DAAzF对DAAF热性能的影响
2022-07-13庄思琪付小林陈建波
庄思琪,付小林,于 谦,陈建波,刘 渝,金 波,黄 辉,
(1. 西南科技大学材料科学与工程学院,四川 绵阳 621010;2. 中国工程物理研究院化工材料研究所,四川 绵阳 621999)
1 Introduction
Insensitive energetic materials based on furazan rings have received wide attention in the last decade due to their favorable properties including high energy density,good safety,and high nitrogen content[1-3].Among them,3,3'-diamino-4,4'-azoxyfurazan(DAAF)is regarded as one of the most promising explosives due to its good thermal stability,high positive enthalpy,high detonation velocity of 8.02 km·s-1,and detonation pressure of 30.6 GPa[4-5]. DAAF,as an excellent explosive,is expected to be used in place of 1,3,5-triamino-2,4,6-trinitrobenzene(TATB)in insensitive booster explosives and hexahydro-1,3,5-trinitro-1,3,5-triazine(RDX)in melt cast explosives[6-7]. However,it is reported that the impurities have a negative effect on the thermal performance of DAAF,as the onset temperature of DAAF measured by differential scanning calorimetry (DSC) is decreased from 250 ℃to 128 ℃by the impurities[8].Therefore,it is necessary to study the change of DAAF's thermal performance in the presence of the impurities.
For the preparation of nano-explosives and plastic-bonded explosives[7,9],DAAF is mainly synthesized based on the Oxone oxidation method[8],which yields as well various impurities such as unreacted precursors,intermediates,and side-products.In our previous study,we found that the impurities of DAAF could not be ignored with a relatively high content of 6.62% of DAAF final product[10]. It is very difficult to remove 3,3'-diamino-4,4'-azofurazan(DAAzF)from DAAF via common purification due to the similar structure between DAAzF and DAAF.Compared to DAAF,DAAzF only lacks an oxygen atom that bound to the azo group. Therefore,DAAzF is regarded as a major impurity of DAAF[11-12]. Some properties of DAAzF itself have been investigated in the past. The vacuum thermal stability test of DAAzF is 5.87 mL·g-1,which is much higher than that of DAAF(0.69 mL·g-1)[13-15].Besides,the detonation velocity and pressure of DAAzF are 7.42 km·s-1and 26.2 GPa,respectively,which are lower than that of DAAF[16]. However,there is no study about the effect of DAAzF as the coexisting impurity on the thermal performance of DAAF until now.
In this study, we investigated the effect of DAAzF on the thermal performance of DAAF for the first time. DAAzF was firstly synthesized,and then several composite DAAF@DAAzF explosives were obtained by doping different content of DAAzF into DAAF. Subsequently, the doping process of DAAF@DAAzF explosives was investigated by analyzing the DAAzF content and the characterizing the morphology. Further,the thermal performances of DAAF@DAAzF explosives,including melting point,thermal decomposition temperature,and activation energy,were investigated via simultaneous thermogravimetry and differential scanning calorimetry(TG-DSC). In addition, the thermal stability of DAAF explosives in the presence of DAAzF was obtained through the study of isothermal thermal decomposition.
2 Experimental Section
2.1 Reagents and instrumentations
3,4-Diaminofurazan(DAF),DAAF and DAAzF were synthesized in our laboratory. Analytical reagent(AR)N,N-dimethylformamide(DMF)was purchased from Kelong Chemical(Chengdu,Sichuan).
High-performance liquid chromatography(HPLC)grade acetonitrile was purchased from Merck Chemicals(Darmstadt,Germany). Ultrapure water was purified by a Millipore-Q system(Bedford,USA)with the resistivity of 18.2 MΩ. Thermal analysis was performed on Mettler Toledo TG-DSC 3+(Zurich, Switzerland). Chromatography analysis was carried out with an Agilent 1260 infinity ultrahigh-performance liquid chromatography(HPLC) system (Waldbronn,Germany). Powder X-Ray diffraction(PXRD)was measured on a Bruker D8 Advance diffractometer(Karlsruhe,Germany).Scanning electron microscopy(SEM)was conducted on a Zeiss high-resolution field emission scanning electron microscopy instrument(Oberkochen,Germany).
