构筑先进二维异质结构Ag/WO3-x用于提升光电转化效率
2019-10-14任玉美许群
任玉美,许群
郑州大学材料科学与工程学院,郑州 450001
1 Introduction
Utilization of renewable solar energy in chemical transformations has been regarded as one of the most promising approach to satisfy the rising global energy demand and simultaneously solve the corresponding environmental problems1-5. Among various forms for solar energy utilization,photocatalysis, which can promote the chemical reactions by the light-matter interaction, represents an efficient way to realize the converting of solar energy to chemical energy6. In view of the abundant and renewable nature of solar and water resources,hydrogen and oxygen production from photoelectrochemical(PEC) water splitting using semiconductor photocatalysts have caught the attention of researchers from academics and industry7-10. However, current photocatalysts suffer from insufficient light absorption, inefficient charge separation, and high charge recombination rate as well. Therefore, exploring photoelectrode materials with highly photoactive and longer carrier lifetime are urgently demanded.
Ideal solar-to-fuel photocatalysts must effectively harvest solar energy to enhance conversion efficiency. Recently,incorporating plasmonic metal nanoparticles into photocatalytic systems holds great promise for dramatically improving the efficiency of sunlight absorption and solar energy conversion, in which the localized surface plasmon resonance (LSPR) effect of the metal nanoparticles plays a significant role in promoting the photoactivity11-13. To date, many semiconductors (such as TiO2,Fe2O3, ZnO and WO3, etc.) and metals (such as Au, Ag, Cu, etc.)have been employed to prepare metal/semiconductor composite photocatalysts for plasmon-enhanced water splitting14-19. In the plasmonic metal/semiconductor systems, the hot electrons transferred from excited plasmonic metal can be directly injected into the conduction band of semiconductor for that they have higher energy than the interfacial Schottky barrier (φSB), and then contribute to the production of photocurrent20-22. However, if simply mixing plasmonic metal nanoparticles with semiconductor, their interface interaction will be very weak and moreover the metal nanoparticles cannot be well-controlled,resulting in the plasmon effect is greatly reduced23. Thus,rational designing peculiar plasmonic metal/semiconductor heterojunction is necessary.
On the basis of our previous study24, loading the plasmonic metal Ag nanoparticles into a peculiar 2D amorphous substoichiometric tungsten trioxide (a-WO3-x), in this work, we further annealed the obtained 2D heterostructure of Ag/a-WO3-xat 400 °C in N2to obtain the expected nanocomposites with local crystalline-amorphous interface (Scheme 1), moreover the plasmonic metals are uniformly dispersed and have intimate contact with the WO3-xnanosheets.
2 Experimental
2.1 Supercritical CO2 (SC CO2)-assisted in inducing chemical reaction
WS2nanosheets with single or few layers can be prepared by exfoliating bulk WS2powder (99%, Sigma-Aldrich Reagent Inc., Product Number: 243639) with assistance of SC CO225-27.Then, amorphous nanosheets can be further obtained by supercritical reaction conditions at 473.2 K, 16 MPa.
2.2 Synthesis of Ag/WO3-x heterostructure
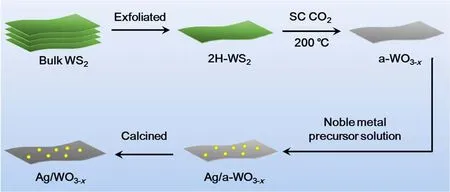
Scheme 1 Schematic preparation process of the as-prepared heterostructure.
Ag nanoparticles were prepared by a facile in situ redox at room temperature. Ag nanoparticles were produced by quickly addition of sodium citrate solution (45.6 mg, GR, Sinopharm Chemical Reagent Co., Ltd) and AgNO3(6.8 mg, AR,Sinopharm Chemical Reagent Co., Ltd) solution to the aqueous solution. The mixture was stirred vigorously at room temperature, following by addition of a certain amount of ascorbic acid solution (200 μL, 0.1 mmol·L-1, AR, Sinopharm Chemical Reagent Co., Ltd). The reaction lasted for 1 h. Next in order to improve the stability of the as-prepared catalyst sample,the obtained sample was further annealed at a temperature of 400°C in N2for 1 h.
