藏猪和杜藏猪生长、肉质性状及脂肪沉积相关基因的表达差异分析
2022-07-14杨烨城黄秋艳辛海云李宝红杜宗亮孟繁明朱向星李剑豪王塑天唐冬生
杨烨城 黄秋艳 辛海云 李宝红 杜宗亮 孟繁明 朱向星 李剑豪 王塑天 唐冬生
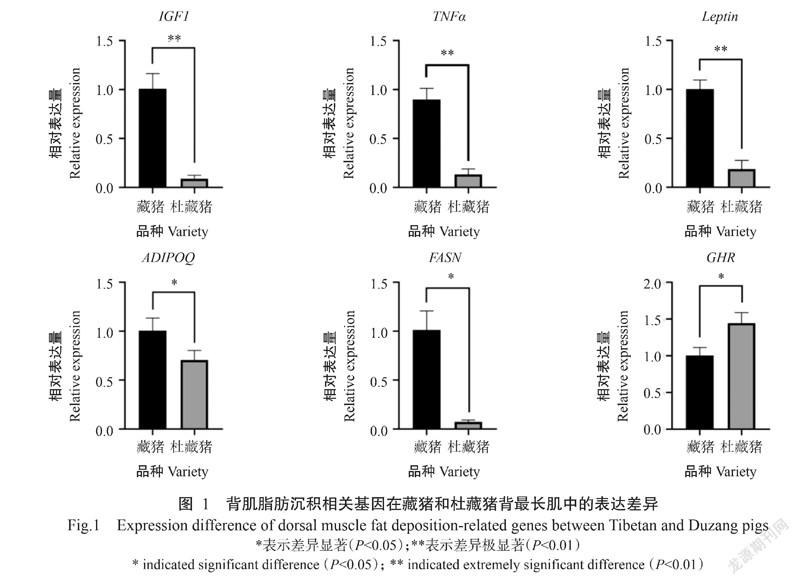
摘要:【目的】明确藏猪和杜藏猪(杜洛克父本与藏猪母本杂交F1代)的生长和胴体性状及肉质指标等表型差异,并分析背最长肌中脂肪沉积相关基因的表达差异,为藏猪种质资源利用及猪脂肪沉积规律研究提供参考依据。【方法】以藏猪和杜藏猪为研究对象,分别测定其体长、体高和胸围等生长性状,屠宰率、背膘厚及眼肌面积等胴体性状,以及pH、肉色、蒸煮损失及肌内脂肪含量等肉质指标;并通过荧光实时定量PCR检测脂肪沉积相关基因在2种猪背最长肌中的表达情况。【结果】杜藏猪的左胴体重、右胴体重、屠宰前重、胴体长、体长、体高、管围和胸围均高于藏猪,且差异显著(P<0.05,下同),眼肌面积(33.00±3.84 cm2)也显著大于藏猪(16.83±1.81 cm2),背膘厚(13.15±2.48 mm)则显著低于藏猪(22.83±2.80 mm),藏猪与杜藏猪的皮厚、腹围和屠宰率无显著差异(P>0.05,下同);杜藏猪肌肉的pH-24 h、熟肉率、剪切力和含水率显著高于藏猪肌肉,而杜藏猪肉色的a-24 h值(10.30±1.06)和肌内脂肪含量[(2.48±1.44)%]显著低于藏猪。除GHR基因外,其他脂肪沉积相关基因的相对表达量均表现为杜藏猪低于藏猪,其中,ADIPOQ基因和FASN基因的相对表达量显著低于藏猪,IGF1基因、TNFa基因和Leptin基因的相对表达量极显著低于藏猪(P<0.01)。相关分析结果显示,GHR基因相对表达量与杜藏猪肌内脂肪含量呈显著正相关,FASN基因相对表达量与藏猪肌内脂肪含量呈显著正相关,IGF1基因相对表达量与藏猪背膘呈显著负相关。【结论】杜藏猪的肉质性状与藏猪相似,但杜藏猪具有更佳的生长性能,说明以杜洛克为父本与藏猪母本杂交得到的杜藏猪具有更优的生产效率。藏猪和杜藏猪的肌内脂肪含量差异与脂肪沉积相关基因的表达差异有关,即猪的肌内脂肪含量受多种脂肪沉积相关基因调控。
关键词: 藏猪;杜藏豬;生长性状;肉质性状;脂肪沉积相关基因
中图分类号: S828.89 文献标志码: A 文章编号:2095-1191(2022)04-0926-09
Analysis of differences in growth, meat quality traits and expression of genes related to fat deposition between Tibetan and Duzang pigs
YANG Ye-cheng 1,2,HUANG Qiu-yan1,2, XIN Hai-yun2, LI Bao-hong2, DU Zong-liang2,MENG Fan-ming2, ZHU Xiang-xing1, LI Jian-hao 2, WANG Su-tian 2*,TANG Dong-sheng1*
(1School of Life Science and Engineering, Foshan University/Guangdong Key Laboratory of Animal Molecular Design and Precise Breeding/Guangdong Research Center of Gene Editing Engineering Technology, Foshan, Guangdong 528225, China; 2Institute of Animal Science, Guangdong Academy of Agricultural Sciences/State Key Laboratory
of Livestock and Poultry Breeding/Guangdong Key Laboratory of Animal Breeding and Nutrition,
Guangzhou, Guangdong 510610, China)
Abstract:【Objective】To clarify the phenotypic differences in growth, carcass traits and meat quality indexes between Tibetan and Duzang pigs (F1 generation crossed between Duroc male and Tibetan pig female) and to analyze the differences in the expression of fat depositing-related genes in longissimus dorsi muscle, so as to provide a reference for the utilization of Tibetan pig germplasm resources and the study of pig fat deposition. 【Method】Tibetan and Duzang pigs were used as the research objects, and their growth traits such as body length, height, chest circumference, slaughter rate, back fat thickness, eye muscle area and meat quality indicators such as pH, meat colour, cooking loss and intramuscular fat content were measured respectively. The expressions of fat deposition-related genes in the longissimus dorsi of the two pigs were also detected by real-time quantitative PCR. 【Result】The left carcass weight, right carcass weight, pre-slaughter weight, carcass length, body length, body height, tube circumference, and chest circumference of Duzang pigs were higher than those of Tibetan pigs. The difference was significant (P<0.05, the same below), and the eye muscle area (33.00±3.84 cm2) was also considerably larger than that of Tibetan pig (16.83±1.81 cm2), the thickness of back fat (13.15±2.48 mm) was significantly lower than that of Tibetan pigs (22.83±2.80 mm). At the same time, there was no significant difference in skin thickness, abdominal circumference and slaughter rate between Tibetan pigs and Duzang pigs (P>0.05, the same below). The pH-24 h, cooked meat rate, shearing force and water content of Duzang pig muscle were significantly higher than those of Tibetan pig muscle. The colour a-24 h value (10.30±1.06) and intramuscular fat content [(2.48±1.44)%] of Duzang pigs were significantly lower than those of Tibetan pigs. Except for GHR, the relative expression of ADIPOQ and FASN were significantly lower in Duzang pigs than in Tibetan pigs, and the relative expression of IGF1, TNFa and Leptin were extremely lower than in Tibetan pigs (P<0.01). The results of correlation analysis showed that the relative expression of GHR was significantly positively correlated with the intramuscular fat content in Duzang pigs. Furthermore, the relative expression of FASN was significantly positively correlated with the intramuscular fat content in Tibetan pigs. In addition,the relative expression of IGF1 was significantly negatively correlated with the back fat of Tibetan pigs. 【Conclusion】The meat quality traits of Duzang pigs are similar to those of Tibetan pigs, but Duzang pigs have better growth performance, indicating that Duzang pigs obtained by crossing Duroc male and Tibetan pig female have better production efficiency. The difference in intramuscular fat content between Tibetan and Duzang pigs is related to the difference in the expression of fat deposition-related genes. That is, a variety of fat deposition-related genes regulate the intramuscular fat content of pigs.