2.2 Sample preparation
DAF,DAAF and DAAzF were synthesized according to the reported literature[17].
DAAF(10 g)and different weights of DAAzF(0-1.0 g)were added into DMF(10 mL),and then the mixture was stirred at 60 ℃. After complete dissolution,cool water was added quickly into the above solution,and then the precipitates were filtered, washed, and dried to obtain DAAF@DAAzF explosives.The composite explosives doped with 0.5%,1.0%,2.5%,5.0%,and 10% mass fractions were named as DAAF@DAAzF-1,DAAF@DAAzF-2,DAAF@DAAzF-3,DAAF@DAAzF-4,and DAAF@DAAzF-5,respectively. As a control experiment,raw DAAF without the addition of DAAzF was performed by the above method.
2.3 The characterization of DAAF@DAAzF
DAAF@DAAzF explosives were measured by PXRD. The tube current and voltage of PXRD were set at 40 mA and 40 kV,respectively,and the images were scanned in a range of 2θfrom 10° to 40° by using a Vantec detector with Cu Kα as radiation(λ=1.54180 Å). Meanwhile,the morphology and particle size of DAAF@DAAzF were characterized by SEM.
The solution of DAAF@DAAzF explosives was prepared with the concentration of 1.0 mg·mL-1in acetonitrile and then diluted to the concentration of 1.0 μg·mL-1for chromatographic analysis. The above solution was filtered through a 0.45 μm membrane before the HPLC analysis. Then,the diluted solution of DAAF@DAAzF explosives was analyzed by HPLC with reversed-phase Hypersil Gold C18column (100 mm×2.1 mm,1.9 μm). The mobile phase consisted of acetonitrile and ultrapure water.The flow rate was set at 0.5 mL·min-1. The temperature of HPLC column oven was set at 35 ℃. The detection wavelength of HPLC was set at 230 nm.
2.4 Thermal analysis of raw DAAF and DAAF@DAAzF explosives
Thermal analysis of both raw DAAF and DAAF@DAAzF explosives were performed by TG-DSC. All the experiments were measured in encapsulated aluminum pans with a low-sized pinhole.The mass of all explosives was about 1.5 mg for each measurement. Nitrogen was chosen as the shield gas with a flow rate of 30 mL·min-1. For the programmed heating measurements,all explosives were heated from 30 ℃to 350 ℃under different heating rates of 2,5,10 K·min-1and 20 K·min-1,respectively. All data of thermal analysis was processed by using the Netzsch kinetics Neo Trial software[18].
3 Results and Discussion
3.1 The doping of DAAzF in DAAF
It would be more homogeneous to dope DAAzF in DAAF through the dissolution-precipitation method compared to the direct mixing of two different solid powders,because all molecules of DAAF and DAAzF can be homogeneously dispersed in the same solvent(DMF)before the precipitation. With that in mind,DAAF explosives were doped with different mass fractions of DAAzF from 0.5% to 10% to prepare DAAF@DAAzF explosives via the dissolution-precipitation method,which is shown in Fig.1.