2.3 Characterization
The morphology and structure of the materials were characterized by transmission electron microscopy (TEM)(JEM-2100, JOEL). X-ray diffraction (XRD) patterns of samples were measured on a Y-2000 X-ray Diffractometer with copper Kαradiation (λ = 0.15406 nm) operating at 40 kV and 40 mA. X-ray photoelectron spectroscopy was performed using a Thermo ESCALAB 280 system with Al/K (photon energy = 1486.6 eV)anode mono X-ray source. UV-Vis spectra (Shimadzu UV-240/PC) were measured to evaluate the light adsorption.
2.4 Photoelectrochemical (PEC) measurements
The PEC measurements were tested using an electrochemical workstation (CHI660E, Shanghai Chenhua Co., Ltd., China)with a typical three-electrode cell. The as-prepared sample was used as the working electrode, a Ag/AgCl electrode and Pt wire were used as reference and counter electrode, respectively. 0.5 mol·L-1Na2SO4was used as the electrolyte. The working electrodes were prepared by dropping the suspension onto the surface of a clean fluorine-doped tin oxide (FTO) conductive glass substrate. The light ON-OFF switches were set as 100 s when measuring the I-t curves of the absolute values under visible light. The bias for the measurement was set as 0.8 V. The reversible hydrogen potential can be converted from the Ag/AgCl reference electrode potential as ERHE= EvsAg/AgCl+EoAg/AgCl+ 0.059 × pH, where EoAg/AgClis 0.1976 V at 25 °C.
The incident photon-to-current conversion efficiency (IPCE)spectra was collected by a solar simulator (Newport 66984,USA) coupled with a filter (Newport 71260) and an aligned monochnromator (Newport 1-800-222-6440). All the electrochemical measurements were carried out by an electrochemical workstation (CHI 660E). IPCE can be expressed by the equation: IPCE = (1240 × I)/(λ × Jlight), where I (mA·cm-2)is the measured photocurrent density at a specific wavelength, λ(nm) is the wavelength of incident light, and Jlight(mW·cm-2) is the measured irradiance at a specific wavelength.
The PEC degradation of methyl orange (MO) was performed in a 100 mL of two electrode quartz cell system with 300 W Xe lamp equipped with a UV cut-off filter (420 nm) on a CHI 660E Electrochemical Workstation, and the light intensity was kept as 100 mW·cm-2. 0.5 mol·L-1Na2SO4was used as electrolyte solution. The initial concentration of MO in the solution was 20 mg·L-1. The as-prepared sample (20 mg) was dispersed into the MO solution. The graphite electrode was connected to the working electrode, and a Pt wire was used as counter electrode.The absorbance of MO was measured at a wavelength of 464 nm. The PEC degradation of MO was performed with a voltage of 1.0 V versus Ag/AgCl. The system was illuminated after stirring in dark for 30 min to reach equilibrium of complete adsorption-deposition for the photoelectrode. Samples were then taken from the reactor every 15 min, and the concentration of MO was determined by a UV-Vis spectrophotometry.
