湖北省葡萄主产区灰葡萄孢菌多样性分析
2022-07-14王泽琼刘勇王榕馨姜婷龚林忠孙中海吕亮
王泽琼 刘勇 王榕馨 姜婷 龚林忠 孙中海 吕亮

摘要:【目的】了解湖北省葡萄主產区灰葡萄孢菌(Botrytis cinerea)多样性,为葡萄灰霉病防治提供科学依据。【方法】采用常规微生物分离法对从湖北省8个葡萄主产区采集的葡萄灰霉病样本进行灰葡萄孢菌分离,在PDA培养基上观察分离物菌落培养形态并测定菌丝生长速率;利用特异引物对各分离物的Flipper和Boty转座子片段进行扩增,区分转座子类型;扩增Bc-hch基因并用Hha I酶切检测分离物的多态性以区分组群;依据菌丝生长速率、菌落形态及转座子类型挑选4株典型分离物在4个葡萄品种果实上进行致病力测定。【结果】从病害样本中共分离获得51株分离物,菌落培养形态表明30株分离物为菌核型,20株为孢子型,仅1株为菌丝型,出现频率分别为58.82%、39.22%和1.96%。所有分离物菌丝生长速率均较高,在10.86~13.94 mm/d。分离物只存在2种转座子类型,其中50株为Transposa型,仅1株为Flipper型。Bc-hch基因Hha I酶切多态性鉴定结果表明,所有菌株均为Group II,为狭义的灰葡萄孢菌。分离物致病力测定结果表明,菌丝生长速率最低的WH3在供试葡萄品种上的致病力均最强,其次是Flipper型分离物SX1,菌丝生长速率最高的XN2和菌丝型WH6分离物致病力均较弱,且不同分离物在不同品种上致病力趋势不同。【结论】湖北省葡萄主产区灰葡萄孢菌菌落培养形态较丰富,所有分离物均为狭义灰葡萄孢菌,只存在Transposa和Flipper 2种转座子类型,前者占绝对优势,典型分离物间致病力差异明显。
关键词: 葡萄;灰霉病;灰葡萄孢菌;多样性;致病力;湖北
中图分类号: S436.631.1 文献标志码: A 文章编号:2095-1191(2022)04-1049-08
Diversity of Botrytis cinerea in major grape production
regions of Hubei Province
WANG Ze-qiong1, LIU Yong1, WANG Rong-xin2, JIANG Ting2, GONG Lin-zhong1*,
SUN Zhong-hai1*, LYU Liang3
(1Institute of Fruit Tree and Tea, Hubei Academy of Agricultural Science, Wuhan, Hubei 430064, China; 2 Wuhan Institute of Bioengineering, Wuhan, Hubei 430415, China; 3Key Laboratory of Integrated Pest Management on
Crops in Central China, Ministry of Agriculture and Rural Affairs, R. P. China/Hubei Key Laboratory
of Crop Diseases, Insect Pests and Weeds Control,Wuhan, Hubei 430064, China)
Abstract:【Objective】To assess the diversity of Botrytis cinerea in major grape production regions of Hubei Provin-ce, so as to provide scientific support for grape gray mold control. 【Method】Isolates of B. cinerea from 8 main grape production regions in Hubei Province were isolated by conventional microbial separation method. All the isolates were cultured on PDA medium to observe the morphological characteristics and measure the mycelium growth rates. Transposon segments Flipper and Boty were amplified using specific primers to detect the transposon types. Bc-hch gene was amplified and detected by Hha I digestion to identify the group. Four representative isolates were used for pathogenicity analysis by inoculating on fruits of 4 grape varieties. 【Result】Totally, 51 isolate were obtained. Results of the morphological analysis showed that:Among them, 30 isolates were the sclerotia type with the frequency of 58.82%, which was the most prevalent type; twenty isolates were the conidial type with the frequency of 39.22%; only 1 isolate was the mycelium type with the frequency of 1.96%. Mycelium growth rates of all isolates were high and ranged from 10.86 mm/d to 13.94 mm/d. Only two transposon types were found in isolates: 50 were Transposa and only 1 was Flipper. All the isolates were classified into group II according to the restriction polymorphism analysis of enzyme Hha I of Bc-hch gene. Pathogencity analysis revealed that, isolate WH3 which had the lowest mycelium growth rate had the strongest pathogenicity. SX1, the only Flipper type isolate, had the second strongest pathogenicity. Pathogenicity of XN2 which had the highest growth rate and WH6 which was the only mycelium type were weaker. Pathogenicity regulations of isolates on different varieties were different. 【Conclusion】Morphological characteristics of B. cinerea in the major grape production regions of Hubei Provin-ce is abundant. All the isolates are identified as B.cinerea in a narrow sense. There are two genotypes of transposable elements:Transposa and Flipper types. The Transposa type is predominant. The differentiation of pathogenicity is obvious between representative isolates.