Fig.1 The process of doping DAAzF into DAAF
3.2 The analysis of DAAzF content
PXRD was used to characterize the doping of DAAzF in DAAF@DAAzF explosives. PXRD patterns of raw DAAF and DAAF@DAAzF explosives are shown in Fig. 2a. It is shown that the signal of raw DAAF at 27.70° disappears after doping 0.5% DAAzF.When 10% DAAzF is doped in DAAF@DAAzF explosives,two signals of DAAzF at 20.08° and 28.02° are observed obviously, which indicates that DAAF@DAAzF explosives contain DAAzF. To obtain the quantitative analysis of the doping content of DAAzF,the content of DAAzF in DAAF@DAAzF explosives was further analyzed by HPLC in Fig. 2b.Chromatographic peaks of DAAF and DAAzF are separated fully by HPLC,because DAAF and DAAzF have different retention times of 8.70 min and 9.36 min,respectively. Although raw DAAF has a very weak signal of DAAzF due to raw DAAF itself containing tiny DAAzF as a byproduct before the doping(Table 1),the signals of DAAzF in DAAF@DAAzF explosives become stronger gradually with the increase of DAAzF content. After the deduction of background signal of DAAzF in raw DAAF,DAAF@DAAzF explosives including DAAF@DAAzF-1,DAAF@DAAzF-2,DAAF@DAAzF-3,DAAF@DAAzF-4,DAAF@DAAzF-5 were doped with 0.5%,1.0%,2.5%,5.0%,and 10% DAAzF,respectively. Moreover,the SEM images in Fig.2c show the morphology of DAAF@DAAzF explosives is more homogeneous compared to raw DAAF,and the particle size of DAAF@DAAzF explosives is less than 1.0 μm. Therefore,different contents of DAAzF are homogeneously doped in DAAF@DAAzF explosives.

Table 1 HPLC data of different content of DAAzF doped in DAAF@DAAzF explosives

Fig.2 PXRD,HPLC and SEM analysis of raw DAAF,raw DAAzF and DAAF@DAAzF explosives
3.3 Mass loss
All TG/DTG curves of raw DAAF and DAAF@DAAzF explosives were determined by TG-DSC with the heating rate at 10 K·min-1. According to the TG curves of Fig.3a,DAAF@DAAzF explosives display a mass loss in one step for their thermal decomposition. The mass loss begins at about 244.3 ℃,and then there is about 60% mass loss observed after 10 min. Through the analysis of DTG curves in Fig.3b,an obvious difference is found between raw DAAF and DAAF@DAAzF explosives.The peak temperature in the DTG curve is 256.7 ℃for raw DAAF. The DTG peak temperature of DAAF@DAAzF explosives decreases with the increase of DAAzF content. The peak temperature is 255.8 ℃ for DAAF@DAAzF-2 with 1.0% DAAzF,and 254.8 ℃ for DAAF@DAAzF-4 with 5.0%DAAzF. Meanwhile, the mass loss rate of DAAF@DAAzF explosives increases with the increase of DAAzF content at the initial stage of thermal decomposition. However,the mass loss rate becomes slow under higher content of DAAzF doped in DAAF@DAAzF explosives at the end of thermal decomposition. Therefore,the doping of DAAzF can slightly lower the peak temperature of DAAF-based explosives in DTG curves.
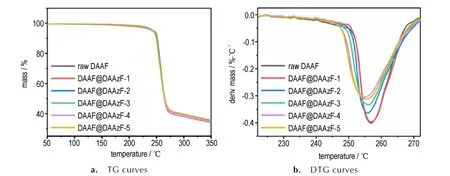
Fig.3 TG and DTG curves of raw DAAF and DAAF@DAAzF explosives at 10 K·min-1
3.4 Melting point and melting heat
The DSC curves of DAAF@DAAzF explosives were investigated under the heating rate of 10 K·min-1in Fig. 4a. An endothermic peak of raw DAAF is observed at 249.0 ℃,which indicates there is an endothermic process before thermal decomposition.Through the endothermic reaction,DAAF melts to provide a liquid phase for its thermal decomposition,which is similar to RDX and HMX[15,19-20]. Meanwhile,melting point of DAAF@DAAzF explosives decreases with increasing DAAzF content,which is shown in Fig.4b. The melting point of raw DAAF is 246.4 ℃. At the heating rate of 10 K·min-1,melting points of DAAF@DAAzF explosives with 0.5%,1.0%,2.5%,5.0%,and 10% DAAzF are 245.9,245.1,244.4,242.9 ℃,and 239.3 ℃,respectively,which indicates that DAAzF as the impurity obviously decreases the melting point of DAAF-based explosives. A linear relationship(y=-0.6862x+246.27)is found between the melting point of DAAF@DAAzF explosives(y)and the content of DAAzF(x)with anR2value of 0.9930,which is in agreement with Raoult's law of colligative property in dilute solution[21]. Compared to solid-phase decomposition,DAAF@DAAzF explosives display much faster thermal decomposition at the initial melting stage due to the decrease of melting points by DAAzF,which is in good agreement with the change of mass loss of DAAF@DAAzF explosives.