3 Results and Discussion
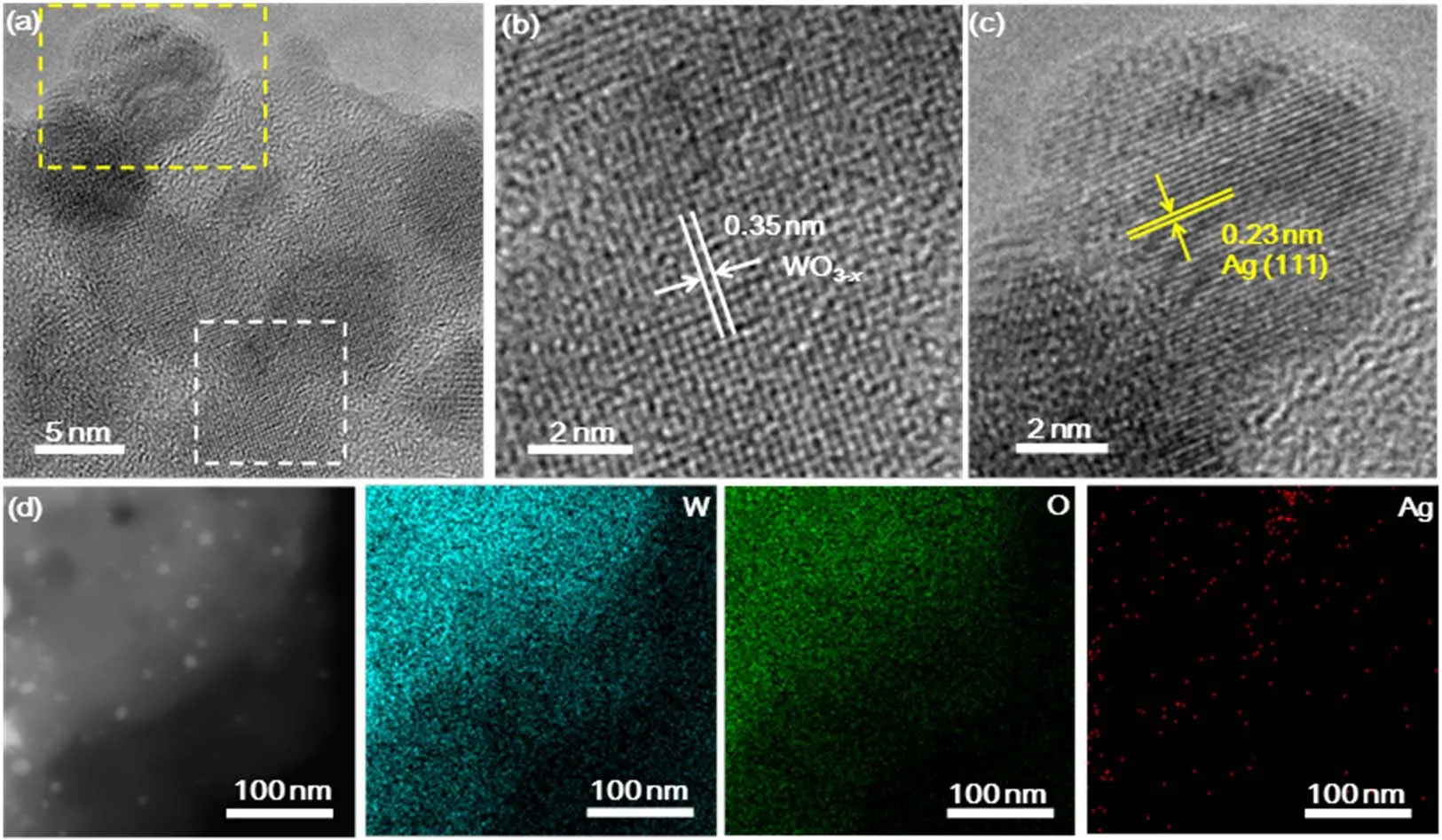
Fig. 1 (a) TEM image of Ag/WO3-x heterostructure. (b, c) Magnified images of the regions enclosed by the white and yellow squares in (a),respectively. (d) Bright-field STEM image and EELS elemental mapping of W (blue), O (green) and Ag (red) of Ag/WO3-x heterostructure.
The TEM image of the Ag/WO3-xheterostructure is shown in Fig. 1a, it can be seen that the as-prepared heterostructure has successfully constructed with local crystalline and amorphous interface. The crystalline structure with the lattice fringes of 0.35 nm corresponds to WO3-x(Fig. 1b)28. The well-resolved lattice fringes with d-spacing of 0.23 nm correspond to the (111) lattice plane of Ag (Fig. 1c)29. Elemental mappings (Fig. 1d) clearly reveal the homogeneous distribution of W, O and Ag atoms over the entire nanosheets.