Key words: grape; gray mold disease; Botrytis cinerea; diversity; pathogenicity; Hubei
Foundation items:National Modern Agricultural Industry Technology System Construction Project (CARS-29-19); Hubei Technology Innovation Major Project (2019ABA093); Open Fund of Key Laboratory of Comprehensive Pest Mana-gement of Crops in Central China of Ministry of Agriculture and Rural Affairs (2018ZTSJJ9)
0 引言
【研究意义】灰葡萄孢菌(Botrytic cinerea)引起的灰霉病是湖北省葡萄生产上的一种重要病害,主要危害葡萄花序和果实,导致落花落果,每年因此造成的损失在20%~40%。灰葡萄孢菌寄主范围广,能侵染1000多种植物(Veloso and van Kan,2018),其遗传变异大,在表型及分子水平上均表现出丰富的多样性,环境适应性强,易发生变异或产生抗药性(郑媛萍,2018;Saito et al.,2019;贾爽爽,2020;孔琼等,2020;DeLong et al.,2020)。湖北省属亚热带季风气候,葡萄生长季降水量充沛,高湿的气候条件极易造成葡萄灰霉病流行。因此,针对湖北葡萄主产区灰葡萄孢菌开展多样性研究,对掌握本产区葡萄上灰葡萄孢菌发生规律,并据此制定综合防控方案具有重要指导意义。【前人研究进展】灰葡萄孢菌表型多样性丰富,其菌落培养形态一般可划分为菌丝型(M)、菌核型(S)和孢子型(C)(张静,2010);也可分为菌丝型和菌核型两大类,再分别细分为M1~M4亚表型和S1~S5亚表型(Mirzaei et al.,2010;Kuzmanovska et al.,2012)共9种表型。灰葡萄孢菌在分子水平上也表现出较强的遗传变异。在系统发育水平上,灰葡萄孢菌包含2个群(Fournier et al.,2003),Group I为假灰葡萄孢(B. pseudocinerea),Group II是狭义的灰葡萄孢(B. cinerea)(Walker et al.,2011),2个群可根据粗糙脉孢菌het-c营养体不亲和位点同源基因Bc-hch位点的Hha I酶切多态性来划分;Group II分布更普遍,侵染力更强(Johnston et al.,2014;Muñoz et al.,2016)。真菌转座子是一类跳跃性元件,因其在真核基因组中的分布、插入位置及拷贝数的不同会导致菌株间或群体内出现遗传多样性从而更好地适应环境。灰葡萄孢菌中已报道2个转座子Boty(Diolez et al.,1995)和Flipper(Levis et al.,1997),根据转座子存在特点可划分为4种类型:同时含有2种转座子的Transposa型、仅含有Flipper的Flipper型、仅含有Boty的Boty型和不含这2种转座子的Vacuma型。研究认为转座子类型与病原菌致病力及样品采集时期具有一定相关性(Muñoz and Campos,2013;Johnston et al.,2014;Kumari et al.,2014),且一般認为Transposa型分离物致病力及抗药性较强(Martinez et al.,2003;Muñoz and Campos,2013;Johnston et al.,2016)。大部分灰葡萄孢菌分离菌株为异宗配合,被2个单独的交配型基因 MAT1-1和MAT1-2所控制,当2种交配型基因在种群中以1∶1存在时,病原菌有性重组概率提高,有利于增强真菌的生活力和适应性(乔广行等,2015;Pei et al.,2019;周默等,2020),以此可评估灰葡萄孢群体的变异潜力。此外,SSR分析(王帆帆等,2020;DeLong et al.,2020;Diao et al.,2020),3-磷酸甘油醛脱氢酶基因(G3PDH)、热激蛋白60基因(HSP60)和依赖DNA的RNA聚合酶亚基II基因(RPB2)等基因序列的同源进化分析(张静,2010;Muñoz et al.,2016),多位点测序分型(Plesken et al.,2021)等也有报道应用于遗传多样性分析。【本研究切入点】湖北省葡萄上灰霉病发生较普遍,而其病原灰葡萄孢菌多样性及群体研究尚未见报道。【拟解决的关键问题】采用常规微生物分离法对从湖北省8个葡萄主产区采集的葡萄灰霉病样本进行灰葡萄孢菌分离,通过对灰葡萄孢菌菌落培养形态观察及菌丝生长速率测定、转座子类型检测、Bc-hch基因的Hha I酶切多态性分析及典型菌株的致病力测定,了解湖北省葡萄主产区灰葡萄孢菌的多样性,为科学防治葡萄灰霉病打下基础。
