盐胁迫对砂糖橘幼树生长、生物量积累及光合生理的影响
2022-07-14郭雁君吉前华杜鹏飞尚荷斌钟雅玲
郭雁君 吉前华 杜鹏飞 尚荷斌 钟雅玲
摘要:【目的】明確砂糖橘对不同种类和水平的盐胁迫的适应性,为砂糖橘引种栽培和果园土壤管理提供科学支撑。【方法】分别以CaCO3、NaHCO3和NaCl模拟石灰质土壤、碱土和盐土的主要胁迫成分,并设0.3%和0.7% 2种含盐量水平,以基质中不加盐为对照,栽培90 d后,测试分析盐胁迫对植株生长和生物量分配的影响及其基础生理响应。【结果】盐胁迫下砂糖橘单株落叶量较对照大1.85~17.66倍,盐分含量越高落叶量越大,NaCl胁迫下落叶量显著大于CaCO3和NaHCO3胁迫(P˂0.05,下同)。盐胁迫对株高生长的抑制明显大于对地径和冠幅生长的抑制,对植株生物量积累的影响以对叶生物量的影响最明显,其次是对根生物量积累的抑制;2种含盐量水平的NaHCO3、NaCl胁迫下各自植株根和叶生物量均较对照显著降低。不同种类和水平的盐胁迫均可导致砂糖橘叶片叶绿素含量降低,但0.7% CaCO3胁迫与0.3% NaHCO3胁迫、0.7% NaHCO3胁迫与0.3% NaCl胁迫的效应相当。砂糖橘叶片光合速率、蒸腾速率和气孔导度对盐胁迫敏感,盐分含量越高3项光合生理指标的降低幅度越大;同种性质、2种水平的胁迫间植株叶片水分饱和亏缺均有显著差异,含盐量达0.3%即可使叶片丙二醛含量显著升高、细胞质膜透性显著增大。【结论】砂糖橘对CaCO3含量较高的土壤具较强适应性,对NaCl为主的盐土及NaHCO3为主的碱土适应性很差;对砂糖橘叶片叶绿素的破坏、光合生理的抑制及伴随的水分亏缺和膜脂过氧化是不同种类盐胁迫共同的作用特性。
关键词:砂糖橘;盐胁迫;光合生理;水分亏缺;膜脂过氧化
中图分类号:S666.2;Q945.78 文献标志码: A 文章编号:2095-1191(2022)04-1112-09
Effects of salt stress on plant growth, biomass accumulation and photosynthetic physiology of Shatangju saplings
GUO Yan-jun1, 2, JI Qian-hua1, 2*, DU Peng-fei2, SHANG He-bin2, ZHONG Ya-ling2
(1Fruit Research Institute, Zhaoqing University, Zhaoqing, Guangdong 526061, China; 2College of Life Sciences, Zhaoqing University, Zhaoqing, Guangdong 526061, China)
Abstract:【Objective】To investigate the adaptability of Shatangju sapling to salt stress, so as to provide scientific support for the introduction and cultivation of Shatangju and orchard soil management. 【Method】CaCO3, NaHCO3 and NaCl were used to simulate the main stress components of calcareous soil, alkaline soil and saline soil respectively, and the content levels of 0.3% and 0.7% were set. With no salt in the substrate as the control, the effects of salt and alkali types and contents on plant growth and biomass allocation and their basic physiological responses were tested and analyzed 90 days after cultivation. 【Result】For Shatangju saplings under salt stress, the amount of fallen leaves per plant was 1.85 to 17.66 times larger than that of the control. The amount of fallen leaves under NaCl stress was significantly greater than that under CaCO3 or NaHCO3 stress(P˂0.05, the same as below). The inhibition of salt stress on plant height growth was significantly greater than that on ground diameter and crown width growth, and the effect on plant biomass accumulation was the greatest on leaf biomass, followed by the inhibition on root biomass accumulation. The root and leaf biomass of each plant under the two treatment intensities of NaHCO3 and NaCl stress were significantly reduced compared with the control, Different types and levels of salt stress could reduce the chlorophyll content of Shatangju leaves, but the effects of 0.7 % CaCO3 stress and 0.3 % NaHCO3 stress, 0.7 % NaHCO3 stress and 0.3 % NaCl stress were similar. The photosynthetic rate, transpiration rate and stomatal conductance of Shatangju leaves were sensitive to salt stress, and the higher the salt content, the greater the decrease of three photosynthetic physiological indexes. There were significant differences in leaf water saturation deficits between the same nature and two levels of stress. When the salt content reached 0.3%, the MDA content in leaves increased significantly and the membrane permeability of cytoplasm increased significantly. 【Conclusion】Shatangju leaves has strong adaptability to soil with high CaCO3 content, but poor adaptability to saline soil dominated by NaCl and alkaline soil dominated by NaHCO3. The damage to chlorophyll, the inhibition of photosynthetic physiology, and the accompanying water deficit and membrane lipid peroxidation in Shatangju leaves are the common characteristics of different types of salt stress.