The XRD patterns shown in Fig. 2a demonstrate the crystallinity of the amorphous substrate enhanced obviously. As can be seen the XRD pattern of a-WO3-xnanosheets, only a bread-shape peak can be found, suggesting the as-obtained product is amorphous. After annealing at 400 °C, the intensity of the diあraction peaks increases. The peaks at 2θ = 22.9°, 31.7°and 45.5° both in Ag/WO3-xheterostructure can be indexed to WO2.9(JCPD card No. 05-0386) and WO2.72(JCPD card No. 05-0392), respectively. Moreover, another two relatively weak peaks appearing at 2θ = 28.6° and 33.5° correspond to WO3(JCPD card No. 43-1035). Beyond the diffraction peaks of WO3-x, the other new diffraction peaks are indexed to face centered cubic structure Ag (JCPD card No. 65-2871)30.
X-ray photoelectron spectroscopy (XPS) characterization was always employed to investigate the surface composition and chemical states of the elements. Fig. 2b, c show that the binding energies of O 1s and W 4f in Ag/WO3-xare negatively shifted, as compared to the ones for a-WO3-x. Moreover, the binding energy of Ag 3d in Ag/WO3-xis positively shifted compared to that of Ag nanoparticles (Fig. 2d). All of these results confirm that the electrons transfer from Ag and WO3-x28,31. The binding energies of W 4f and O 1s more negative shift and Ag 3d more positive shift in Ag/WO3-xthan that of Ag nanoparticles demonstrate better contact may be formed between the metals and the matrix during the process of calcination, facilitating the transformation of more electrons from metals to the substrate. In the O 1s region of the spectra, the peak locating at 532.4 eV is attributed to nonstoichiometric tungsten oxides, while the binding energy at ca. 532 and 533.4 eV are related to adsorbed H2O molecules inside and on the surface of the tungsten oxide32-34. And the increase in peak area at 532.4 eV and the decrease in peak area at 532 and 533.4 eV indicate an increase in crystallinity of the as-prepared sample. From the XPS spectra analysis, we also verify that the doped Ag nanoparticles are both in metallic state.The peaks observed at around 368 and 374 eV are ascribed to metallic Ag35.
The effect of metal nanoparticles on the optical properties of as-prepared substrate materials was studied by optical absorption spectroscopy. The UV-Vis absorption spectra of a-WO3-x,Ag/WO3-xand Ag nanoparticles are compared in Fig. 3. For a-WO3-xnanosheets, they exhibit strong peaks below 400 nm assigned to the inter-band absorbance36,37. It can be seen that Ag/WO3-xshows a broad peak centered on 480 nm,corresponding to the LSPR of Ag nanoparticles36. The broaden LSPR peaks of the metal nanoparticles in these nanocomposites obviously mainly attribute to the electronic interaction between the embedded metal nanoparticles and the a-WO3-xnanosheets31.