1 材料与方法
1. 1 试验材料
2017—2018年,从湖北省不同葡萄产区采集病害样本,样本信息见表1。
1. 2 病原菌分离
采用常规微生物分离方法对病原菌进行分离纯化,每株葡萄保留1个单孢分离物,保存于-80 ℃冰箱。
1. 3 菌落形态观察
将保存菌株接种到新鲜PDA培养基上活化3 d,用直径5 mm的灭菌打孔器沿菌落边缘打取菌饼,接种到PDA培养基上,每株菌株设3个重复,20 ℃恒温暗培养,15 d后观察培养形态。以培养24~48 h的菌落半径增长量为菌丝生长速率,菌丝生长速率(mm/d)=(48 h菌落直径-24 h菌落直径)/2。
1. 4 DNA提取
将活化后的菌株接种至铺有灭菌玻璃纸的PDA培养基中20 ℃下倒置培养,3 d后刮取菌丝,参照DNA提取试剂盒(杭州新景生物试剂开发有限公司)操作说明提取病原菌总DNA。取1 μL DNA样品用NanoDrop 2000超微量分光光度计进行浓度检测,样品稀释至浓度为50 ng/μL备用。
1. 5 灰葡萄孢菌转座子检测
Flipper转座子检测引物为Flipper-F(5'-GCAC AAAACCTACAGAAGA-3')/Flipper-R(5'-ATTCGT TTCTTGGACTGTA-3') (Levis et al.,1997);Boty转座子检测引物为Boty-F(5'-TTAGCCAAGGGATGG ATCAG-3')/Boty-R(5'-TTCGAGCACTGCCTTAAC CT-3')(Johnston et al.,2014)。引物由生工生物工程(上海)股份有限公司合成。PCR反应体系25.0 μL:2×PCR Mix(Coolaber,北京)12.5 μL,DNA 模板1.0 μL,上、下游引物各1.0 μL,灭菌去离子水补足至25.0 μL。2种转座子PCR扩增程序均为:94 ℃预变性5 min;94 ℃ 30 s,55 ℃ 30 s,72 ℃ 90 s,进行35个循环;72 ℃延伸5 min。PCR产物经1.2%琼脂糖凝胶电泳检测。
1. 6 Bc-hch基因序列扩增及酶切
Bc-hch基因扩增引物为Bc-hch-F(5'-AAGCCC TTCGATGTCTTGGA-3')/Bc-hch-R(5'-ACGGATTC CGAACTAAGTAA-3')(Fournier et al.,2003)。PCR反应体系同1.5。PCR扩增程序:94 ℃预变性5 min;94 ℃ 30 s,55 ℃ 30 s,72 ℃ 90 s,进行35个循环;72 ℃延伸10 min。将扩增得到的基因片段用Hha I(TaKaRa,大连)酶切。酶切反应体系20.0 μL:PCR 产物8.0 μL,Hha I 1.0 μL,10×Buffer 2.0 μL,灭菌去离子水补足至20.0 μL。37 ℃酶切4 h,产物经1.5% 琼脂糖凝胶电泳检测。
1. 7 致病力测定
结合菌落形态、菌丝生长速率及转座子类型,挑选4株典型分离物进行致病力测定。将新鲜采摘近成熟期的阳光玫瑰、夏黑、甬优和红地球葡萄健康果粒剪下,清水洗净后用75%乙醇表面消毒30 s,无菌水清洗3次,自然晾干。沿菌丝边缘打取直径5 mm的菌饼接种于葡萄果实上,菌丝面朝下,每个果实接种1块,以PDA培养基块为对照,重复3次,每个重复3个果实。将接种后的果实置于无菌吸水纸上保湿,20 ℃培养4 d后十字交叉法测量病斑直径并计算病斑面积。
2 结果与分析
2. 1 灰葡萄孢菌不同分离物培养形态特征
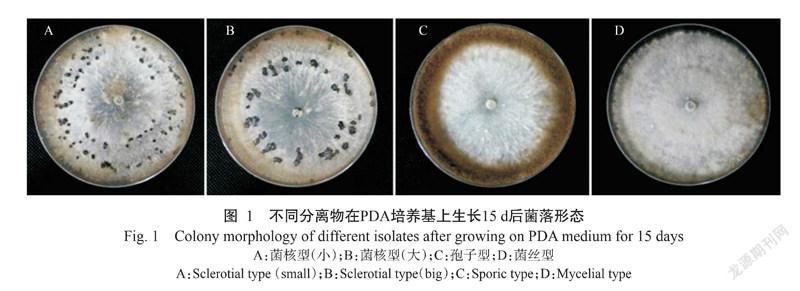