Key words: Shatangju; salt stress; photosynthetic physiology; water deficit; membrane lipid peroxidation
Foundation items: National Modern Agricultural Industrial Technology System Construction Special Project (CARS-26); Guangdong Rural Science and Technology Specialist Project (2021-1056-9-4)
0 引言
【研究意义】砂糖橘(Citrus reticulata Shatangju)果实皮薄多汁,果肉鲜滑脆嫩、甜酸适中、爽口化渣,深受消费者欢迎,是华南地区栽培面积最大、产量最多的柑橘品种(吴文等,2020)。廣东肇庆砂糖橘栽培历史悠久,是当地农业的重要支柱产业(Wu et al.,2016;徐呈祥等,2021)。由于砂糖橘优质、早果、丰产,全国多地引种栽培,涉及的园地土壤化学类型和立地条件多样,包括滨海盐土、次生盐渍化土壤和内陆的石灰质土壤,且引种栽培后时有不良报道。因此,开展砂糖橘果园土壤化学生态研究极有必要,特别是栽培中对盐胁迫的适应性。深入探究砂糖橘树对土壤关键化学胁迫成分的适应性,可为引种栽培和果园管理提供科学依据。【前人研究进展】柑橘是世界大宗栽培的园艺植物。我国长江以南尤其五岭以南是柑橘类植物起源中心、栽培起源地和现代栽培中心之一,柑橘栽培历史悠久(王刘坤和祁春节,2018)。盐胁迫是世界植物栽培生产中的重要非生物逆境,盐胁迫下农作物的生物学响应、耐盐性和机制,国内外已有广泛而深入的研究(Munns and Tester,2008;Ismail and Horie,2017;Song et al., 2021),在指导科学栽培、高效生产中发挥了重要作用。目前,国内外对柑橘耐盐生理已有一定研究,但深度和广度远不及农作物,主要研究盐胁迫下柑橘生长响应和光合生理(马翠兰等,2004;Anjum,2007;Brito et al.,2016)、柑橘活性氧代谢和抗氧化物质积累特性(吴强盛等,2010;Khoshbakht et al., 2018;Nayem et al.,2020)、柑橘砧木或诱导的柑橘愈伤组织的耐盐性(Balal et al.,2011;蔡小东等,2012;朱世平等,2014;Etehadpour et al.,2020)及柑橘体内盐分离子含量和矿质营养状况(Hussain et al., 2012;魏清江等,2016)。上述研究表明,柑橘耐盐性因种质不同而存在明显差异,总体上对盐胁迫敏感,盐胁迫显著抑制柑橘生长和生理,易导致柑橘植株发生水分亏缺,对盐胁迫最敏感的基因型体内积累高浓度Na+和Cl–并产生毒害效应;盐胁迫还显著影响柑橘植株对其他矿质元素的吸收,从而使体内元素含量显著失衡;糖类、醇类和氨基酸的积累有助渗透调节,酶促系统和非酶类氧化剂能降低活性氧伤害,二者在柑橘对盐胁迫的适应中起重要作用。柑橘耐盐性的调控机制近年来受到关注并取得一定进展,相关研究得出,柑橘6个多胺氧化酶(Polyamine oxidase,PAO)基因中CsPAO4(定位于质外体,以亚精胺和精胺为底物)参与H2O2产生,引起盐胁迫下柑橘幼苗氧化损伤,下调PAO基因参与多胺末端分解代谢可能是提高柑橘耐盐性的一种途径(Wang and Liu,2016);50 mg/L腐胺(Putrescine,Put)、250 mg/L多效唑(Paclobutrazol,PBZ)单独或联合处理的柑橘砧木幼苗在盐胁迫下具有较高的抗氧化酶活性、脯氨酸含量和K+、Ca2+等矿质积累,减少Na+和Cl-在根和叶中的积累、降低膜损伤指数(Sharma et al., 2014);接种球囊霉(Glomus mosseae,G. intraradices,G. hoi)等丛枝菌根真菌(Arbuscular mycorrhizal fungi,AMF)是盐胁迫下提高柑橘产量的一种实用方法,可改善盐胁迫下柑橘植株生长和土壤结构(Zhang et al., 2017;Bourazza et al., 2021),显著促进柑橘植株胁迫小蛋白(CaSISP)基因的表达(Hadian-Deljou et al., 2020)、诱导参与水分和小分子有机物运输的水通道蛋白(AQPs)(Cheng et al., 2021),从而提高细胞膜的透水性,刺激其水分运输。【本研究切入点】目前,对柑橘耐盐生理的研究主要针对中性盐(NaCl)胁迫,未见针对碱性盐(CaCO3、NaHCO3)胁迫的研究,相关的生长响应和生理特性尚不清楚。【拟解决的关键问题】以砂糖橘为研究对象,把碱性盐纳入耐盐性研究范围,以CaCO3、NaHCO3和NaCl分别模拟石灰质土壤、碱土和盐土的主要胁迫成分,分析探究不同种类和水平的盐胁迫下砂糖橘植株的落叶特性、生长量和生物量差异以及光合作用、水分代谢、膜脂过氧化作用等基础生理响应,揭示砂糖橘对盐胁迫的适应性及主要机制,为砂糖橘引种栽培和土壤管理提供科学支撑。