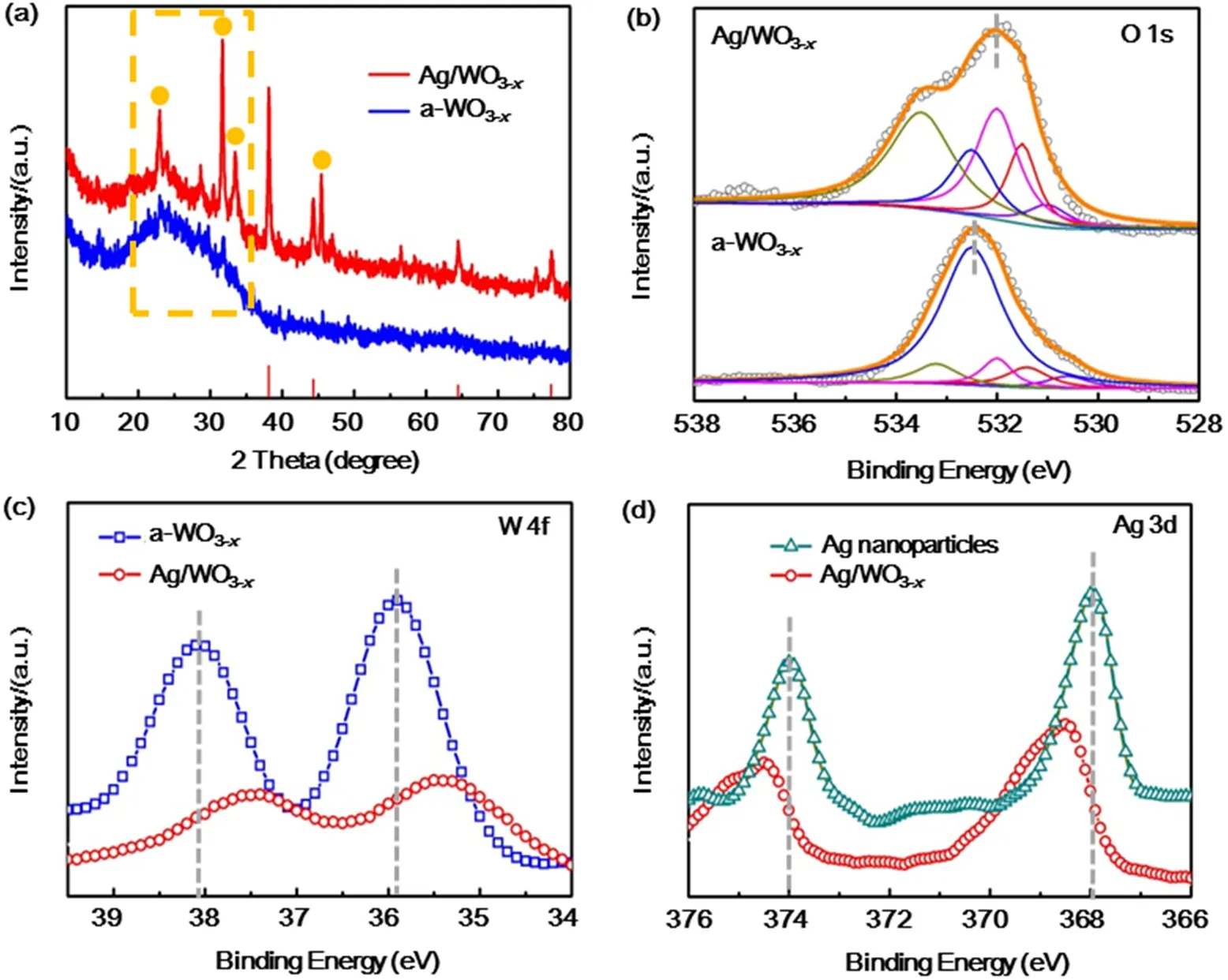
Fig. 2 (a) XRD patterns of a-WO3-x nanosheets and Ag/WO3-x heterostructure. Deconvoluted high-resolution XPS of selected core level peak region: (b) O 1s and (c) W 4f XPS spectra of a-WO3-x and Ag/WO3-x. (d) Deconvoluted high-resolution XPS of selected core level peak regions: Ag 3d for Ag nanoparticles and Ag/WO3-x.
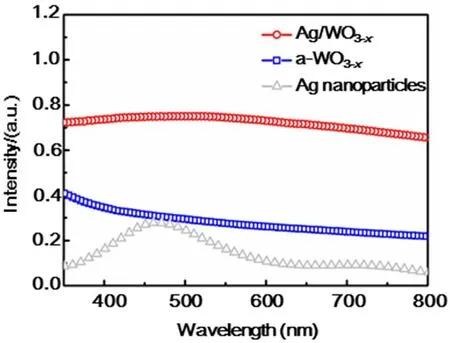
Fig. 3 Absorption spectra of Ag/WO3-x, a-WO3-x and Ag nanoparticles.