Key words: Tibetan pig; Duzang pig; growth traits; meat quality traits; fat deposition-related genes
Foundation items:Guangdong Basic and Applied Basic Research Foundationand Wen’s Joint Fund Project (2019 B1515210013);Guangdong General Universities Key Fields Special Project (2021ZDZX2050);Guangdong Academy of Agricultural Sciences Scientific Innovation Strategy Special Fund (R2019YJ-YB2004)
0 引言
【研究意义】生长和肉质性状是地方猪遗传改良最常关注的生理指标,而脂肪沉积是影响这2个性狀指标的重要因素,脂肪沉积过多会降低猪的生长效率和猪肉品质,导致养猪生产效益不高(Zhang et al.,2019)。脂肪沉积过程受遗传选择、营养水平及环境条件等因素的共同影响(相德才等,2021;邢宝松等,2021;Nematbakhsh et al.,2021),因此,探究藏猪和杜藏猪生长、肉质性状及脂肪沉积相关基因的表达模式,可为我国地方猪的遗传改良提供科学依据。【前人研究进展】藏猪是我国珍贵的物种遗传资源,主要分布在海拔2500~4300 m的青藏高原半农半牧区,具有四肢坚实、脂肪沉积能力强及肉质风味鲜美等特点,但存在体型短小、肥育周期长和背膘较厚等缺点(强巴央宗等,2001;Chen et al.,2014)。为了充分利用我国宝贵的动物遗传资源,加快地方猪的遗传改良,常通过引进外种猪与本土猪进行杂交。苑洪霞等(2017)以杜洛克父本与贵州地方品种白洗猪母本进行杂交,结果发现与纯种白洗猪相比,杜白猪F1代的不饱和脂肪酸含量及肌内脂肪含量均上调,但背膘厚和鲜味氨基酸含量显著下降。刘莹等(2018)以杜洛克为父本分别与陆川猪和隆陆猪(隆林公猪×陆川母猪)进行杂交,结果发现杜隆陆三元杂具有生长速度快、胴体品质好等优点。脂肪沉积作为猪遗传改良中重要的目标经济性状,直接影响其生长效率和猪肉品质(Suzuki et al.,2009;Bertolini et al.,2018)。脂肪是由大量成熟的脂肪细胞汇集而成,成熟脂肪细胞来源于前脂肪细胞的分裂和终端分化,而前脂肪细胞是由间充质干细胞增殖分化而来(Lefterova and Lazar,2009)。脂肪沉积过程较复杂,调控细胞生长与分化及脂滴形成等因素均参与脂肪沉积,脂肪沉积相关基因的表达则决定着肌内脂肪沉积速度(吕亚宁等,2020;欧秀琼等,2021)。其中,生长激素受体(Growth hormone receptor,GHR)是I类细胞因子受体家族的跨膜糖蛋白,敲除GHR基因后小鼠表现出全身脂肪量增加,脂肪细胞体积变大(List et al.,2013;Chhabra et al.,2021);胰岛素样生长因子1(Insulin-like growth factor 1,IGF1)在脊椎动物的胚胎发育过程中发挥重要作用,与细胞分裂及凋亡相关(Allan et al.,2000);脂联素(Adiponectin)是一种由脂肪细胞分泌的激素,可促进胎儿的脂肪沉积和脂肪细胞分化,敲除脂联素基因(ADIPOQ)能降低幼鼠初生重(Čikoš et al.,2010),猪脂肪细胞中ADIPOQ基因沉默能抑制细胞分化(Qiao et al.,2012);瘦素(Leptin)是一种细胞分泌激素,主要在白色脂肪组织中产生,在胎盘、胃底和骨骼肌中也有表达,具有调节体重、生殖及免疫功能(李恭贺等,2021),体外研究表明其可促进3T3-L1细胞中脂肪生成相关基因的表达(Palhinha et al.,2019);脂肪酸合成酶(Fatty acid synthase,FASN)被认为是一种多功能的蛋白酶,可催化饱和脂肪酸的从头合成与调节脂质代谢,其多态性会影响猪肌肉中的胆固醇水平、背脂厚及皮下脂肪组织中的多不饱和脂肪酸含量(Piórkowska et al.,2020);肿瘤坏死因子-α(Tumor necrosis factor α,TNFα)是一种多功能细胞因子,参与细胞分化、增殖、凋亡和能量代谢等多种生物学过程,同时调控脂肪酸摄取、脂肪诱导生成及脂肪分解等过程(Cawthorn and Sethi,2008)。【本研究切入点】至今,已有学者在不同动物中对上述的脂肪沉积相关基因进行研究,并证实其在不同物种间存在明显的表达差异,但针对藏猪及其杂交后代脂肪沉积相关基因表达的研究鲜见报道。【拟解决的关键问题】通过测定藏猪和杜藏猪(杜洛克父本与藏猪母本杂交F1代)的生长和胴体性状及肉质指标,发掘二者的表型差异,同时利用实时荧光定量PCR检测背最长肌中脂肪沉积相关基因的转录水平,以期为藏猪种质资源利用及猪脂肪沉积规律研究提供参考依据。