1 材料与方法
1. 1 植物材料及培养
试验材料为砂糖橘嫁接苗(2015年夏季嫁接),砧木为枳壳(Poncirus trifoliata),于2017年1月下旬(18月龄)栽植于盆高40 cm、上口直径35 cm、下口直径25 cm的陶瓷盆中,每盆1株。栽培基质为普通耕作土+150 g/kg草炭+50 g/kg河沙,拍碎、混匀、过筛。盆栽基质中含有机质1.72%、速效氮55.6 mg/kg、速效磷32.5 mg/kg、速效钾115.8 mg/kg,pH 6.5。称量基质重量,保持每盆一致。在温室中避雨栽培,期间视季节和天气情况,每周浇水1~2次、每次每株1000 mL,每30 d施复合肥1次、每次每株5~7 g。盆底放置圆形浅壁塑料托盘。温室内气温高于30 ℃,湿帘降温系统自动启动。于2018年7月下旬选择生长势及树体大小相当的3年生嫁接苗进行盐胁迫。处理前7 d暂停浇水。
1. 2 试验设计
设6种不同含盐量水平的CaCO3、NaHCO3和NaCl胁迫:(1)0.3% CaCO3;(2)0.7% CaCO3;(3)0.3% NaHCO3;(4)0.7% NaHCO3;(5)0.3% NaCl;(6)0.7% NaCl。以在温室中常规栽培、不进行盐胁迫的植株为对照(CK)。每处理3次重复,每重复3株,参试植株共63株,共栽培90 d。CaCO3、NaHCO3和NaCl均为化学纯(国药集团产品),添加量基于盆中基质干重,充分溶解在1000 mL自来水中后缓缓浇入盆中,1 h内偶有渗漏至托盘中的浇灌液返浇入盆。至90 d时收获(从盆中取出)。处理后的水分管理同常规管理,第30和60 d时每株各施复合肥7 g,期间每天定时收集落叶,分别记录每株的落叶数并称重;收获前3 d,测试叶片叶绿素含量和气体交换参数、叶片含水量和膜脂过氧化状况以及植株生长量;收获后,立即按植株分别称量根、茎、叶鲜生物量。
1. 3 測定项目及方法
以植株为单位收集落叶,统计叶片数量,及时烘至恒干重,以FA1004型电子天平(0.001 g)称重,按1~30、31~60、61~90和1~90 d汇总。生长量指标株高、冠幅用WK331025型钢卷尺测量,地径用530-118型游标卡尺测量。使用VP1002型电子天平称重生物量,根、茎和叶生物量之和即为全株生物量;收获时,立即称量鲜生物量,编号带回实验室分摊开存放45 d(自然干燥)后再次称重(干重)。本研究中使用的生物量均为干重。
叶绿素提取及含量测定:每一株植株取中部叶片3片,擦净表面污物,去除中脉后用打孔器(内径1.15 cm)打取叶圆片,每份3片,每处理3份,剪成宽2~3 mm的细丝,用95%乙醇浸提至白色为止。以UV-6300B型紫外可见分光光度计测定浸提液A665 nm、A649 nm的值,分别计算叶绿素a和叶绿素b浓度(Ca、Cb,mg/L),其中:Ca=13.95A665 nm-6.88A649 nm,Cb=24.96A649 nm-7.32A665 nm。叶绿素浓度为Ca与Cb之和。叶绿素(Chl)含量(mg/dm2)=(叶绿素浓度×提取液体积×稀释倍数)/样品面积。
叶片气体交换参数净光合速率[Pn,μmol/(m2·s)]、蒸腾速率[Tr,mmol/(m2·s)]和气孔导度[Gs,mmol/(m2·s)],采用LI-6400 XT便携式光合仪测定。测定条件:温度为20~25 ℃,光强为800 µmol/(m2·s),叶室CO2浓度400 µmol/mol,流速500 μmol/s。测定时间为上午9:30—11:30、下午2:00—4:00,在不同处理的砂糖橘植株上各选取5片叶进行。各处理的测试结果取上午和下午的平均值。
按饱和重量法测定叶片相对含水量(Relative water content,RWC),RWC(%)=(Wf–Wd)/(Wt–Wd)×100。式中,Wf指叶片自然鲜重;Wt指叶片被水分饱和后的重量;Wd指叶片恒干重;以OLABO型电子天平(0.0001 g)称重;每处理3次重复,每样取2片完整叶片。水分饱和亏(Water saturation deficiency,WSD)=1–RWC。叶片丙二醛(Malondialdehyde,MDA)含量采用硫代巴比妥酸反应法测定(张朝坤等,2018);叶片细胞质膜相对透性(Relative permeability of plasma membrane,RPP)使用电导仪法测定(林丽仙等,2013)。