The photocurrent responses of the PEC devices based on a-WO3-xand Ag/WO3-xalong with Ag nanoparticles and blank FTO are recorded to compare their PEC behavior for several ON-OFF cycles under simulated solar light illumination (AM 1.5, 100 mW·cm-2) (Fig. 4a). It can be obviously observed that Ag/WO3-xexhibits enhanced photocurrent density as compared with a-WO3-xand Ag nanoparticles. The photoresponse of Ag/WO3-xheterostructure is about 5 times higher than that of a-WO3-x. Electrochemical impedance spectroscopy (EIS) was commonly used to investigate the electrode kinetics of the catalytic processes on the samples (Fig. 4b)38. The representative Nyquist plots display a remarkably decreased charge transfer resistance (Rct) for Ag/WO3-xcompared to a-WO3-x, indicating that the incorporation of metal nanoparticles and the improved conductivity can enhance the electron mobility by suppressing the recombination of photogenerated electrons and holes, thus the photogenerated electrons and holes are effectively separated and the interfacial electron transport is increased31.
To better evaluate the PEC efficiency of these as-prepared samples as a function of illumination wavelength, the incident photo-to-current conversion efficiency (IPCE) measurements conducted at a bias potential of 0.8 V vs Ag/AgCl was displayed from 360 to 650 nm (Fig. 4c). The Ag/WO3-xshows much higher IPCE than that of a-WO3-x, which is well-matched with their corresponding LSPR absorption peaks in the visible region. This signifies that excitation of the metal LSPR is responsible for the improved visible-light photoactivity of Ag doped a-WO3-x. And this enhancement is ascribed to more incident photons provided by the plasmonic noble metal nanoparticles via multiple scattering39. Moreover, the time dependence curve of current density at 0.8 V vs Ag/AgCl shown in Fig. 4d demonstrates that the as-prepared sample has good stability.