Fig.4 DSC curves and melting points of raw DAAF and DAAF@DAAzF explosives at 10 K·min-1,DSC curves of raw DAAF under different heating rates,and melting heat of raw DAAF and DAAF@DAAzF explosives at 20 K·min-1
Further,the DSC curves of raw DAAF were measured under different heating rates in Fig.4c. The melting heat of raw DAAF under 5,10,15 K·min-1,and 20 K·min-1is 211.09,184.34,146.93 J·g-1,and 125.59 J·g-1,respectively. Normally,the melting point of raw DAAF keeps constant under different heating rates. However,the decomposition temperature of raw DAAF at a higher heating rate of 20 K·min-1apparently lags behind that at a low heating rate of 5 K·min-1. So,the melting heat of raw DAAF decreases with the increase of the heating rate. As shown in Fig. 4d,the melting heat of DAAF@DAAzF explosives after the doping of DAAzF was also investigated at 20 K·min-1. The melting heat of DAAF@DAAzF explosives increases with the increase of DAAzF content,when the doping content of DAAzF is less than 5.0%.DAAF@DAAzF-4 containing 5.0% DAAzF displays the maximum melting heat with the value of 337.38 J·g-1. The melting heat of DAAF@DAAzF explosives decreases with the increase of DAAzF content,when DAAzF content is higher than 5.0%.Therefore,the change of melting heat indicates the eutectic mixture is formed between 5.0% DAAzF and 95% DAAF.
Besides,the melting process of DAAF@DAAzF-3 containing 2.5% DAAzF is successfully observed by a microscopic melting point meter in Fig.5. During the melting process,solid samples began to spin and move,and then melted with the bubbles appearing,which indicates that thermal decomposition of DAAF@DAAzF explosives includes solid decomposition,melting,and liquid decomposition.Thus,it is further demonstrated that the thermal process of DAAF-based explosives containing DAAzF includes both melting and thermal decomposition.
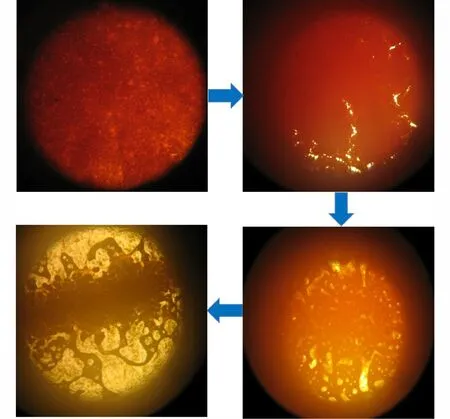
Fig.5 The melting process of DAAF@DAAzF-3 containing 2.5% DAAzF
3.5 Kinetic analysis
Kinetic parameters of thermal decomposition of DAAF explosives in the presence of DAAzF were calculated by Friedman method(Eq.(1))based on TG curves at different heating rates[20]:

whereEais the apparent activation energy,kJ·mol-1;Ais the pre-exponential(frequency)factor,s-1;αis the conversion fraction;βis the heating rate,K·min-1;R is the gas constant,8.314 J·mol-1·K-1;Tis the absolute temperature,K;f(α)is the differential expression of the reaction model function.The pre-exponential factor(A)can be found by model-free analysis only assumption of known functionf(α),which is often used in the view of reaction ofnthorder in model-free analysis.