采用常规微生物分离法从病害样本中共分离获得51株分离物。分离物在PDA上培养15 d后,菌落培养形态可明显分为3种类型,其中菌核型30株,有大、小粒2种形态,菌丝短,产生黑色菌核,环状生长,外周均有少量分生孢子产生;孢子型20株,菌丝生长较菌核型丰富,分生孢子由外缘向中间生长,培养21 d铺满整个培养基表面,没有菌核产生;菌丝型仅1株,为WH6,菌丝生长浓密蓬松,产生少量分生孢子,没有菌核产生(图1)。菌核型出现频率为58.82%,孢子型为39.22%,菌絲型仅为1.96%;8个采集地中,只有仙桃的3株分离物形态均为孢子型,其余地区的分离物菌核型和孢子型均有出现;分离物菌丝生长速率在10.86~13.94 mm/d,其中生长最慢的为WH3,最快的为XN2(表1)。
2. 2 灰葡萄孢菌不同分离物的转座子分类
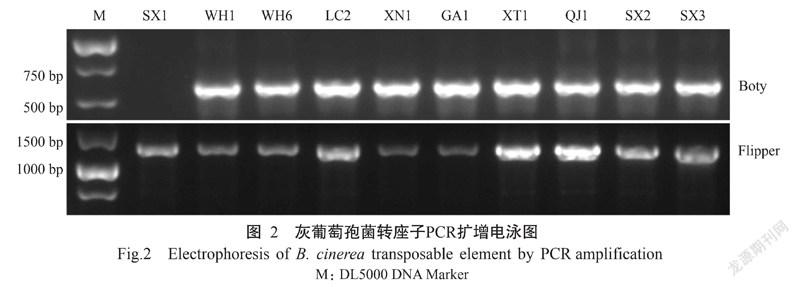
用Boty和Flipper 2种转座子的特异引物进行PCR扩增,结果(图2)表明,51株分离物中只含有2种转座子类型,除分离物SX1为Flipper型外,其余均为Transposa型,未发现Boty和Vacuma型分离物(表1)。
2. 3 Bc-hch基因酶切多态性分析结果
51个灰葡萄孢菌分离物Bc-hch基因位点扩增后均能得到1171 bp大小的目标片段,PCR产物经Hha I酶切后均出现6条带,最大片段为517 bp(图3)。参照Fournier等(2003)结果,本研究中所有分离物均属于Group II,是狭义的灰葡萄孢菌。
2. 4 不同分离物对不同葡萄品种果实的致病力分析结果
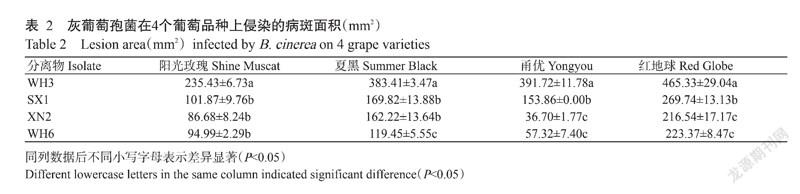
挑选编号为WH6、WH3、SX1和XN2菌株进行致病力测定,其中SX1是唯一的转座子类型为Flipper的分离物,WH6是唯一的菌丝型分离物,XN2的生长速率最快,WH3最慢,分离物生长速率表现为WH3<WH6<SX1<XN2。由表2可知,4株分离物在4个品种葡萄果实上均能侵染造成病斑并产生分生孢子。在葡萄果实上致病力分化较大,病斑面积36.70~465.33 mm2,其中,WH3的致病力最强,病斑面积为235.43~465.33 mm2,显著高于其他分离物(P<0.05,下同),SX1次之,WH6和XN2的致病力均较弱,在甬优葡萄上表现最明显。
从分离物特性与致病力的关系来看,4株分离物中,只有SX1转座子类型为Flipper,在阳光玫瑰上致病力与XN2和WH6相当,在夏黑上的致病力与XN2相当,在红地球和甬优上的致病力显著强于XN2和WH6,致病力较强。但由于得到的Flipper型分离物仅SX1一株,无法评估转座子类型与致病力间的关系。WH6是唯一的菌丝型分离物,其致病力与XN2相当,仅在夏黑上显著弱于XN2。WH3菌丝生长最慢,但致病力显著强于其他分离物;XN2菌丝生长最快,但致病力与生长速率较慢的WH6相当。
同一分離物在不同葡萄品种上的致病力趋势存在一定差异,如4个分离物在甬优和红地球上的致病力趋势相同,均为WH3最强,其余依次为SX1、WH6和XN2;在阳光玫瑰上WH3最强,在其余品种上的致病力相当;在夏黑上WH3的致病力最强,SX1和XN2次之,WH6最弱。
3 讨论
灰葡萄孢菌具有丰富的表型多样性,一般可分为菌核型、孢子型和菌丝型3种,也可分为菌核型和菌丝型2种,在此基础上再细分为9种表型。无论以哪种分类方法,其分离物大多以菌核型为主(Martinez et al.,2003;Kumari et al.,2014;张艳杰等,2017;Pei et al.,2019;周默等,2020)。本研究51株分离物中菌核型占58.82%,为主要类型,其中又有大、小菌核2种表型,形态较丰富。张艳杰等(2017)将菌丝生长速率在9.28~12.91 mm/d的分离物聚类为高等级,本研究中分离物菌丝生长速率在10.86~13.94 mm/d,有不少分离物超过12.91 mm/d,表明所得到的分离物生长速率快。灰葡萄孢菌变异快,表型不稳定,因此还需从分子水平上对其多样性进行评估。
在分子水平上灰葡萄孢菌遗传多样性受有性重组、繁殖及转座子等的影响。对来源于不同地区及寄主的分离物研究表明,灰葡萄孢菌转座子类型大多以Transposa为主(张静,2010;张佳等,2016;Wahab,2015;Pei et al.,2019;DeLong et al.,2020),该特点在灰葡萄孢菌葡萄分离物中表现明显(Martinez et al.,2005;Kretschmer and Hahn,2008;Esterio et al.,2011;Samuel et al.,2012;Zhang et al.,2018),甚至可达100%(Muñoz et al.,2010)。