌病异位内膜腺体的胞质与胞膜中特异性高表达,且SFRP4的表达与COX-2和痛经均有显著相关性。COX-2的表达与子宫腺肌病痛经具有明显相关,且COX-2与异位内膜细胞的侵袭转移显著相关[24],在子宫腺肌病异位内膜中,SFRP4可能不是通过抑制Wnt/β-catenin信号通路的表达参与子宫腺肌病的发生发展,而是通过其他机制与COX-2相互作用进而促进异位内膜细胞的侵袭和转移,从而参与子宫腺肌病的发生发展,SFRP4在异位内膜中也可能受到甲基化的调节进而失活,因而减少了对Wnt/β-catenin信号通路的抑制,从而促进了子宫腺肌病的发生发展,其具体机制需进一步研究。
子宫腺肌病虽是良性病变,但存在一些恶性肿瘤的特点,如血管生成、间质浸润等。WBP2是一种新兴的肿瘤相关蛋白,其在乳腺癌组织中呈高表达,是乳腺癌中的雌激素受体(estrogen receptor,ER)和Wnt/β-catenin信号通路的潜在激活因子[33-34],且与乳腺癌的预后显著相关。WBP2可能是通过调节β-catenin的表达进而参与调节Wnt/β-catenin信号通路[35]。而WBP2在乳腺癌中以雌激素依赖性方式调节ER的表达[36]。ER的高表达和Wnt信号通路的异常激活是子宫腺肌病发病的重要影响因素[6]。COX-2的表达也可促进子宫腺肌病中雌激素的上调[37],且大量雌激素可促进上皮间质转化(epithelial mesenchymal transformation,EMT)的发生[38]。本研究表明,WBP2在子宫腺肌病异位内膜组织中的表达与COX-2呈正相关,因而本研究推测,在子宫腺肌病组织中,WBP2与COX-2可能相互作用并参与Wnt/β-catenin信号通路的表达进而参与子宫腺肌病的发生发展,其具体机制需进一步探究。
本研究也表明,COX-2、SFRP4和WBP2的表達与子宫腺肌病的月经量和子宫大小均无显著相关性。子宫腺肌病的发生为一个复杂的过程,其中涉及了神经和血管的生成、慢性炎症的存在和细胞异常增殖凋亡等过程[18]。COX-2、SFRP4和WBP2的表达可能促进子宫腺肌病的发生发展,但COX-2、SFRP4和WBP2可能在疾病的早期参与子宫腺肌病的发生,而子宫腺肌病患者的月经量增多及子宫体积的增大则为子宫腺肌病发展到一定程度后发生的后期事件,因而COX-2、SFRP4和WBP2的表达与子宫腺肌病的月经量及体积无明显相关性。
综上所述,COX-2、SFRP4和WBP2可能参与了子宫腺肌病的发生,而COX-2和SFRP4的表达与子宫腺肌病的痛经症状显著相关,COX-2可能是治疗子宫腺肌病的一个潜在的分子靶点。
参考文献
[1] STRUBLE J,REID S,BEDAIWY M A.Adenomyosis:A Clinical Review of a Challenging Gynecologic Condition[J].J Minim Invasive Gynecol,2016,23(2):164-185.
[2] GARCIA-SOLARES J,DONNEZ J,DONNEZ O,et al.
Pathogenesis of uterine adenomyosis: invagination or metaplasia?[J].Fertil Steril,2018,109(3):371-379.
[3] GUO S W.The Pathogenesis of Adenomyosis vis-a-vis Endometriosis[J].J Clin Med,2020,9(2):485.
[4] OH S J,SHIN J H,KIM T H,et al.β-Catenin activation contributes to the pathogenesis of adenomyosis through epithelial-mesenchymal transition[J].J Pathol,2013,231(2):210-222.
[5] BOURDON M,SANTULLI P,JELJELI M,et al.Immunological changes associated with adenomyosis: a systematic review[J].Hum Reprod Update,2021,27(1):108-129.