1. 4 统计分析
采用Microsoft Excel 2010进行统计分析制图;各处理间差异显著性采用Duncan’s多重比较法检验。
2 结果与分析
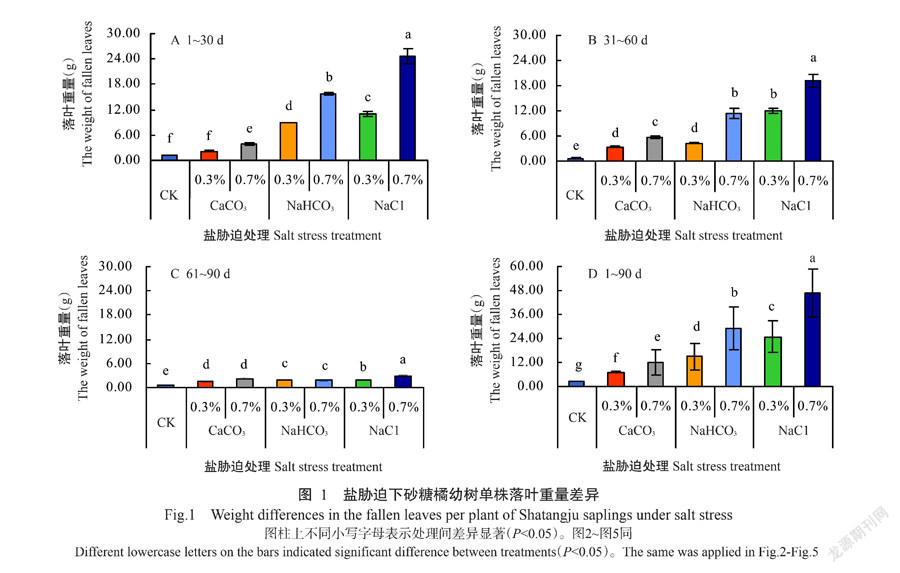
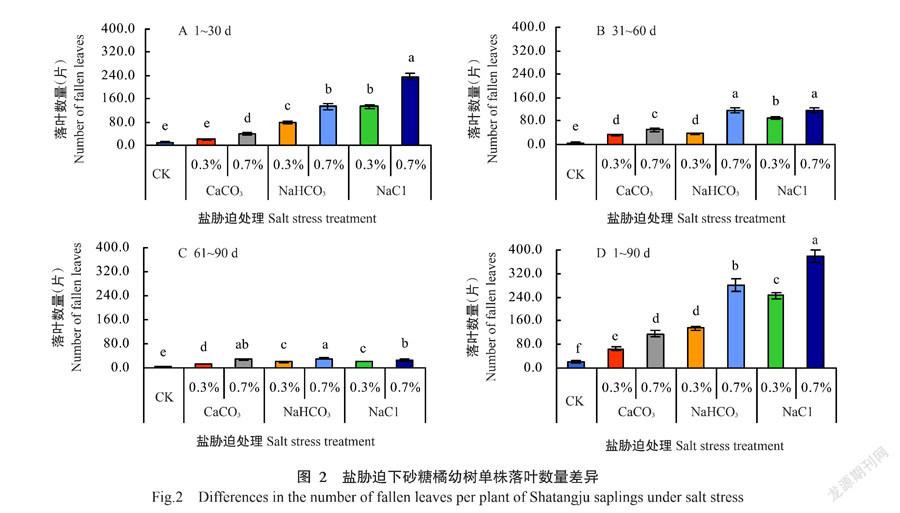
2. 1 盐胁迫下砂糖橘幼树落叶量差异分析
盐胁迫后2周,几乎所有植株每天均有落叶,时期集中在处理后60 d之前(图1-A和图1-B,图2-A和图2-B),处理60 d后基本稳定(图1-C,图2-C)。不同种类的盐胁迫下植株落叶量差异明显:含盐量相同,以NaCl胁迫下落叶量最大,其次为NaHCO3胁迫,CaCO3胁迫下落叶量最小。由图1-D和图2-D可见,盐胁迫90 d,砂糖橘单株的落叶重量和落叶数量在不同种类盐胁迫间均存在显著差异(P˂0.05,下同);期间CK单株落叶重量为2.49 g,同期0.3% CaCO3、0.3% NaHCO3、0.3% NaCl、0.7% CaCO3、0.7% NaHCO3和0.7% NaCl胁迫下单株落叶量分别较CK增加1.85、5.01、8.94、3.75、10.62和17.66倍;期间CK单株落叶数量为21.9片,同期0.3% CaCO3、0.3% NaHCO3、0.3% NaCl、0.7% CaCO3、0.7% NaHCO3和0.7% NaCl胁迫下单株落叶数量分别较CK增加1.99、5.16、11.20、4.34、11.85和16.23倍。植株落叶特点初步表明不同种类盐胁迫各有特性,对砂糖橘的作用机制存在差异。
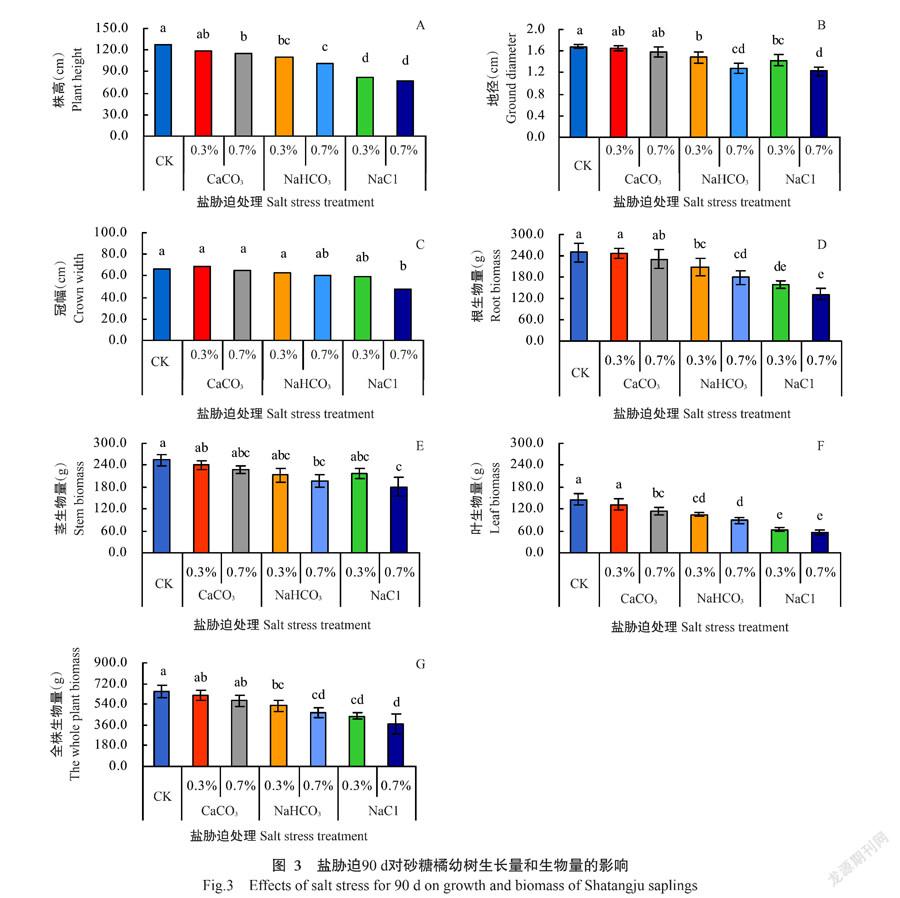
2. 2 盐胁迫下砂糖橘幼树生长量和生物量差异分析
参试的砂糖橘在春季开花时见花即被疏除,故生长主要是营养生长。从图3可知,盐胁迫下栽培90 d,植株冠幅只在0.7% NaCl胁迫下显著降低,其他5种盐胁迫间以及与CK均无显著性差异(P˃0.05,下同)。由图3-A~图3-C可知,盐分含量越高,砂糖橘株高、地径和冠幅的降低幅度越大,株高指标的差异性大于地径,以冠幅的差异性最小;同种盐胁迫下因基质含盐量水平不同而导致的株高、地径和冠幅差异不显著,差异主要因盐胁迫种类不同而导致,但0.3% CaCO3胁迫下株高、地径和冠幅均与CK无显著性差异。
由图3-D~图3-G可知,盐胁迫下砂糖橘植株根、茎、叶及全株生物量的变化同株高、地径的响应相似,但不同处理间以叶生物量的差异性最大,其次是根生物量,茎生物量的差异性最小;差异性主要受盐胁迫种类支配,同种盐胁迫下2种水平间根、茎、叶及全株生物量无显著性差异;茎生物量只在0.7% NaHCO3和0.7% NaCl胁迫下显著小于CK;0.3%和0.7% CaCO3胁迫对根生物量的抑制作用均不显著,但0.7% CaCO3胁迫下叶生物量显著减小,较CK减小22.7%。
与CK相比,NaHCO3和NaCl胁迫下,砂糖橘根、叶及全株生物量均显著减小,其中:0.3% NaHCO3胁迫下分别减少16.7%、28.3%和19.1%,0.7% NaHCO3胁迫下分别减少28.3%、39.4%和28.6%,0.3% NaCl胁迫下分别减少36.4%、56.7%和32.6%,0.7% NaCl胁迫下则分别减少46.9%、62.0%和43.4%。上述结果进一步表明,盐胁迫对砂糖橘的抑制作用主要与盐胁迫种类有关,叶片是响应最灵敏的器官。
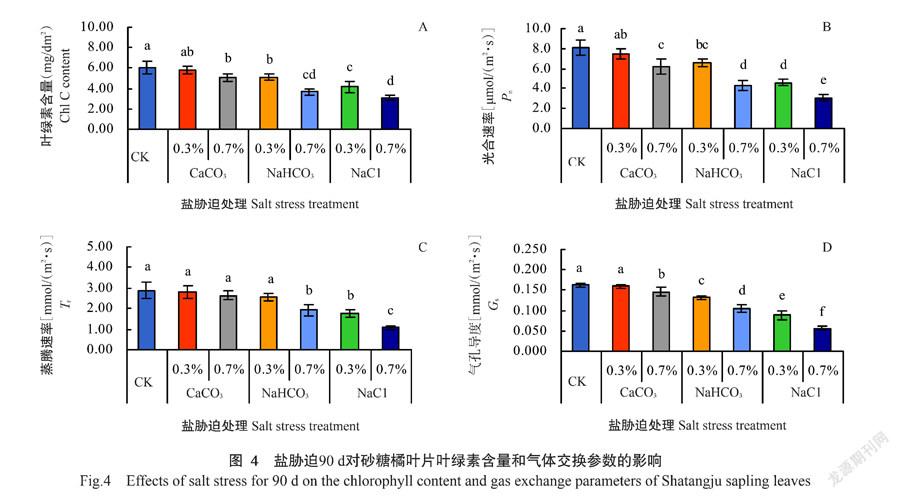
2. 3 盐胁迫对砂糖橘叶片叶绿素含量和气体交换参数的影响
盐胁迫下栽培90 d,砂糖橘叶片Chl含量与3项气体交换参数的响应趋势一致,且较生物量的响应更敏感。由图4-A可知,砂糖橘CK叶片Chl含量为6.05 mg/dm2,除0.3% CaCO3胁迫下降低幅度与CK无显著性差异外,其余盐胁迫处理均与CK存在显著差异,0.7% CaCO3、0.3% NaHCO3、0.7% NaHCO3、0.3% NaCl、0.7% NaCl处理分别较CK降低16.5%、15.4%、39.7%、31.4%和49.4%;同时,NaHCO3和NaCl胁迫内部2种含盐量水平间差异性也达显著水平,但0.7% CaCO3胁迫与0.3% NaHCO3胁迫、0.7% NaHCO3胁迫与0.3% NaCl胁迫效应相当,这种现象也存在于Pn和Tr的响应。由图4-B可知,CK植株叶片Pn值为8.1 μmol/(m2·s),0.3% CaCO3胁迫下降低但与CK无显著性差异,0.7% CaCO3、0.3% NaHCO3、0.7% NaHCO3、0.3% NaCl、0.7% NaCl胁迫下均显著低于CK,分别较CK降低23.5%、18.5%、46.9%、43.2%和65.4%,降低幅度明显大于Chl含量。