本研究中发现的转座子类型只有Transposa和Flipper 2种,前者占绝对优势,后者仅有1株。研究认为灰葡萄孢菌葡萄分离物转座子类型及出现频率可能与采样时期及植株是否显症相关,相比在葡萄花期采样,结果期分离物中Transposa型出现频率明显上升,其他类型频率明显下降,花期Vacuma型出现频率较其他时期高(Martinez et al.,2003,2005;Johnston et al.,2016)。无症状植株中分离物多样性更丰富(Johnston et al.,2014,2016)。转座子出现频率还与药剂施用相关,不同类型转座子分离物表现出对不同药剂的抗性,Transposa型分离物抗药性更广泛(Esterio et al.,2011)。同时,转座子类型与地理位置、寄主(Zhang et al.,2018;Pei et al.,2019)及气候(Zhang et al.,2018)等均存在相关性。灰葡萄孢菌葡萄分离物中Vacuma型主要分布于我国北方,但分离频率也不高(Zhang et al.,2018)。Boty型和Flipper型转座子分离物分别具有地域和寄主专一性(Kumari et al.,2014)。本研究中灰葡萄孢菌分离物的转座子只有Transposa和Flipper 2种,类型单一,可能与分离物均来自湖北省内,地域相近、气候条件相似、施药和管理措施较相似及菌株产生抗药性(郑媛萍,2018)等因素有关。此外,本研究样品均采自灰霉病显症材料,可能影响了其多样性表现。Transposa型占绝对优势,说明湖北省的灰葡萄孢菌可能具有较广的抗药性,后期应注意其抗药性监控及药剂筛选。
来源于不同寄主的灰葡萄孢菌分离物研究表明,Group I出现频率低,Group II更为普遍,侵染力更强(Johnston et al.,2014;Muñoz et al.,2016),在葡萄分离物中也是如此(Esterio et al.,2011;Zhang et al.,2018)。来源于湖北省8个葡萄主产区的灰葡萄孢菌分离物只检测到Group II,表明所有分离物均为狭义的灰葡萄孢菌,没有发现假灰葡萄孢分离物。但湖北省番茄上已有假灰葡萄孢分离物的报道(Li et al.,2015),葡萄上是否存在假灰葡萄孢菌还需进一步收集样品,增加采样地区及次数以验证。
本研究选取的4株典型分离物在葡萄果实上致病力分化明显。大部分研究认为Transposa型转座子分离物致病力更强(Martinez et al.,2003;张静,2010;Muñoz and Campos,2013;Johnston et al.,2016;周默等,2020),也有Boty型分离物致病力最强的报道(张佳等,2016)。WH3是Transposa型转座子分离物,致病力最强,而Flipper型转座子分离物SX1致病力并不弱于另2个Transposa型转座子分离物。受Flipper型分离物样本量限制,无法评估湖北省葡萄上灰葡萄孢菌转座子类型与致病力间的关系。此外,致病力测定结果与选用品种有关,同一分离物在不同葡萄品种上致病力趋势不完全一致,因此有必要选取不同品种或筛选对不同分离物致病力趋势相近的品种进行测定。
4 结论
湖北省葡萄主产区灰葡萄孢菌菌落培养形态较丰富,以菌核型为主。所有分离物均为狭义灰葡萄孢菌,暂未发现假灰葡萄孢分离物。分离物只具有Transposa和Flipper 2种转座子类型,前者占绝对优势,而Transposa型是抗药性和致病力最强的类型,生产中应加强病害防治并监测抗药性变化。典型分离物致病力分化明显,在不同品种上不同分离物致病力趋势存在差异。
参考文献:
贾爽爽. 2020. 我国葡萄灰霉菌对主要杀菌剂的抗药突变型分布与多药抗性机制研究[D]. 北京:中国农业科学院. [Jia S S. 2020. Study on the distribution of resistant mutation to main fungicides and the mechanism of multi-drug resistance of Botrytis cinerea in China[D]. Beijing:Chinese Academy of Agricultural Sciences.] doi:10.27630/ d.cnki.gznky.2020.000891.