1 材料与方法
1. 1 试验材料
选择6头7月龄藏猪(引种自甘肃省合作地区)和6头7月龄杜藏猪,所有猪只均饲养于广东省农业科学院畜牧研究所藏猪养殖基地,采取半开放猪舍的养殖模式,以相同的日粮水平进行饲喂,自由采食。屠宰前禁食12 h但不禁止饮水,屠宰后采集左半边胴体第13肋和第14肋间的背最长肌肉样品,在冰上将样品剪碎后装入细胞冻存管中并迅速置于装有液氮的泡沫箱中,运送回实验室液氮罐保存备用。提取脂肪的石油醚购自天津市百世化工有限公司,TRIzol试剂购自美国Ambion公司,氯仿购自广东广试试剂科技有限公司,RNA反转录试剂盒PrimeScriptTM RT reagent Kit with gDNA Eraser和RT-PCR试剂盒TB Green® Premix Ex TaqTM II购自TaKaRa公司。主要仪器设备:数显式肌肉嫩度仪购自南农畜牧技术(北京)有限公司,皮尺和游标卡尺购自上海得力文具有限公司,便携式pH计购自德图仪表有限公司,肉色测定仪购自柯尼卡美能达有限公司,蒸锅购自美的集团股份有限公司,紫外分光光度计购自美国NanoDrop公司,全自动脂肪分析仪购自北京安科博瑞科技有限公司,实时荧光定量PCR仪(CFX96)购自美国Bio-Rad公司。
1. 2 引物设计与合成
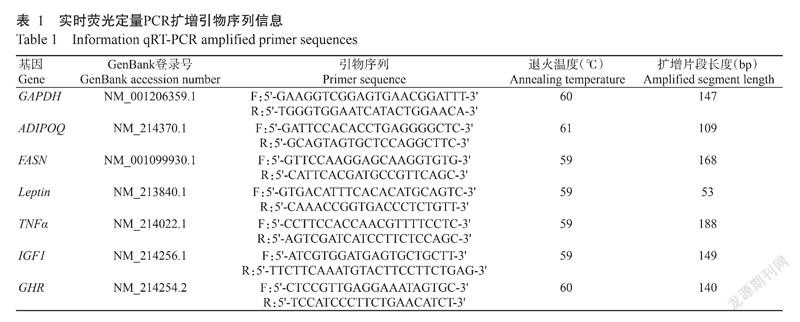
通過NCBI检索猪脂肪沉积相关基因mRNA序列,并根据实时荧光定量PCR引物设计原则设计扩增引物(表1),并委托生工生物工程(上海)股份有限公司合成。
1. 3 生长和胴体性状测定
测定猪的体长、体高和胸围等生长性状,并依照NY/T 825—2004《瘦肉型猪胴体性状测定技术规范》测定屠宰率、背膘厚及眼肌面积等胴体性状。
1. 4 肉用性状指标测定
依照NY/T 821—2004《猪肌肉品质测定技术规范》进行测定肉质指标,包括pH、肉色、剪切力及肌内脂肪含量等指标。
1. 5 猪脂肪沉积相关基因定量分析
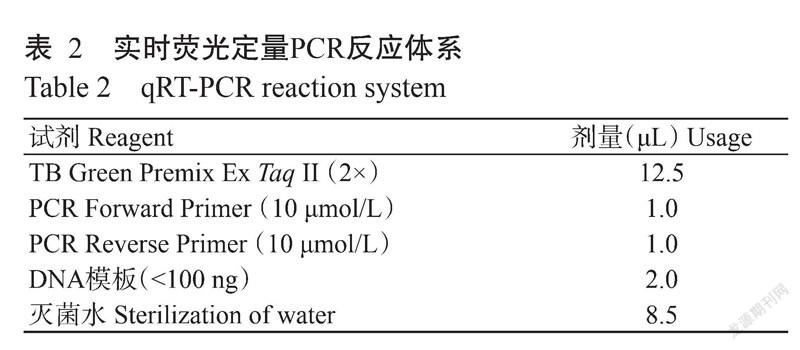
从液氮罐中取出藏猪和杜藏猪的背最长肌样品,采用TRIzol法提取总RNA,经紫外分光光度计测定总RNA浓度后,按照PrimeScriptTM RT reagent Kit with gDNA Eraser试剂盒说明在冰上去除基因组DNA并反转录合成cDNA。反转录体系20.0 μL:去除基因组DNA反应液(5×gDNA Eraser Buffer 2.0 μL,gDNA Eraser 1.0 μL,Total RNA 1.0 μL,RNase Free H2O补足至10.0 μL)10.0 μL,PrimeScript RT Enzyme Mix I 1.0 μL,RT Primer Mix 1.0 μL,5×PrimeScript Buffer 2 4.0 μL,RNase Free H2O 4.0 μL。根据TB Green® Premix Ex TaqTM II试剂盒说明,在冰上配制实时荧光定量PCR反应体系25.0 μL(表2),扩增程序:95 ℃预变性30 s;95 ℃ 5 s,60 ℃ 30 s,进行39个循环。
1. 6 统计分析
采用SPSS 24.0统计分析测定获得的生长、胴体和肉质性状数据,并进行T检验。以GAPDH为内参基因对实时荧光定量PCR检测结果进行校正,以2‒ΔΔCt法换算脂肪沉积相关基因相对表达量,经T检验后采用GraphPad Prism 8制图。同时,以SPSS 24.0中的Bivariate correlation分析肌内脂肪含量、背膘厚与IGF1、TNFa、Leptin、ADIPOQ、FASN和GHR基因相对表达量的相关性。
2 结果与分析
2. 1 生长和胴体性状测定结果
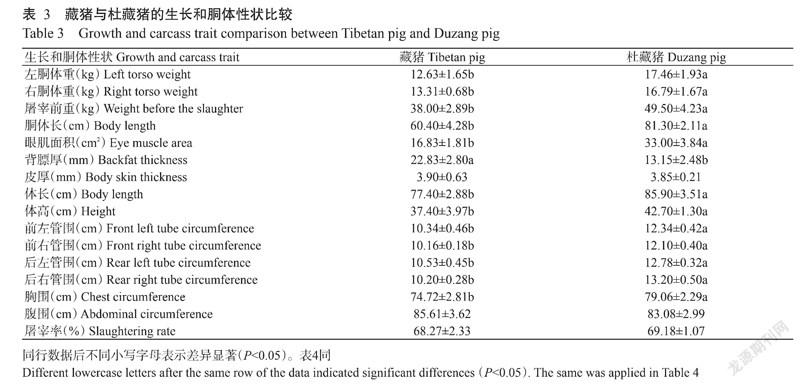
由表3可知,杜藏猪的左胴体重、右胴体重、屠宰前重、胴体长、体长、体高、管围和胸围均高于藏猪,且差异显著(P<0.05,下同),说明杜藏猪整体生长速度较快;杜藏猪的眼肌面积(33.00±3.84 cm2)也显著大于藏猪(16.83±1.81 cm2),说明杜藏猪的背肌更大。此外,杜藏猪的背膘厚为13.15±2.48 mm,显著低于藏猪(22.83±2.80 mm),而藏猪与杜藏猪的皮厚、腹围和屠宰率无显著差异(P>0.05,下同)。
2. 2 肉质性状测定结果