[6] VANNUCCINI S,TOSTI C,CARMONA F,et al.Pathogenesis of adenomyosis: an update on molecular mechanisms[J].Reprod Biomed Online,2017,35(5):592-601.
[7] LI Q,LAI Q,HE C,et al.RUNX1 promotes tumour metastasis by activating the Wnt/beta-catenin signalling pathway and EMT in colorectal cancer[J].J Exp Clin Cancer Res,2019,38(1):334.
[8] WANG Y Y,DUAN H,WANG S,et al.Talin1 Induces Epithelial-Mesenchymal Transition to Facilitate Endometrial Cell Migration and Invasion in Adenomyosis Under the Regulation of microRNA-145-5p[J].Reprod Sci,2021,28(5):1523-1539.
[9] DINICOLA S,MASIELLO M G,PROIETTI S,et al.Nicotine increases colon cancer cell migration and invasion through epithelial to mesenchymal transition (EMT): COX-2 involvement[J].J Cell Physiol,2018,233(6):4935-4948.
[10] OTA H,IGARASHI S,SASAKI M,et al.Distribution of cyclooxygenase-2 in eutopic and ectopic endometrium in endometriosis and adenomyosis[J].Hum Reprod,2001,16(3):561-566.
[11] HASHEMI G N,NAJAFI M,SALEHI E,et al.
Cyclooxygenase-2 in cancer:A review[J].J Cell Physiol,2019,234(5):5683-5699.
[12] CHEN Y J,LI H Y,CHANG Y L,et al.Suppression of migratory/invasive ability and induction of apoptosis in adenomyosis-derived mesenchymal stem cells by cyclooxygenase-2 inhibitors[J].Fertil Steril,2010,94(6):1971-1979.
[13] SONG H,WU T,XIE D,et al.WBP2 Downregulation Inhibits Proliferation by Blocking YAP Transcription and the EGFR/PI3K/Akt Signaling Pathway in Triple Negative Breast Cancer[J].Cell Physiol Biochem,2018,48(5):1968-1982.
[14] CHEN S,ZHANG Y,WANG H,et al.WW domain-binding protein 2 acts as an oncogene by modulating the activity of the glycolytic enzyme ENO1 in glioma[J].Cell Death Dis,2018,9(3):347.
[15] PAWAR N M,RAO P.Secreted frizzled related protein 4 (sFRP4) update: A brief review[J].Cell Signal,2018,45:63-70.
[16] REID P C,COKER A,COLTART R.Assessment of menstrual blood loss using a pictorial chart: a validation study[J].BJOG,2000,107(3):320-322.
[17] KITAMURA Y,ALLISON S J,JHA R C,et al.MRI of adenomyosis: changes with uterine artery embolization[J].AJR Am J Roentgenol,2006,186(3):855-864.
[18] VANNUCCINI S,TOSTI C,CARMONA F,et al.Pathogenesis of adenomyosis:an update on molecular mechanisms[J].Reprod Biomed Online,2017,35(5):592-601.
[19] YE Y,WANG X,JESCHKE U,et al.COX-2-PGE2-EPs in gynecological cancers[J].Arch Gynecol Obstet,2020,301(6):1365-1375.
[20] ZHU Y,SHI C,ZENG L,et al.High COX-2 expression in cancer-associated fibiroblasts contributes to poor survival and promotes migration and invasiveness in nasopharyngeal carcinoma[J].Mol Carcinog,2020,59(3):265-280.
[21] LI B,CHEN M,LIU X,et al.Constitutive and tumor necrosis factor-alpha-induced activation of nuclear factor-kappaB in adenomyosis and its inhibition by andrographolide[J].Fertil Steril,2013,100(2):568-577.
[22] HARMSEN M J,WONG C,MIJATOVIC V,et al.Role of angiogenesis in adenomyosis-associated abnormal uterine bleeding and subfertility: a systematic review[J].Hum Reprod Update,2019,25(5):647-671.