孔瓊,袁盛勇,李珣,薛春丽,李林倩,余朝阳,杨红玉. 2020. 灰葡萄孢蛋白质毒素的致病性研究[J]. 河南农业科学,49(4):72-77. [Kong Q,Yuan S Y,Li X,Xue C L,Li L Q,Yu C Y,Yang H Y. 2020. Study on pathogenicity of protein toxin secreted from Botrytics cinerea[J]. Journal of Henan Agricultural Sciences,49(4):72-77.] doi:10. 15933/j.cnki.1004-3268.2020.04.010.
乔广行,李兴红,黄金宝,林秀敏,周莹. 2015. 灰葡萄孢交配型基因的分析与检测[J]. 菌物学报,34(1): 108-116. [Qiao G H,Li X H,Huang J B,Lin X M, Zhou Y. 2015. Analysis and molecular detection of Botrytis cinerea ma-
ting type genes[J]. Mycosystema,34(1):108-116.] doi:
10.13346/j.mycosystema.130210.
王帆帆,曾佳,郭杰,唐其, 郭晓亮, 段媛媛,游景茂. 2020. 华重楼灰霉病菌灰葡萄孢 ITS 分型及 SSR 遗传多样性分析[J]. 菌物学报,40(2):1-13. [Wang F F,Zeng J, Guo J,Tang Q,Guo X L,Duan Y Y,You J M. 2020. ITS and SSR-PCR analyses reveal the genetic diversity of Botrytis cinerea strains collected from Paris polyphylla var. chinensis[J]. Mycosystema,40(2):1-13.] doi:10.13346/j.mycosystema.200234.
张静. 2010. 湖北省灰霉病病菌区系和灰葡萄孢菌多样性研究[D]. 武汉:华中农业大学. [Zhang J. 2010. Studies on taxonomy of Botrytis species in Hubei Province and diversity of B. cinerea[D]. Wuhan:Huazhong Agricultural University.]
张佳,张晓歌,张璨,张国珍. 2016. 北京地区草莓灰葡萄孢菌的转座子及其分布频率[J]. 植物保护,42(2):177-181. [Zhang J,Zhang X G,Zhang C,Zhang G Z. 2016. Presen-ce and frequency distribution of Transposable elements in Botrytis cinerea from strawberry in Beijing[J]. Plant Protection,42(2):177-181.] doi:10.3969/j.issn.0529-1542. 2016.02.032.
张艳杰,许换平,沈凤英,李兴红,李亚宁,刘大群. 2017. 我国葡萄灰葡萄孢菌形态型和致病力分化[J]. 农业生物技术学报,25(11):1740-1755. [Zhang Y J,Xu H P,Shen F Y,Li X H,Li Y N,Liu D Q. 2017. Phenotypes and viru-lence variability among grape gray mold isolates from grapes (Vitis vinifera) in China[J]. Journal of Agricultural Biotechnology,25(11):1740-1755.] doi:10.3969/j.issn.1674-7968.2017.11.002.
郑媛萍. 2018. 我国葡萄灰霉病菌对主要杀菌剂的抗药性检测[D]. 北京:中国农业科学院. [Zheng Y P. 2018. The detection on the main fungicides resistance of Botrytis cinerea from grape in China[D]. Beijing:Chinese Academy of Agricultural Sciences.]