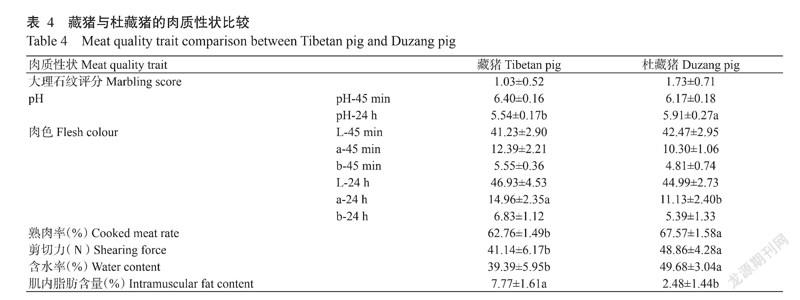
由表4可知,杜藏猪肌肉的pH-24 h、熟肉率、剪切力和含水率显著高于藏猪,而杜藏猪肉色的a-24 h值(10.30±1.06)和肌内脂肪含量[(2.48±1.44)%]显著低于藏猪,其他肉质指标在藏猪和杜藏猪间并无显著差异。
2. 3 背肌脂肪沉积相关基因的表达情况
由图1可看出,除GHR基因的相对表达量呈杜藏猪显著高于藏猪外,其他脂肪沉积相关基因的相对表达量均表现为杜藏猪低于藏猪,其中,ADIPOQ基因和FASN基因的相对表达量显著低于藏猪,IGF1基因、TNFa基因和Leptin基因的相对表达量极显著低于藏猪(P<0.01)。
2. 4 脂肪沉积相关基因表达水平与肌内脂肪含量和背膘厚的相关分析结果
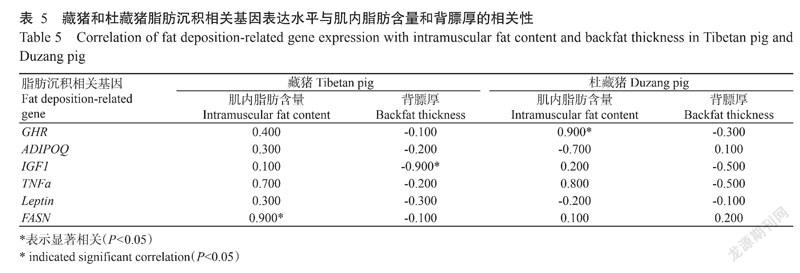
由表5可知,藏猪的背最长肌肌内脂肪含量与FASN基因相对表达量呈显著正相关,而背膘厚与IGF1基因相对表达量呈显著负相关;杜藏猪除了肌内脂肪含量与GHR基因相对表达量呈显著正相关外,肌内脂肪含量和背膘厚与其他脂肪沉积相关基因相对表达量的相关性均未达显著水平。
3 讨论
我国地方猪遗传资源丰富,具有性繁殖力高、抗逆性强、耐粗饲及抗寒冷等优点,但也存在生长速度慢、出栏率低和瘦肉率低等问题,从而限制了地方猪的产业化和规模化发展进程。选用地方猪与外国瘦肉型品种进行杂交可有效改善我国地方猪的生产性能(赵连生,2007),李闯等(2019)选用广东小耳花猪与巴克夏公猪杂交培育出巴花猪,巴花猪既遗传了父本生长快、瘦肉率高的特性,又保留了母本肌肉细嫩、大理石纹丰富的特点;方晓敏等(2021)以巴克夏猪与苏紫黑猪母本杂交获得巴苏猪F1代,且发现6月龄的巴苏猪各项生长指标均显著高于纯种苏紫黑猪。本研究通过对比藏猪和杜藏猪的生长、胴体性状,发现杜藏猪的多个性状优于藏猪,其中,杜藏猪的宰前体重较藏猪显著增重11.50±7.12 kg,胴体长、体长和体高分别较藏猪显著增加20.90±6.39、8.50±6.39和5.30±5.27 cm;杜藏猪的左胴体重、右胴体重、皮厚、管围及胸围等指标也均优于藏猪,而背膘厚较藏猪显著降低9.68±5.29 cm,表明F1代杜藏猪有效遗传了父本的优良生长和胴体性状。
猪肉品质的颜色主要与肌红蛋白有关,肌红蛋白对氧的亲和力高于血红蛋白,高原品种藏猪生长速度较慢,瘦肉含量较少,但肌红蛋白含量高,肉质较好(Liang et al.,2015)。与约克夏猪相比,藏猪的肉质含有相对较低的L值和更高的a值(Gan et al.,2019)。任守文等(2020)研究表明,以大白公猪与中国新配套系苏种猪杂交获得的苏山猪具有更优质的肉质性状。在本研究中,藏猪肉的L-45 min值低于杜藏猪,而a-45 min高于杜藏猪,但均未达差异显著水平,可能与杜藏猪依然保持着母本的高肌红蛋白特性有关。生猪被屠宰后,体内无氧糖酵解过程取代氧化代谢,从而引起肉质产生乳酸并积累,该过程与肉质的pH、亮度、嫩度及含水率密切相关(Luo et al.,2017)。pH直接影响肉质加工和食用的品质,藏猪肉质的pH-45 min略高于杜藏猪,但差异不显著,说明藏猪肉在屠宰后短时间可能有更优的肉品质;藏猪肉质的pH-24 h显著低于杜藏猪,可能与藏猪肉的无氧糖酵解过程积累关。陈映等(2020)研究发现,藏猪背肌嫩度显著小于其他中外杂交猪种;杜长大三元杂背肌熟肉率约64.95%,较本研究中藏猪的熟肉率[(62.76±1.49)%]略高,但低于杜藏猪的熟肉率[(67.57±1.58)%];肌肉嫩度可能与肌纤维直径有关,而肌纤维粗细和密度及肌肉脂肪含量均会影响肉质的细嫩程度。此外,Nikkilä等(2013)研究发现猪的体长越长其眼肌面积越小。本研究结果表明,杜藏猪的眼肌面积和体长均显著高于藏猪,可能与选取的藏猪和杜藏体重存在差异有关。Ohnishi和Satoh(2018)研究表明,眼肌面积与体长无明显的相关性,同时发现眼肌面积与猪的后宽呈正相关,与胸深和胸宽呈负相关。可见,不同猪种间影响眼肌面积的因素仍有待进一步探究。