[23] KOGAN E A,UNANIAN A L,DEMURA T A,et al.Clinical and morphological parallels and molecular aspects of the morphogenesis of adenomyosis[J].Arkh Patol,2008,70(5):8-12.
[24]馬艳鸽,申爱荣,李灿宇,等.子宫内膜异位症和子宫腺肌病在位、异位内膜间质细胞原代培养与形态学观察[J].中国妇幼保健,2015,30(2):287-290.
[25] LI C,CHEN R,JIANG C,et al.Correlation of LOX5 and COX2 expression with inflammatory pathology and clinical features of adenomyosis[J].Mol Med Rep,2019,19(1):727-733.
[26] BOVOLENTA P,ESTEVE P,RUIZ J M,et al.Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease[J].J Cell Sci,2008,121(Pt6):737-746.
[27] FINCH P W,HE X,KELLEY M J,et al.Purification and molecular cloning of a secreted, Frizzled-related antagonist of Wnt action[J].Proc Natl Acad Sci U S A,1997,94(13):6770-6775.
[28] FENG T,WEI S,WANG Y,et al.Rhein ameliorates adenomyosis by inhibiting NF-kappaB and beta-Catenin signaling pathway[J].Biomed Pharmacother,2017,94:231-237.
[29] CHEN K,LIANG H,PENG J,et al.Expression of secreted frizzled-related protein 4 in DNA mismatch repair-deficient and mismatch repair-proficient colorectal cancers[J].Nan Fang Yi Ke Da Xue Xue Bao,2018,38(11):1300-1305.
[30] MURAKAMI T,MITOMI H,SAITO T,et al.Distinct WNT/beta-catenin signaling activation in the serrated neoplasia pathway and the adenoma-carcinoma sequence of the colorectum[J].Mod Pathol,2015,28(1):146-158.
[31] ZOU H,MOLINA J R,HARRINGTON J J,et al.Aberrant methylation of secreted frizzled-related protein genes in esophageal adenocarcinoma and Barrett’s esophagus[J].Int J Cancer,2005,116(4):584-591.
[32] VINCENT K M,POSTOVIT L M.A pan-cancer analysis of secreted Frizzled-related proteins: re-examining their proposed tumour suppressive function[J].Sci Rep,2017,7:42719.
[33] CHEN S,WANG H,HUANG Y F,et al.WW domain-binding protein 2: an adaptor protein closely linked to the development of breast cancer[J].Mol Cancer,2017,16(1):128.
[34] TABATABAEIAN H,RAO A,RAMOS A,et al.The emerging roles of WBP2 oncogene in human cancers[J].Oncogene,2020,39(24):4621-4635.
[35] LI Z,LIM S K,LIANG X,et al.The transcriptional coactivator WBP2 primes triple-negative breast cancer cells for responses to Wnt signaling via the JNK/Jun kinase pathway[J].J Biol Chem,2018,293(52):20014-20028.
[36] LIM S K,ORHANT-PRIOUX M,TOY W,et al.Tyrosine phosphorylation of transcriptional coactivator WW-domain binding protein 2 regulates estrogen receptor alpha function in breast cancer via the Wnt pathway[J].FASEB J,2011,25(9):3004-3018.
[37] JIN Z,WU X,LIU H,et al.Celecoxib,a selective COX-2 inhibitor,markedly reduced the severity of tamoxifen-induced adenomyosis in a murine model[J].Exp Ther Med,2020,19(5):3289-3299.
[38] HU R,PENG G Q,BAN D Y,et al.High-Expression of Neuropilin 1 Correlates to Estrogen-Induced Epithelial-Mesenchymal Transition of Endometrial Cells in Adenomyosis[J].Reprod Sci,2020,27(1):395-403.
(收稿日期:2022-01-24) (本文编辑:占匯娟)