周默,卢宝慧,刘丽萍,白庆荣,高洁. 2020. 人参灰葡萄孢菌Botrytis cinerea的种群表型和基因型多样性[J]. 华中农业大学学报,39(3):45-53. [Zhou M,Lu B H,Liu L P,Bai Q R,Gao J. 2020. Phenotypeic and genetic variability among Botrytis cinerea population isolated from ginseng[J]. Journal of Huazhong Agricultural University,39(3):45-53.] doi:10.13300/j.cnki.hnlkxb.2020.03.006.
DeLong J A,Saito S,Xiao C L,Naegele R P. 2020. Population genetics and fungicide resistance of Botrytis cinerea on Vitis and Prunus spp. in California[J]. Phytopathology,110(3):694-702. doi:10.1094/PHYTO-09-19-0362-R.
Diao Y Z,Larsen M M,Kamvar Z N,Zhang C,Li S,Wang W Z,Lin D,Peng Q, Knaus B J, Foster Z S L,Grünwald N J,Liu X L. 2020. Genetic differentiation and clonal expansion of Chinese Botrytis cinerea populations from tomato and other crops in China[J]. Phytopathology,110:428-439. doi:10.1094/PHYTO-09-18-0347-R.
Diolez A,Marchies F,Fortini D,Brygoo Y. 1995. Boty,a long-terminal-repeat retroelement in the phytopathogenic fungus Botrytis cinerea[J]. Applied and Environment Microbiology,61(1):103-108. doi:10.1128/aem.61.1.103-108.1995.
Esterio M,Muñoz G,Ramos C,Estévez R,Salinas A,Auger J. 2011. Characterization of Botrytis cinerea isolates pre-sent in Thompson seedless table grapes in the central valley of Chile[J]. Plant Disease,95:683-690. doi:10.1094/PDIS-04-10-0298.
Fournier E,Levis C,Fortini D,Leroux P,Giraud T,Brygoo Y. 2003. Characterization of Bc-hch,the Botiytis cinerea homolog of the Neurospora crassa het-c vegetative incompatibility locus,and its use as a population marker[J]. Mycologia,95(2):951-961. doi:10.1080/15572536.2004. 11833110.
Johnston P R,Hoksbergen K,Park D,Beever R E. 2014. Genetic diversity of Botrytis in New Zealand vineyards and the signi?cance of its seasonal and regional variation[J]. Plant Pathology,63(4):888-898. doi:10.1111/ppa.12143.
Johnston P R,Park D,White D,Wilkie J P. 2016. Genetic diversity of Botrytis populations in New Zealand vineyards across seasons and regions[J]. New Zealand Plant Protection,69:25-29. doi:10.30843/nzpp.2016.69.5911.
Kretschmer M,Hahn M. 2008. Fungicide resistance and genetic diversity of Botrytis cinerea isolates from a vineyard in Germany[J]. Journal of Plant Disease and Protection,115 (5):214-219. doi:10.1007/BF03356266.
Kumari S,Tayal P,Sharma E,Kapoor R. 2014. Analyses of genetic and pathogenic variability among Botrytis cinerea isolates[J]. Microbiological Research,169(11):862-872. doi:10.1016/j.micres.2014.02.012.
Kuzmanovska B,Rusevski R,Ljupcho J,Mirjana J,Dario I,Katerina B. 2012. Phenotypic and genetic characterization of Botrytis cinerea isolates from tomato[J]. Genetika,44(3):633-647. doi:10.2298/GENSR1203663K.
Levis C,Fortini D,Brygoo Y. 1997. Flipper,a mobile Fot1-like transposable element in Botrytis cinerea[J]. Molecular and General Genetics,254:674-680. doi:10.1007/s00 4380050465.
Li N,Zhang J,Yang L,Wu M D,Li G Q. 2015. First report of Botrytis pseudocinerea causing gray mold on tomato (Lycopersicon esculentum) in central China[J]. Plant Disea-se,99(2):283. doi:10.1094/PDIS-03-14-0256-PDN.
Martinez F,Blancard D,Lecomte L,Levis C,Dubos B,Fermaud M. 2003. Phenotypic differences between vacuma and transposa subpopulations of Botrytis cinerea[J]. European Journal of Plant Pathology,109:479-488. doi:10. 1023/A:1024222206991.
Martinez F,Dubos B,Fermaud M. 2005. The role of saprotrophy and virulence in the population dynamics of Botrytis cinerea in vineyards[J]. Phytopathology,95:692-700. doi:10.1094/PHYTO-95-0692.