猪肉品质受肌内脂肪含量的影响,而影響肌内脂肪含量的因素包括遗传和营养两大方面。至今,已有大量与动物脂肪沉积相关的基因被发现,包括GHR基因(杨慧等,2014)、FASN基因(Zappaterra et al.,2016)、TNF基因、LEP基因(Stachowiak and Flisikowski,2019)、ADIPOQ基因(Villaplana-Velasco et al.,2021)及IGF1基因(Wang et al.,2021)等。Wang等(2017)研究发现,去势公猪体内的脂肪含量显著增加的同时,GHR基因表达量在皮下脂肪中显著上调,但在肝脏中显著下调,故推测GHR基因表达与猪的脂肪发育相关。本研究结果表明,藏猪肌内脂肪含量显著高于杜藏猪,而藏猪背最长肌GHR基因相对表达量显著低于杜藏猪。IGF1基因表达与脂肪细胞含量间存在一定程度的正相关(Wang et al.,2019)。在本研究中,藏猪肌内脂肪含量和IGF1基因相对表达量均高于杜藏猪,进一步证实IGF1基因作为脂肪沉积相关基因在猪的生长发育过程中扮演着重要角色。ADIPOQ基因通过促进脂肪细胞分化和抑制脂解作用,而调控脂质积累(Kubota et al.,2007)。与杜藏猪相比,藏猪含有更高水平的肌内脂肪和ADIPOQ基因相对表达量,与预期结果一致。本研究还发现,杜藏猪背最长肌中的Leptin基因相对表达量极显著低于藏猪,是由于瘦素主要参与调节动物对食物的摄入和新陈代谢,进而影响到脂肪在背最长肌中的沉积速度。TNF通过调节脂蛋白含量和 PPARG 转录因子的表达,在脂肪发生中发挥重要作用。本研究发现,杜藏猪中的TNFα基因相对表达量极显著低于藏猪,暗示藏猪肌内脂肪沉积高于杜藏猪可能与TNFa基因的表达水平存在关联。FASN与脂肪沉积和脂肪酸组成有关,能间接激活脂肪酸氧化基因而控制脂肪酸分解代谢(Jensen-Urstad and Semenkovich,2012)。张雄等(2021)研究发现,野柯杂交猪背肌中的FASN基因表达量与背膘厚呈正相关,由此推测FASN基因表达量越高猪的背膘越厚。在本研究中,具有更厚背膘的藏猪FASN基因表达量显著高于杜藏猪,与焦明慧等(2014)的研究一致。此外,本研究的相关分析结果显示,GHR基因相对表达量与杜藏猪肌内脂肪含量呈显著正相关,FASN基因相对表达量与藏猪肌内脂肪含量呈显著正相关,IGF1基因相对表达量与藏猪背膘呈显著负相关,与赵小琪等(2018)、Yu等(2018)、张斌等(2019)的研究结果一致。同一基因在藏猪和杜藏猪中与肌内脂肪含量和背膘厚的相关性并不完全相同,可能是受到品种差异的影响,但具体分子机制有待进一步探究。
4 结论
杜藏猪的肉质性状与藏猪相似,但杜藏猪具有更佳的生长性能,说明以杜洛克为父本与藏猪母本杂交得到的杜藏猪具有更优的生产效率。藏猪和杜藏猪的肌内脂肪含量差异与脂肪沉积相关基因的表达差异有关,即猪的肌内脂肪含量受多种脂肪沉积相关基因调控。
参考文献:
陈映,葛桂华,徐旭,李强,丁俊仁,曾仰双,王小强,刘一辉,张顺华,朱砺. 2020. 品种和肌纤维类型对猪肉质性状的影响[J]. 中国畜牧杂志,56(11):52-55. [Chen Y,Ge G H,Xu X,Li Q,Ding J R,Zeng Y S,Wang X Q,Liu Y H,Zhang S H,Zhu L. 2020. Effect of different muscle fiber types on meat quality in pigs[J]. Chinese Journal of Animal Science,56(11):52-55.] doi:10.19556/j.0258-7033. 20191230-04.
方晓敏,王丽,涂枫,赵为民,李碧侠,王学敏,任守文. 2021. 导入巴克夏猪血统对苏紫黑猪生产性能的影响[J]. 江苏农业科学,49(9):138-142. [Fang X M,Wang L,Tu F,Zhao W M,Li B X,Wang X M,Ren S W. 2021. Effects of introduced Berkshire pigs lineage on performance of Suzi black pigs[J]. Jiangsu Agricultural Sciences,49(9):138-142.] doi:10.15889/j.issn.1002-1302.2021.09.025.
焦明慧,周梅,陈宏权,陈公伟,谢亚男,潘中婷,王学故,马帮军. 2014. 不同产脂能力猪脂肪合成相关基因表达谱分析[J]. 安徽农业大学学报,41(3):367-370. [Jiao M H,Zhou M,Chen H Q,Chen G W,Xie Y N,Pan Z T,Wang X G,Ma B J. 2014. Gene expression analysis related to the fatty synthesis for the different fat deposition capacity in pigs[J]. Journal of Anhui Agricultural University,41(3):367-370.] doi:10.13610/j.cnki.1672-352x.20140423. 020.