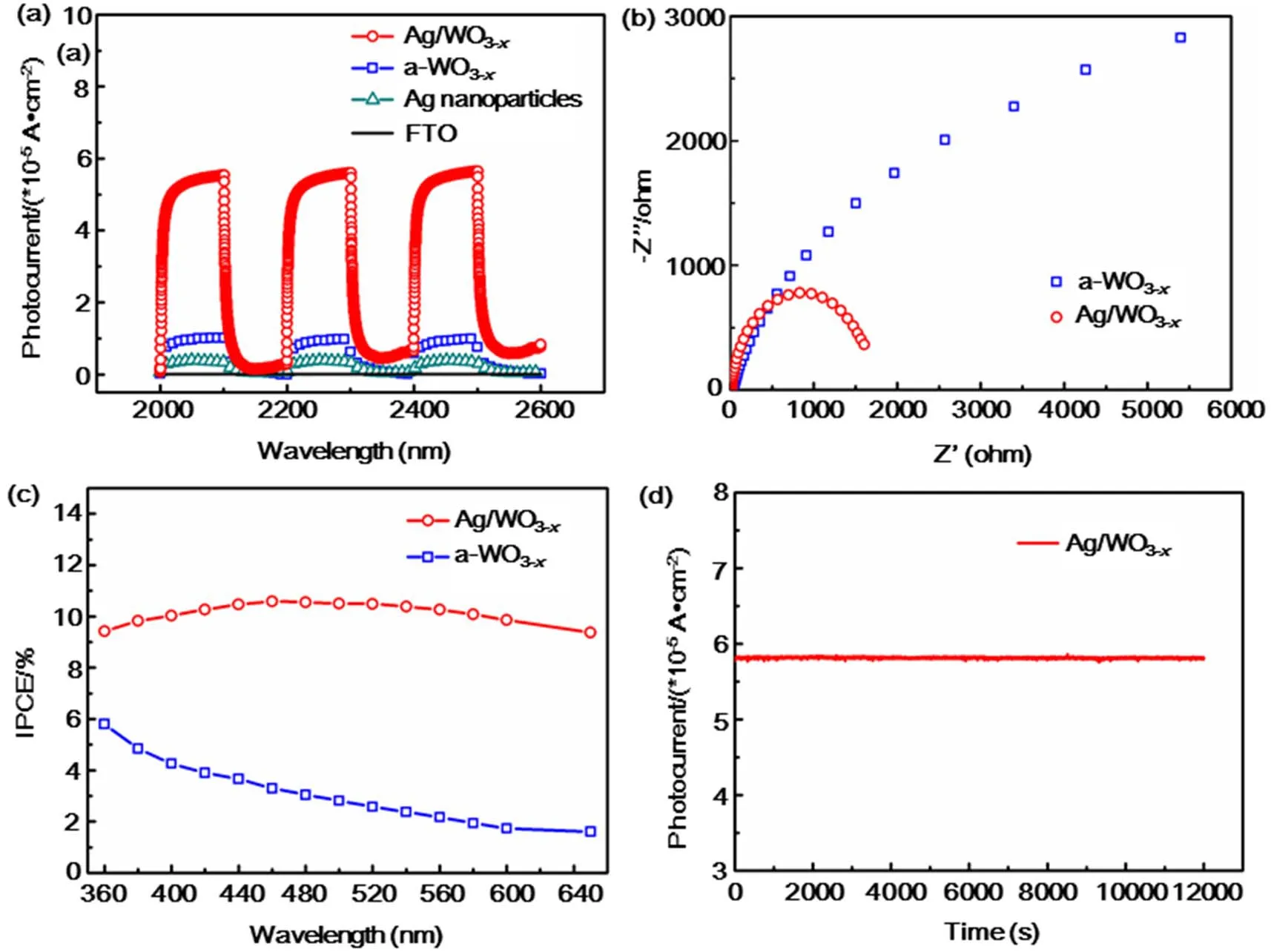
Fig. 4 (a) The photocurrent response (0.8 V bias) of bare FTO glass, Ag nanoparticles, a-WO3-x and Ag/WO3-x coated FTO electrodes in 0.5 mol·L-1 Na2SO4 under simulated solar light illumination (AM 1.5, 100 mW·cm-2). (b) EIS plots of a-WO3-x and Ag/WO3-x electrode in 0.5 mol·L-1 Na2SO4 illuminated by simulated solar light (AM 1.5, 100 mW·cm-2). (c) IPCE spectra of a-WO3-x and Ag/WO3-x heterostructure measured at 0.8 V vs Ag/AgCl. (d) The Ag/a-WO3-x photoanode at a constant bias of 0.8 V vs Ag/AgCl in 0.5 mol·L-1 Na2SO4 under AM 1.5G simulated sunlight for 200 min.
Prompted by the unique structural advantages of the asprepared Ag/WO3-xnanocomposites, a comparative study was carried out on the degradation of methyl orange (MO)measurement as a probe reaction to further confirm the advantages of the as-prepared photoelectrode in the use of sunlight. Fig. 5 show the changes in relative concentration(C/Co) of MO under UV and Vis light illumination with a-WO3-xand Ag/WO3-xheterostructure as electrode individually and the initial MO concentration of 20 mg·L-1. From Fig. 5, it is found that the Ag/WO3-xhas a higher PEC degradation efficiency than that of the a-WO3-xno matter under UV or Vis light illumination.After reaction for 120 minutes under Vis light illumination, the PEC degradation efficiency of Ag/WO3-xcan reach 96.7% for MO, while the PEC degradation efficiency of WO3-xis only 63.6% (Fig. 5b). Moreover, under the illumination of UV light,the PEC degradation efficiency of Ag/WO3-xis only 60.3% for MO, which is a little higher than that of WO3-x(47.7%). The significant difference of the PEC degradation efficiency of Ag/WO3-xunder UV and Vis light illumination can be attributed to the strong broadened absorption at visible light region arising from the SPR effect of the metallic Ag nanoparticles40,41.