As shown in Fig.6a,a high activation energy((560.9±60.8)kJ·mol-1)of raw DAAF is obtained at the initial thermal decomposition (α<0.30),which may be attributed to several coexisting processes of solid decomposition:melting and liquid decomposition. The doping of DAAzF can decrease the activation energy of DAAF-based explosives,which is similar to the decrease of melting point and mass loss rate of DAAF@DAAzF explosives. After doping with 0.5% DAAzF,the activation energy of DAAF@DAAzF-1 is decreased to(423.2±6.9)kJ·mol-1.The activation energy decreases as the reaction goes on.The change of activation energy of DAAF@DAAzF explosives becomes slow when the conversion is over 0.30. The activation energy values of all DAAF@DAAzF explosives range from (155.3±9.9) kJ·mol-1to(213.2±44.2) kJ·mol-1under high conversion(α≥0.30),which is in agreement with that of the reported DAAF explosive[4,16]. As shown in Fig. 6b,pre-exponential factors(logA)of all DAAF-based explosives show similar trends with their activation energies. Before the doping of DAAzF,raw DAAF shows a high pre-exponential factor with logAof(39.3±3.3)s-1. However,the pre-exponential factor of DAAF@DAAzF explosives is decreased with logAranging from(31.7±1.3)s-1to(23.4±1.5)s-1during the initial decomposition when the doping content of DAAzF is over 0.5%.

Fig.6 Activation energies and pre-exponential factors of raw DAAF and DAAF@DAAzF explosives under different conversion(α),linear relationship between pre-exponential factor(ln(A))and activation energy(Ea),and isothermal thermal decomposition of raw DAAF and DAAF@DAAzF explosives
Further,there is a kinetic compensation effect existing between apparent activation energy and pre-exponential factor,which means a linear relationship(lnA=a+bEa)between lnAandEa[22-23]. As shown in Fig.6c and Table 2,different content of DAAzF shows nearly the same slopes of 0.227~0.229 by performing a plot of lnAagainstEawith a good linear relationship ofR2=0.9999,which indicates raw DAAF and DAAF@DAAzF explosives have the same decomposition mechanism. According to Eq.(2)[24],the decomposition rate constant(k)of raw DAAF is(1.11×10-3)s-1at 250 ℃. After the doping of DAAzF,the rate constant of thermal decomposition increases with the increase of DAAzF content,and thekvalue of DAAF@DAAzF-5 containing 10% DAAzF is(3.59×10-3)s-1:

Table 2 The parameters of linear equations between pre-exponential factors and activation energies

where dα/dtis the reaction rate,s-1;αis the conversion fraction;k(T)is the rate constant,s-1;tis time,s;Tis temperature,K;f(α)is the reaction model.
In addition,the isothermal thermal decomposition of DAAF@DAAzF explosives was investigated before the melting. Fig. 6d displays the isothermal DSC curves of all DAAF-based explosives at 230 ℃.It can be found that the decomposition peak time of raw DAAF is located at 41.72 min. After doping with 0.5% and 5.0% DAAzF,the DSC peak time of DAAF@DAAzF-1 and DAAF@DAAzF-4 is decreased to 27.09 min and 21.75 min,respectively. Compared to raw DAAF,isothermal thermal decomposition of DAAF@DAAzF explosives occurs in advance due to the doping of DAAzF. Therefore,the doping of DAAzF decreases the thermal stability of DAAF-based explosives.
4 Conclusions
(1)The effect of DAAzF on the thermal performance of DAAF@DAAzF explosives were studied comprehensively by TG-DSC after doping different content of DAAzF from 0.5% to 10% in DAAF explosives.
(2) DAAzF decreases the melting points of DAAF@DAAzF explosives,with the largest decline of 7.1 ℃in the presence of 10% DAAzF. The doping of 5.0% DAAzF in DAAF can lead to the formation of the eutectic mixture between them.
(3)The coexistence of DAAzF also decreases the activation energies and pre-exponential factors of DAAF@DAAzF explosives during the initial decomposition. Meanwhile,DAAzF can increase the rate constant of thermal decomposition of DAAF-based explosives.
(4) Through isothermal thermal decomposition,the decomposition peak time of DAAF@DAAzF explosives is advanced clearly due to the presence of DAAzF. Therefore,DAAzF as an impurity accelerates the thermal decomposition of DAAF-based explosives and decreases their thermal stability.
Acknowledgements:This work was financially supported by the National Natural Science Foundation of China(No. 21975235).