盐胁迫下栽培90 d,砂糖橘叶片Tr、Gs均较CK降低,分别为2.89和0.163 mmol/(m2·s),变化趋势近乎一致,但Gs降低幅度更大。由图4-C可知,0.3% CaCO3、0.7% CaCO3和0.3% NaHCO3胁迫下,砂糖橘叶片Tr间差异性均不显著,分别较CK降低3.8%、8.3%和11.8%,但0.7% NaHCO3、0.3% NaCl和0.7% NaCl胁迫下与CK的差异均达显著水平,分别降低32.9%、38.4%和62.9%。由图4-D可知,0.3% CaCO3胁迫下,砂糖橘叶片Gs较CK的降低幅度很小(2.5%),但0.7% CaCO3、0.3% NaHCO3、0.7% NaHCO3、0.3% NaCl和0.7% NaCl胁迫下与CK差异显著,分别降低10.4%、19.0%、36.2%、46.0%和65.6%。盐胁迫种类和含盐量水平显著影响砂糖橘叶片Chl含量和气体交换状况,尤其是叶片Pn和Gs。
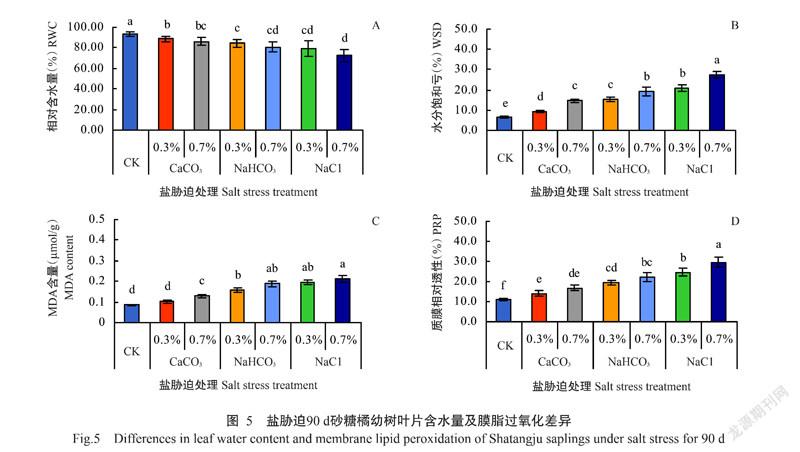
2. 4 盐胁迫对砂糖橘叶片水分亏缺和膜脂过氧化的影响
盐胁迫下栽培90 d,与CK相比,砂糖橘叶片RWC降低、WSD加重、MDA含量升高、PRP增大,但不同处理间降低或增大的幅度不同。如图5-A所示,与CK(叶片RWC为93.5%)相比,盐胁迫植株叶片RWC均显著降低,0.3% CaCO3、0.3% NaHCO3、0.3% NaCl、0.7% CaCO3、0.7% NaHCO3和0.7% NaCl脅迫下降幅分别为5.2%、9.8%、13.8%、7.6%、9.7%、15.5%和22.5%,响应与叶片Chl含量和Pn、Tr、Gs的响应基本一致。
不同处理间砂糖橘叶片WSD的差异较明显,同种盐胁迫的2种含盐量水平间差异显著(图5-B);叶片MDA含量(图5-C)和PRP(图5-D)对盐胁迫的响应趋势同叶片WSD的响应趋势基本一致,均表现为增大趋势,胁迫愈重增幅愈大。与CK相比,0.3% CaCO3、0.3% NaHCO3、0.3% NaCl、0.7% CaCO3、0.7% NaHCO3和0.7% NaCl胁迫处理叶片WSD分别增大46.2%、124.6%、136.9%、198.5%、223.1%和323.1%,MDA含量分别增大11.1%、14.4%、77.8%、111.1%、122.2%和133.3%,叶片PRP分别增大25.9%、50.9%、74.1%、99.1%、120.5%和163.4%。
3 讨论
引起植物器官非正常脱落的主因是逆境胁迫、内源激素及相关酶类的代谢,尤以非生物胁迫导致的脱落最易发生且危害严重(Goldental-Cohen et al., 2017)。至今,对于植物器官异常脱落研究最多的是落花落果,主要因为落花落果直接影响果实产量甚至导致严重减产(Yang et al.,2015;Chersicola et al.,2017;Einhorn and Arrington,2018)。本研究结果表明,砂糖橘植株以NaCl胁迫下落叶量最大、落叶的单叶重量最重,CaCO3胁迫下落叶量最小、落叶的单叶重量最轻,NaHCO3胁迫下居中;盐胁迫加重,这些落叶特性更为突出,暗示这三类盐胁迫的作用机制有差异。落叶特性应是评价砂糖橘植株对盐胁迫适应性的重要方面之一。
生长状况是植物对胁迫响应的综合表现,特别是新梢生长对胁迫很敏感。果树对胁迫的适应性或耐逆性,研究者们大多基于生理生化指标鉴评,结合生长量特别是生物量等基础生物学响应作为鉴评指标的为数不多(马翠兰等,2004;Anjum,2007;Brito et al., 2016)。本研究对盐胁迫下砂糖橘植株生长量和生物量响应的分析结果表明,生物量较生长量更能准确地反映其植株对盐胁迫的响应,但不同指标的响应差异显著:生长量指标,不同处理间以冠幅的差异性最小、株高差异性最大、地径差异性居中;生物量指标,不同处理间以叶生物量的差异性最大、是对胁迫响应最敏感的器官,其次是根生物量,茎生物量差异性最小,全株生物量的差异性居中;株高应是反映其胁迫响应的生长量关键指标,叶和根生物量是反映其胁迫响应的生物量关键指标。盐胁迫下砂糖橘表现出的这些生长特性对于果树是否具有普适性需更多测试,但无疑给现在的栽培生产提供了重要鉴评指标。