On the basis of above analysis, the possible mechanism for the enhanced photoelectrocatalytic efficiency of the Ag/WO3-xphotoelectrode was proposed and illustrated in Fig. 6. The enhanced crystallinity can effectively improve the conductivity and electrochemical stability as well. Under the irradiation of simulated solar light (AM 1.5, 100 mW·cm-2), photogenerated electrons of WO3-xfrom the valence band are excited to the conduction band, leaving the same amount of holes in the valence band. On the other hand, the incorporation of plasmonic Ag nanoparticles can act as photosensitizers to enhance the optical absorption of the metal/semiconductor heterostructere,the hot plasmonic electrons of Ag can transfer to the conduction band (CB) of WO3-xover the metal/semiconductor Schottky barrier and the small size of metal nanoparticles allows the hot electrons to reach the metal/semiconductor interface before decay2. For the plasmonic holes, they can readily accumulate at the interface between Ag and WO3-x. Meanwhile, the LSPR-induced electromagnetic field enhancement effects facilitated photogenerated electrons and holes generation and separation42.For PEC water splitting43, the electrons are promptly transferred from the working electrode via the FTO substrate toward the Pt counter electrode, where the H+in water is reduced to generate H2. While the remaining holes on the Ag/WO3-xsurface will oxidize OH-to generate O2. For PEC degradation of MO44,45,the photogenerated electrons (e-) could combine with the dissolved O2to yield the superoxide anion radicals (·O2-), and further form the hydroxyl radicals (·OH) for the MO degradation into CO2, H2O and other products. Meanwhile, the consumption of electrons can also inhibit the recombination of the electron/hole pairs to some extent. Moreover, the photogenerated holes (h+) could easily seize the H2O molecules to generate high active species of the OH radicals, which also contribute much to the MO degradation. Thus, both the unique substrate and the introduction of metal nanoparticles contribute to the efficient charge transfer and reduced recombination,resulting in enhanced PEC performance.
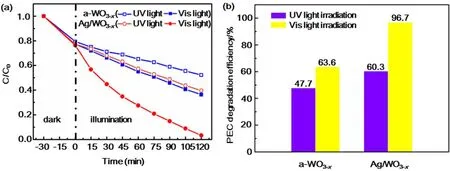
Fig. 5 (a) PEC degradation of MO with a-WO3-x and Ag/WO3-x at 1.0 V vs Ag/AgCl.(b) The PEC degradation efficiency of a-WO3-x and Ag/WO3-x heterostructure.
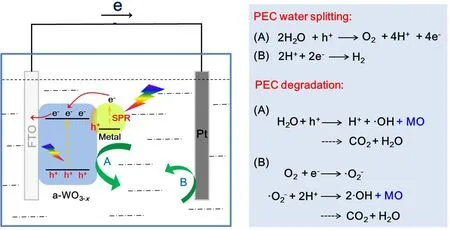
Fig. 6 Schematic of the proposed photoelectrocatalysis mechanism for the Ag/WO3-x system.
4 Conclusions
In summary, we have demonstrated that 2D a-WO3-xnanosheets can be used as effective support for metal nanoparticles, and the resultant unique heterostructure exhibit a much superior PEC activity. The enhanced PEC performance can be attributed to the construction of special local crystallineamorphous interface, which can increase the specific surface area and active sites, and improve the electrical conductivity as well. Moreover, the introduction of Ag nanoparticles can induce LSPR effect, and the excellent contact between the Ag nanoparticles and WO3-xcan promote the transfer and separation of charge carriers effectively. Therefore, we believe this designing strategy will lead to more impossibilities for design and fabrication of high-performance catalyst materials in the future.