李闯,韦明飞,莫德林,陈建伟,王永江,刘小红,阳林芳,曾检华. 2019. 巴克夏与广东小耳花猪杂交效果分析[J]. 中国畜牧杂志,55(4):62-65. [Li C,Wei M F,Mo D L,Chen J W,Wang Y J,Liu X H,Yang L F,Zeng J H. 2019. Analysis of crossbreeding effect between Berkshire and Guangdong small-eared pigs[J]. Chinese Journal of Animal Science,55(4):62-65.] doi:10.19556/j.0258-7033. 2019-04-062.
李恭贺,陈秋玉,吴文德,郑喜邦. 2021. 水牛瘦素基因的真核表达及生物学特性分析[J]. 河南农业科学,50(10):132-137. [Li G H,Chen Q Y,Wu W D,Zheng X B. 2021. Eukaryotic expression and biological characterization of leptin gene in buffalo[J]. Journal of Henan Agricultural Sciences,50(10):132-137.] doi:10.15933/j.cnki.1004-3268.2021.10.017.
刘莹,龙欢,牛丽珠,马婷婷,刘炎,张欣怡,王目凤,林德源,黄华伟,李新云,赵书红,徐学文. 2018. 杜陆与杜隆陆猪生长、胴体及肉质性状的比较分析[J]. 畜牧兽医学报,49(12):2576-2583. [Liu Y,Long H,Niu L Z,Ma T T,Liu Y,Zhang X Y,Wang M F,Lin D Y,Huang H W,Li X Y,Zhao S H,Xu X W. 2018. Comparison of growth,carcass and meat quality traits between Dulu and Dulonglu pigs[J]. Acta Veterinaria et Zootechnica Sinica,49(12):2576-2583.] doi:10.11843/j.issn.0366-6964.2018.12.006.
吕亚宁,贺琛昕,兰旅涛. 2020. 猪肌内脂肪与肉品质的关系及其影响因素的研究进展[J]. 中国畜牧兽医,47(2):554-563. [Lü Y N,He C X,Lan L T. 2020. Research advances on the relationship between intramuscular fat and meat quality and influence factor of intramuscular fat in pigs[J]. China Animal Husbandry and Veterinary Medicine,47(2):554-563.] doi:10.16431/j.cnki.1671-7236. 2020.02.027.
歐秀琼,李睿,张晓春,钟正泽,解华东,李星,布丽君,景绍红,彭鸿. 2021. 猪肌纤维性状形成和肌内脂肪沉积的遗传机制[J]. 中国畜牧兽医,48(3):925-931. [Ou X Q,Li R,Zhang X C,Zhong Z Z,Xie H D,Li X,Bu L J,Jing S H,Peng H. 2021. Genetic mechanism of pig muscle fiber property formation and intramuscular fat deposition[J]. China Animal Husbandry and Veterinary Medicine,48(3):925-931.] doi:10.16431/j.cnki.1671-7236.2021.03.016.
强巴央宗,谢庄,田发益. 2001. 高原藏猪现状与保种策略[J]. 中国畜牧杂志,37(6):46-47. [Chamba Y Z,Xie Z,Tian F Y. 2001. Current situation and conservation strategy of Tibetan pigs in Plateau[J]. Chinese Journal of Animal Science,37(6):46-47.] doi:10.3969/j.issn.0258-7033.2001. 06.020.
任守文,李碧侠,葛云山,王学敏,方晓敏,付言峰,赵为民. 2020. 苏山猪选育研究[J]. 中国畜牧杂志,56(11):47-51. [Ren S W,Li B X,Ge Y S,Wang X M,Fang X M,Fu Y F,Zhao W M. 2020. Study on breeding of Sushan pig[J]. Chinese Journal of Animal Science,56(11):47-51.] doi:10.19556/j.0258-7033.20191121-07.
相德才,张斌,韩敏,刘韶娜,赵智勇,吴国权. 2021. 迪庆藏猪ADFP基因多态性及其组织表达特征[J]. 南方农业学报,52(10):2872-2879. [Xiang D C,Zhang B,Han M,Liu S N,Zhao Z Y,Wu G Q. 2021. The polymorphism and its expression characteristics in the tissues of ADFP gene in Diqing Tibetan pig[J]. Journal of Southern Agriculture,52(10):2872-2879.] doi:10.3969/j.issn.2095-1191.2021.10.028.
邢宝松,王璟,白献晓,陈俊峰,张家庆,任巧玲,郭红霞,张华,曹海. 2021. 不同养殖模式对淮南猪屠宰和肉质性状的影响[J]. 河南农业科学,50(1):160-165. [Xing B S,Wang J,Bai X X,Chen J F,Zhang J Q,Ren Q L,Guo H X,Zhang H,Cao H. 2021. Influence of different feeding styles on slaughter and meat quality traits of Huainan pigs[J]. Journal of Henan Agricultural Sciences,50(1):160-165.] doi:10.15933/j.cnki.1004-3268.2021.08.019.
楊慧,陈宝剑,兰干球,郭亚芬,蒋钦杨,杨秀荣. 2014. 猪GHR基因两个SNPs位点多态性与生长性状的关联分析[J]. 南方农业学报,45(11):2052-2057. [Yang H,Chen B J,Lan G Q,Guo Y F,Jiang Q Y,Yang X R. 2014. Correlation analysis on 2 SNPs sites polymorphism in GHR gene and corresponding growth traits of pig[J]. Journal of Southern Agriculture,45(11):2052-2057.] doi:10.3969/ j.issn.2095-1191.2014.11.2052.
苑洪霞,王鑫,孙振梅,李鹏程,冯文武,陈祥. 2017. 白洗猪与杜洛克猪杂交F1代猪肉品质特性研究[J]. 中国畜牧兽医,44(3):761-766. [Yuan H X,Wang X,Sun Z M,Li P C,Feng W W,Chen X. 2017. The meat quality and cha-racteristics of Baixi pig×Turok hybrid F1 generation[J]. China Animal Husbandry and Veterinary Medicine,44(3):761-766.] doi:10.16431/j.cnki.1671-7236.2017.03.020.