植物对土壤盐度的反应,即使同一植物种的不同品种间也可能存在显著差异。淡土植物耐盐阈值一般在0.60%以下。柑橘对盐渍环境敏感,主要源于土壤中含量过高的盐分离子。砧木是柑桔商业化栽培的重要元素,对盐分离子的吸收、积累主要通过砧木控制,使用耐盐砧木是解决其栽培中盐害问题的关键(周心智和张云贵,2019)。国内外柑橘耐盐性研究中使用最多的材料是柑橘砧木,但尚未见有研究涉及柑橘种质的绝对耐盐性。目前,通过传统育种手段获得的柑橘耐盐砧木和栽培品种很有限,主要使用NaCl模拟胁迫,处理液中NaCl浓度多为50~80 mmol/L,即0.295%~0.468% NaCl。本研究使用CaCO3、NaHCO3和NaCl等单质盐模拟盐胁迫,在栽培基质中的施用量均为0.3%和0.7%量级,这是砂糖橘生物学研究中首次开展,含盐量0.3%处理的植株生长量和生物量关键指标显著减小,同时,对NaCl胁迫最敏感,其次为NaHCO3胁迫,对CaCO3胁迫抗性强,说明砂糖橘和大多数宽皮柑橘类品种一样不耐盐,对NaCl为主的盐土及NaHCO3为主的碱土适应性差,但对钙质土壤具有潜在的较强适应性。
盐胁迫对植物的伤害及植物的适应机制,至今已有大量研究。盐胁迫下柑橘类果树叶绿素含量和光合生理指标的降低幅度与盐胁迫水平、砧木耐盐性以及是否接种菌根菌等的影响有关(Wei et al., 2018;Shahid et al., 2019;Nayem et al.,2020)。本研究中虽只使用了1种砧木,但设计了2种含盐量水平、3种胁迫成分不同的盐类在胁迫下进行90 d栽培,其结果对实际生产具有重要的指导意义。综观砂糖橘在盐胁迫下的生物学响应,不同种类和水平的盐胁迫对其幼树生长、生物量积累的抑制作用有着深刻的生理基础:光合作用是其植株正常生长发育所需的能量和物质基础,盐胁迫导致的植株水分亏缺是胁迫效应的重要生理生态因子,而膜脂过氧化作用进一步加剧光合及水分生理失调。但这些方面在设定的试验条件下主要受盐胁迫种类支配,其次是盐分含量的影响,暗示栽培基质的化学性状、盐分组成很重要。盐胁迫对砂糖橘盐分离子吸收分配的影响今后将进一步研究。
4 结论
砂糖橘对CaCO3含量较高的土壤具较强适应性,对NaCl为主的盐土及NaHCO3为主的碱土适应性很差。对砂糖橘叶片叶绿素的破坏、光合生理的抑制及伴随的水分亏缺和膜脂过氧化是不同种类盐胁迫共同的作用特性。
参考文献:
蔡小东,黄颖,曹文娟. 2012. 盐胁迫对柑橘愈伤组织生理效应的影响[J]. 湖北农业科学,51(12): 2493-2495. [Cai X D,Huang Y,Cao W J. 2012. Effect of salt stress on physio-logical characteristics of cotrus callus[J]. Hubei Agricultural Sciences,51(12):2493-2495.] doi:10.14088/j.cnki.issn0439-8114.2012.12.058.
林丽仙,李惠华,张雪芹,张庆美. 2013. 甲醛对吊兰等植物细胞质膜相对透性和光合特性的影响[J]. 热带作物学报,34(4):719-726. [Lin L X,Li H H,Zhang X Q,Zhang Q M. 2013. Effect of formaldehyde on relative permeability of plasma membrane and photosynthetic characteristics of several plants[J]. Chinese Journal of Tropical Crops,34(4):719-726.] doi:10.3969/j.issn.1000-2561.2013.04.025.
馬翠兰,刘星辉,王湘平. 2004. 盐胁迫下柚实生苗生长、矿质营养及离子吸收特性研究[J]. 植物营养与肥料学报,10(3):319-323. [Ma C L,Liu X H,Wang X P. 2004. Study on the growth,mineral nutrition and ion absorption characteristics of pomelo seedlings under salt stress[J]. Plant Nutrition and Fertilizer Science,10(3):319-323.] doi:10.3321/j.issn:1008-505X.2004.03.019.