Mirzaei S,Goltapeh E M,Shams-Bakhsh M,Safaie M,Chaichi M. 2010. Genetic and phenotypic diversity among Botrytis cinerea isolates in Iran[J]. Journal of phytopathology,157(7-8):474-482. doi:10.1111/j.1439-0434.2008. 01518.x.
Muñoz C,Talquenca S G,Oriolani E,Combina M. 2010. Genetic characterization of grapevine-infecting Botrytis cinerea isolates from Argentina[J]. Revista Iberoamericana de Micología,27(2):66-70. doi:10.1016/j.riam.2009.12. 006.
Muñoz G,Campos F. 2013. Genetic characterization of Botrytis cinerea,isolates collected from pine and eucalyptus nurse-ries in Bio-Bio Region,Chile[J]. Forest Pathology,43(6):509-512. doi:10.1111/efp.12057.
Muñoz G,Campos F,Salgado D,Galdames R,Gilchrist L,Chahin G,Andrade O. 2016. Molecular identification of Botrytis cinerea,Botrytis paeoniae and Botrytis pseudocinerea associated with gray mould disease in peonies (Paeo-nia lactiflora Pall.) in southern Chile[J]. Revista Iberoa-mericana de Micología,33(1):43-47. doi:10.1016/j.riam. 2015.02.002.
Pei Y G,Tao Q J,Zheng X J,Li Y,Sun X F,Li Z F,Qi X B,Xu J,Zhang M,Chen H B,Chang X L,Tang H M,Sui L Y,Gong G S. 2019. Phenotypic and genetic characterization of Botrytis cinerea population from kiwifruit in Si-chuan Province,China[J]. Plant Disease,103:748-758. doi:10.1094/PDIS-04-18-0707-RE.
Plesken C,Pattar P,Reiss B,Noor Z N, Zhang L,Klug K,Huettel B,Hahn M. 2021. Genetic diversity of Botrytis cinerea revealed by multilocus sequencing,and identification of B. cinerea populations showing genetic isolation and distinct host adaptation[J]. Frontiers in Plant Science,12:e663027. doi:10.3389/fpls.2021.663027.
Saito S,Michailides T J,Xiao C L. 2019. Fungicide-resistant phenotypes in Botrytis cinerea populations and their impact on control of gray mold on stored table grapes in California[J]. European Journal of Plant Pathology,154(2):203-213. doi:10.1007/s10658-018-01649-z.
Samuel S,Veloukas T,Papavasileiou A,Karaoglanidis G S. 2012. Differences in frequency of transposable elements presence in Botrytis cinerea populations from several hosts in Greece[J]. Plant Disease,96:1286-1290. doi:10.1094/PDIS-01-12-0103-RE.
Veloso J,van Kan J A L. 2018. Many shades of grey in Botrytis-host plant interactions[J]. Trends in Plant Scien-ce,23(7):613-622. doi:10.1016/j.tplants.2018.03.016.
Wahab H A. 2015. Characterization of Egyptian Botrytis cinerea isolates from different host plants[J]. Advances in Microbiology,5(3):177-189. doi:10.4236/aim.2015.53017.
Walker A S,Gautier A,Confais J,Martinho D,Viaud M,Le Pêcheur P,Dupont J,Fournier E. 2011. Botrytis pseudocinerea,a new cryptic species causing gray mold in French vineyards in sympatry with Botrytis cinerea[J]. Phytopathology,101(12):1433-1445. doi:10.1094/PHYTO-04-11-0104.
Zhang Y J,Li X H,Shen F Y,Xu H P,Li Y N,Liu D Q. 2018. Characterization of Botrytis cinerea isolates from grape vineyards in China[J]. Plant Disease,102:40-48. doi:10.1094/PDIS-01-17-0062-RE.
收稿日期:2021-08-02
基金項目:国家现代农业产业技术体系建设专项(CARS-29-19);湖北省技术创新重大专项 (2019ABA093);农业农村部华中作物有害生物综合治理重点实验室开放基金项目(2018ZTSJJ9)
通讯作者:龚林忠(1977-),https://orcid.org/0000-0001-6042-0769,研究员,主要从事葡萄、桃等果树遗传育种与栽培研究工作,E-mail:gcs325@126.com;孙中海(1961-),https://orcid.org/0000-0001-5412-3274,博士,研究员,主要从事果树及经济林果育种与栽培研究工作,E-mail:hbfruit@126.com
第一作者:王泽琼(1981-),https://orcid.org/0000-0002-7361-1561,博士,主要从事植物保护研究工作,E-mail:wangzeqiong@126.com