张斌,相德才,赵智勇,吴国权,刘韶娜,胡清泉,陈吉红,韩敏,常雅洁,杨仁灿,赵彦光. 2019. IGFs和MSTN基因表达量与种猪生长性状的相关性研究[J]. 云南农业大学学报(自然科学),34(2):263-270. [Zhang B,Xiang D C,Zhao Z Y,Wu G Q,Liu S N,Hu Q Q,Chen J H,Han M,Chang Y J,Yang R C,Zhao Y G. 2019. Correlation analysis on expression of IGFs and MSTN genes and growth traits in breeding pigs[J]. Journal of Yunnan Agricultural University(Natural Sciences),34(2):263-270.] doi: 10.12101/j.issn.1004-390X(n).201801022.
张雄,史开志,张勇,郭勇,陈大军,尚以顺. 2021. 猪FASN基因表达与胴体及肉质性状相关性研究[J]. 黑龙江畜牧兽医,(9):64-67. [Zhang X,Shi K Z,Zhang Y,Guo Y,Chen D J,Shang Y S. 2021. Study on the correlation between FASN gene expression and carcass and meat quality traits in pigs[J]. Heilongjiang Animal Science and Vete-rinary Medicine,(9):64-67.] doi:10.13881/j.cnki.hljxmsy.2020.05.0352.
赵连生. 2007. 我国地方猪种在产业化发展中的作用[J]. 中国畜牧兽医,34(10):151-153. [Zhao L S. 2007. The role of local pig breeds in the development of industria-lization in China[J]. China Animal Husbandry and Veterinary Medicine,34(10):151-153.] doi:10.3969/j.issn. 1671-7236.2007.10.057.
赵小琪,谢宇潇,潘洪彬,黄英,杨明华,赵素梅. 2018. 不同品种猪肌内脂肪合成代谢相关基因表达水平的研究[J]. 黑龙江畜牧兽医,(19):6-10. [Zhao X Q,Xie Y X,Pan H B,Huang Y,Yang M H,Zhao S M. 2018. Study of intramuscular fat synthesis metabolism related genes expression level in the different varieties of pigs[J]. Heilongjiang Animal Science and Veterinary Medicine,(19):6-10.] doi:10.13881/j.cnki.hljxmsy.2017.12.0051.
Allan G J,Flint D J,Darling S M,Geh J,Patel K. 2000. Altered expression of insulin-like growth factor-1 and insulin like growth factor binding proteins-2 and 5 in the mouse mutant Hypodactyly(Hd) correlates with sites of apoptotic activity[J]. Anatomy and Embryology,202(1):1-11. doi:10.1007/pl00008239.
Bertolini F,Schiavo G,Galimberti G,Bovo S,D'Andrea M,Gallo M,Buttazzoni L,Rothschild M F,Fontanesi L. 2018. Genome-wide association studies for seven production traits highlight genomic regions useful to dissect dry-cured ham quality and production traits in Duroc heavy pigs[J]. Animal,12(9):1777-1784. doi:10.1017/S17517 31118000757.
Cawthorn W P,Sethi J K. 2008. TNF-α and adipocyte biology[J]. FEBS Letter,582(1):117-131. doi:10.1016/j.febslet.2007.11.051.
Chen A D,Hao L L,Fang X B,Lu K,Liu S C,Zhang Y L. 2014. Polymorphism analysis of IGFBP-5 gene exon 1 in Tibet mini-pig and Junmu No.1 white pig[J]. Genetics and Molecular Research,13(1):1643-1649. doi:10.4238/2014.March.12.17.
Chhabra Y,Lee C M M,Müller A F,Brooks A J. 2021. GHR signalling:Receptor activation and degradation mechanisms[J]. Molecular and Cellular Endocrinology,520:111075. doi:10.1016/j.mce.2020.111075.
Čikoš S,Burkuš J,Bukovská A,Fabian D,Rehák P,Koppel J. 2010. Expression of adiponectin receptors and effects of adiponectin isoforms in mouse preimplantation embryos[J]. Human Reproduction,25(9):2247-2255. doi:10.1093/ humrep/deq193.
Gan M L,Shen L Y,Fan Y,Guo Z X,Liu B,Chen L,Tang G Q,Jiang Y Z,Li X W,Zhang S H,Bai L,Zhu L. 2019. High altitude adaptability and meat quality in Tibetan pigs:A reference for local Pork processing and genetic improvement[J]. Animals (Basel),9(12):1080. doi:10. 3390/ani9121080.
Jensen-Urstad A P L,Semenkovich C F. 2012. Fatty acid synthase and liver triglyceride metabolism:Housekeeper or messenger?[J]. Biochimica et Biophysica Acta,1821(5):747-753. doi:10.1016/j.bbalip.2011.09.017.
Kubota N,Yano W,Kubota T,Yamauchi T,Itoh S,Kumagai H,Kozono H,Takamoto I,Okamoto S,Shiuchi T,Suzuki R,Satoh H,Tsuchida A,Moroi M,Sugi K,Noda T,Ebinuma H,Ueta Y,Kondo T,Araki E,Ezaki O,Nagai R,Tobe K,Terauchi Y,Ueki K,Minokoshi Y,Kadowaki T. 2007. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake[J]. Cell Metabolism,6(1):55-68. doi:10.1016/j.cmet.2007. 06.003.
Lefterova M I,Lazar M A. 2009. New developments in adipogenesis[J]. Trends in Endocrinology & Metabolism,20(3):107-114. doi:10.1016/j.tem.2008.11.005.
Liang Y,Yang X M,Gu Y R,Tao X,Zhong Z Z,Gong J J,Chen X H,Lü X B. 2015. Developmental changes in the expression of the GLUT2 and GLUT4 genes in the longissimus dorsi muscle of Yorkshire and Tibetan pigs[J]. Genetics and Molecular Research,14(1):1287-1292. doi:10.4238/2015.February.13.7
List E O,Berryman D E,Funk K,Gosney E S,Jara A,Kelder B,Wang X Y,Kutz L,Troike K,Lozier N,Mikula V,Lubbers E R,Zhang H,Vesel C,Junnila R K,Frank S J,Masternak M M,Bartke A,Kopchick J J. 2013. The role of GH in adipose tissue:Lessons from adipose-specific GH receptor gene-disrupted mice[J]. Molecular Endocrinology,27(3):524-535. doi:10.1210/me.2012-1330.