王刘坤,祁春节. 2018. 中国柑橘主产区的区域比较优势及其影响因素研究——基于省级面板数据的实证分析[J]. 中国农业资源与区划,39(11):121-128. [Wang L K,Qi C J. 2018. Research on the comparative advantage and its influencing factors of in Chinese citrus main produ-cing region—Empirical analysis based on inter-provincial panel data[J]. Chinese Journal of Agricultural Resources and Regional Planning,39(11):121-128.] doi:10.7621/cjarrp.1005-9121.20181117.
魏清江,冯芳芳,古湘,宁少君,苏受婷,辜青青. 2016. NaCl胁迫对水培枳和枸头橙幼苗生长及盐离子分布的影响[J]. 中国南方果树,45(5): 7-11. [Wei Q J,Feng F F,Gu X,Ning S J,Su S T,Gu Q Q. 2016. Effect of NaCl stress on seedling growth and saline ions distribution in trifoliate and Citrus aurantium cv. Goutoucheng[J]. South China Fruits,45(5):7-11.] doi:10.13938/j.issn.1007-1431.2016 0282.
吳强盛,柳威,翟华芬,叶贤锋,赵伦杰. 2010. 盐胁迫下AM真菌对枳实生苗生长和根系抗氧化酶的影响[J]. 江西农业大学学报,32(4):759-762. [Wu Q S,Liu W,Zhai H F,Ye X F,Zhao L J. 2010. Influences of AM fungi on growth and root antioxidative enzymes of trifoliate orange seedlings under salt stress[J]. Acta Agriculturae Universitatis Jiangxiensis,32(4):759-762.] doi:10.3969/j.issn. 1000-2286.2010.04.023.
吴文,张瑞敏,朱从一,曾继吾,马培恰,黄永敬. 2020. 隔年交替结果对砂糖橘产量、品质的影响及效益研究[J]. 广东农业科学,47(8):30-36. [Wu W,Zhang R M,Zhu C Y,Zeng J W,Ma P Q,Huang Y J. 2020. Effect of alternate year bearing technology on yield and quality of Shatangju mandarin and its economic benefits analysis[J]. Guangdong Agricultural Sciences,47(8):30-36.] doi:10.16768/j.issn.1004-874X.2020.08.005.
徐呈祥,郑福庆,马艳萍,张少平,陈小婷,叶思敏. 2021. 贮藏温度对耐贮性不同的柑橘品种果皮蜡质含量及其化学组成的影响[J]. 食品科学,42(13):223-232. [Xu C X, Zheng F Q,Ma Y P,Zhang S P,Chen X T,Ye S M. 2021. Effect of storage temperature on peel wax content and chemical composition of citrus cultivars with different storability[J]. Food Science,42(13):223-232.] doi:10. 7506/spkx1002-6630-20190617-167.
张朝坤,陈洪彬,康仕成,刘剑锋,周文龙. 2018. 不同品种番石榴果实耐藏性和采后品质变化比较[J]. 南方农业学报,49(7):1409-1414. [Zhang C K,Chen H B,Kang S C,Liu J F,Zhou W L. 2018. A comparative study of fruit storability and postharvest quality changes among different Psidium guajava L. cultivars[J]. Journal of Sou-thern Agriculture,49(7):1409-1414.] doi:10.3969/j.issn. 2095-1191.2018.07.23.
周心智,张云贵. 2019. NaCl胁迫对5种柑橘砧木生长及生理特性的影响[J]. 西南大学学报(自然科学版),41(11): 1-6. [Zhou X Z,Zhang Y G. 2019. Evaluation of salinity tolerance in five citrus rootstock seedlings[J]. Journal of Southwest University(Natural Science),41(11):1-6.] doi:10.13718/j.cnki.xdzk.2019.11.001.
朱世平,陈娇,刘小丰,曹立,陆智明,赵晓春. 2014. 15种柑橘砧木出苗期耐盐碱性评价[J]. 西南大学学报(自然科学版),36(6):1-7. [Zhu S P,Chen J,Liu X F,Cao L,Lu Z M,Zhao X C. 2014. Evaluation of salinity and alkalinity tolerances of 15 citrus rootstocks by in vitro culture[J]. Journal of Southwest University(Natural Science),36(6):1-7.] doi:10.13718/j.cnki.xdzk.2014.06.008.
Anjum M A. 2007. Effect of NaCl concentration in irrigation water on growth and polyamine metabolism in two citrus rootstocks with different levels of salinity tolerance[J]. Acta Physiologia Plantrum,30(1):43-52. doi:10.1007/s11738-007-0089-3.
Balal R M,Ashraf M Y,Khan M M,Jaskani M J,Ashfaq M. 2011. Influence of salt stress on growth and biochemical parameters of citrus rootstocks[J]. Pakistan Journal of Botany,43(4):2135-2141. doi:10.1186/1746-4811-7-25.
Bourazza M,Chetto O,Talha A,Farih A,Douira A,Benyahia H. 2021. The influence of arbuscular mycorrhizal colonization on key growth parameters of five citrus cultivars under salt stress[J]. Plant Cell Biotechnology and Mole-cular Biology,22(41-42):125-138.
Brito M E B,da Silva Sá F V,Filho W D S,de Andrade S L,Fernandes P D. 2016. Gas exchange and chlorophyll fluorescence of citrus rootstock varieties under salt stress[J]. Revista Brasileira de Fruticultura,38(2). doi:10.1590/01 00-29452016951.
Cheng X F,Wu H H,Zou Y N,Wu Q S,Kuča K. 2021. Mycorrhizal response strategies of trifoliate orange under well-watered,salt stress,and waterlogging stress by regulating leaf aquaporin expression[J]. Plant Physiology and Biochemistry,162:27-35. doi:10.1016/j.plaphy.2021. 02.026.
Chersicola M,Kladnik A,Tušek-Žnidarič M,Mrak T,Gruden K,Dermastia M. 2017. 1-aminocyclopropane 1-carboxylate oxidase induction in tomato flower pedicel phloem and abscission related processes are differentially sensitive to ethylene[J]. Frontiers in Plant Science,8:464. doi:10.3389/fpls.2017.00464.
Einhorn T C,Arrington M. 2018. ABA and shading induce ‘Bartlett’pear abscission and inhibit photosynthesis but are not additive[J]. Journal of Plant Growth Regulation,37(1):300-308. doi:10.1007/s00334-017-9729-z.
Etehadpour M,Fatahi R,Zamani Z,Golein B,Naghavi M R,Gmitter F. 2020. Evaluation of the salinity tolerance of Iranian citrus rootstocks using morph-physiological and molecular methods[J]. Scientia Horticulturae,261:109012. doi:10.1016/j.scienta.2019.109012.
Goldental-Cohen S,Burstein C,Biton I,Ben Sasson S,Sadeh A,Many A,Doron-Faigenboim A ,Zemach H,Mugira Y,Schneider D,Birger R,Meir S,Philosoph-Hadas S,Irihomovitch V,Lavee S,Avidan B,Ben-Ari G. 2017. Ethephon induced oxidative stress in the olive leaf abscission zone enables development of a selective abscission compound[J]. BMC Plant Biology,17(1): 87. doi:10.1186/s12870-017-1035-1.
Hadian-Deljou M,Esna-Ashari M,Mirzaie-asl A. 2020. Alleviation of salt stress and expression of stress-responsive gene through the symbiosis of arbuscular mycorrhizal fungi with sour orange seedlings[J]. Scientia Horticulturae,268: 109373. doi:10.1016/j.scienta.2020.109373.
Hussain S,Luro F,Costantino G,Ollitrault P,Morillon R. 2012. Physiological analysis of salt stress behaviour of citrus species and genera: low chloride accumulation as an indicator of salt tolerance[J]. South African Journal of Botany,81: 103-112. doi:10.1016/j.sajb.2012.06.004.
Ismail A M,Horie T. 2017. Genomics,physiology,and molecu-lar breeding approaches for improving salt tolerance[J]. Annual Review of Plant Biology,68(1): 405-434. doi:10.1146/annurev-arplant-042916-040936.
Khoshbakht D,Asghari M R,Haghithi M. 2018. Effects of foliar applications of nitric oxide and spermidine on chlorophyll fluorescence,photosynthesis and antioxidant enzyme activities of citrus seedlings under salinity stress[J]. Photosynthetica,56(4):1313-1325. doi:10.1007/s11099-018-0839-z.
Munns R,Tester M. 2008. Mechanisms of salinity tolerance[J]. Annual Review of Plant Biology,59:651-681. doi:10.1146/annurev.arplant.59.032607.092911.
Nayem S A,M. Chowdhury M S M,Sultana N,Masum G Z H,Rahman Md S,Jamal M A H M. 2020. Combined effect of salt stress and Xanthomonas axonopodis pv citri on citrus(Citrus aurantifolia)[J]. Heliyon,6(2): e03403. doi:10.1016/j.heliyon.2020.e03403.
Shahid M A,Balal R M,Khan N,Simón-Grao S,Alfosea-Simón M,Cámara-Zapata J M,Mattson N S,Garcia-Sanchez F. 2019. Rootstocks influence the salt tolerance of Kinnow mandarin trees by altering the antioxidant defense system,osmolyte concentration,and toxic ion accumulation[J]. Scientia Horticulturae,250:1-11. doi:10.1016/j.scienta.2019.02.028.
Sharma D K,Dubey A K,Srivastav M,Singh A K,Sairam R K,Pandey R N,Dahuja A,Kaur C. 2014. Effect of putrescine and paclobutrazol on growth,physiochemical parameters,and nutrient acquisition of salt-sensitive citrus rootstock Karna khatta(Citrus karna Raf.) under NaCl stress[J]. Journal of Plant Growth Regulation,30:301-311. doi:10.1007/s00344-011-9192-1.
Song T Z,Shi Y Y,Shen L K,Cao C J,Shen Y,Jing W,Tian Q X,Lin F,Li W Y,Zhang W H. 2021. An endoplasmic reticulum-localized cytochrome b5 regulates high-affinity K+ transport in response to salt stress in rice[J]. Procee-dings of the National Academy of Sciences,118(50): e2114347118. doi:10.1073/pnas.2114347118.
Wang W,Liu J H. 2016. CsPAO4 of Citrus sinensis functions in polyamine terminal catabolism and inhibits plant growth under salt stress[J]. Scientific Reports,6:31384. doi:10.1038/srep31384.
Wei Q J,Ma Q L,Ning S J,Su S T,Gu Q Q. 2018. Molecular characterization and functional analysis of a cation-chloride cotransporter gene from trifoliate orange(Poncirus trifoliate L.)[J]. Trees: Struction and Function,32(1):165-173. doi:10.1007/s00468-017-1621-8.
Wu X L,Zheng F Q,Xu C X,Tang W W. 2016. Genetic diversity analysis of Shatangju mandarin(Citrus reticulata) by SCoT-PCR[J]. Agricultural Science & Technology,17(1): 34-37. doi:10.16175/j.cnki.1009-4229.2016.01.010.
Yang Z Q,Zhong X M,Fan Y,Wang H C,Li J G,Huang X M. 2015. Burst of reactive oxygen species in pedicel mediated fruit abscission after carbohydrate supply was cut off in longan(Dimocarpus longan)[J]. PhytoKeys,6: 360. doi:10.3389/fpls.2015.00360.
Zhang Y C,Wang P,Wu Q H,Zou Y N,Bao Q,Wu Q S. 2017. Arbuscular mycorrhizas improve plant growth and soil structure in trifoliate orange under salt stress[J]. Archives of Agronomy and Soil Science,63(4):491-500. doi:10.1080/03650340.2016.1222609.
收稿日期:2021-09-30
基金項目:国家现代农业产业技术体系建设专项(CARS-26);广东省农村科技特派员项目(2021-1056-9-4)
通讯作者:吉前华(1972-),https://orcid.org/0000-0003-2280-2074,博士,教授,主要从事柑橘遗传育种及生理研究工作,E-mail:qhgee@163.com
第一作者:郭雁君(1973-),https://orcid.org/0000-0003-3127-148X,副教授,主要从事柑橘栽培及生理研究工作,E-mail:yjguo@163.com