Luo J,Shen Y L,Lei G H,Zhu P K,Jiang Z Y,Bai L,Li Z M,Tang Q G,Li W X,Zhang H S,Zhu L. 2017. Correlation between three glycometabolic-related hormones and muscle glycolysis,as well as meat quality,in three pig breeds[J]. Journal of the Science of Food and Agriculture,97(9):2706-2713. doi:10.1002/jsfa.8094.
Nematbakhsh S,Chong P P,Selamat J,Nordin N,Idris L H,Abdull Razis A F. 2021. Molecular regulation of lipogene-sis,adipogenesis and fat deposition in chicken[J]. Genes (Basel),12(3):414. doi:10.3390/genes12030414.
Nikkilä M T,Stalder K J,Mote B E,Rothschild M F,Gunsett F C,Johnson A K,Karriker L A,Boggess M V,Serenius T V. 2013. Genetic parameters for growth,body composition,and structural soundness traits in commercial gilts[J]. Journal of Animal Science,91(5):2034-2046. doi:10. 2527/jas.2012-5722.
Ohnishi C,Satoh M. 2018. Estimation of genetic parameters for performance and body measurement traits in Duroc pigs selected for average daily gain,loin muscle area,and backfat thickness[J]. Livestock Science,214:161-166. doi:10.1016/j.livsci. 2018.05.022.
Palhinha L,Liechocki S,Hottz E D,da Silva Pereira J,de Almeida C J,Moraes-Vieira P M M,Bozza P T,Maya-Monteiro C M. 2019. Leptin induces proadipogenic and proinflammatory signaling in adipocytes[J]. Frontiers in Endocrinology (Lausanne),10:841. doi:10.3389/fendo.2019. 00841.
Piórkowska K,Malopolska M,Ropka-Molik K,Szyndler-Nedza M,Wiechniak A,Żukowski K,Lambert B,Tyra M. 2020. Evaluation of SCD,ACACA and FASN mutations:Effects on pork quality and other production traits in pigs selected based on RNA-Seq results[J]. Animals (Basel),10(1):123. doi:10.3390/ani10010123.
Qiao L P,Yoo H S,Madon A,Kinney B,Hay Jr W W,Shao J H. 2012. Adiponectin enhances mouse fetal fat deposition[J]. Diabetes, 61(12):3199-3207. doi:10.2337/db12-0055.
Stachowiak M,Flisikowski K. 2019. Analysis of allele-speci-fic expression of seven candidate genes involved in lipid metabolism in pig skeletal muscle and fat tissues reveals allelic imbalance of ACACA,LEP,SCD,and TNF[J]. Journal of Applied Genetics,60(1):97-101. doi:10.1007/s13353-019-00485-z.
Suzuki K,Inomata K,Katoh K,Kadowaki H,Shibata T. 2009. Genetic correlations among carcass cross-sectional fat area ratios,production traits,intramuscular fat,and serum leptin concentration in Duroc pigs[J]. Journal of Animal Science,87(7):2209-2215. doi:10.2527/jas.2008-0866.
Villaplana-Velasco A,Noguera J L,Pena R N,Ballester M,Munoz L,González E,Tejeda J F,Ibánez-Escriche N. 2021. Comparative transcriptome profile between Iberian pig varieties provides new insights into their distinct fat deposition and fatty acids content[J]. Animals (Basel),11(3):627. doi:10.3390/ani11030627.
Wang B B,Li P H,Zhou W D,Gao C,Liu H,Li H X,Niu P P,Zhang Z P,Li Q,Zhou J,Huang R H. 2019. Association of twelve candidate gene polymorphisms with the intramuscular fat content and average backfat thickness of Chinese Suhuai pigs[J]. Animals (Basel),9(11):858. doi:10.3390/ani9110858.
Wang J,Chen J F,Zhang J Q,Gao B W,Bai X X,Lan Y L,Lin P,Guo H X,Gao Y,Xing B S. 2017. Castration-induced changes in the expression profiles and promoter methylation of the GHR gene in Huainan male pigs[J]. Animal Science Journal,88(8):1113-1119. doi:10.1111/asj.12739.
Wang W J,Guo Y Q,Xie K J,Li Y D,Li Z W,Wang N,Xiao F,Guo H S,Li H,Wang S Z. 2021. A functional variant in the promoter region of IGF1 gene is associated with chicken abdominal fat deposition[J]. Domestic Animal Endocrinology,75:106584. doi:10.1016/j.domaniend. 2020.106584.
Yu H H,Long W H,Zhang X Z,Xu K X,Guo J X,Zhao H,Li H H,Qing Y B,Pan W R,Jia B Y,Zhao H Y,Huang X X,Wei H J. 2018. Generation of GHR-modified pigs as Laron syndrome models via a dual-sgRNAs/Cas9 system and somatic cell nuclear transfer[J]. Journal of Trans-lational Medicine,16(1): 41. doi:10.1186/s12967-018-1409-7.
Zappaterra M,Deserti M,Mazza R,Braglia S,Zambonelli P,Davoli R. 2016. A gene and protein expression study on four porcine genes related to intramuscular fat deposition[J]. Meat Science,121:27-32. doi:10.1016/j.meatsci.2016. 05.007.
Zhang Y F,Zhang J J,Gong H F,Cui L L,Zhang W C,Ma J W,Chen C Y,Ai H S,Xiao S J,Huang L S,Yang B. 2019. Genetic correlation of fatty acid composition with growth,carcass,fat deposition and meat quality traits based on GWAS data in six pig populations[J]. Meat Science,150:47-55. doi:10.1016/j.meatsci.2018.12.008.
收稿日期:2021-07-18
基金項目:广东省基础与应用基础研究基金温氏联合基金项目(2019B1515210013);广东省普通高校重点领域专项(2021ZDZX2050);广东省农业科学院科技创新战略专项(R2019YJ-YB2004)
通讯作者:王塑天(1989-),https://orcid.org/0000-0002-3554-5022,博士,副研究员,主要从事抗病基因功能研究与抗病育种工作,E-mail:wstlyt@126.com;唐冬生(1962-),https://orcid.org/0000-0001-9466-6687,博士,教授,主要从事基因编辑与生物治疗技术研究工作,E-mail:tangdsh@163.com
第一作者:杨烨城(1995-),https://orcid.org/0000-0002-5745-7445,研究方向为动物遗传育种,E-mail:fdyangyecheng@163.com
