二甲醚、氨气和甲烷混合燃料的层流预混火焰损特性研究
2022-06-25孙国军付忠广卢茂奇
孙国军,付忠广,卢茂奇
【碳中和专栏】
孙国军,付忠广,卢茂奇
(华北电力大学能源动力与机械工程学院,北京 102206)


面对能源日益短缺、环境污染日益严重的两大世界难题,国内外一直致力于开发利用风能、太阳能等可再生能源,然而风能、太阳能受气候、季节、地理条件等诸多因素影响,其能量供给具有间歇性及波动性.为了提高可再生能源利用率,诸多国家及地区相继提出了“Power-to-X”概念[1],其中氢气[2]、氨气(NH3)[3]、醇类[4]及醚类[5]等替代燃料作为可再生能源的化学能载体获得了诸多关注.
二甲醚(DME)作为一种很有前途的清洁替代燃料,它可以利用可再生能源、废弃的生物质能进行大规模催化制备[6].与此同时,优异的着火和雾化性 能[7-8]、运输的便利性[9]以及低排放特性[10]使得DME作为纯燃料或掺混燃料在内燃机、燃气轮机等领域广泛研究,特别是CH4/DME二元燃料系统[11-12].类似地,完善的制备工艺、安全及便利的储运性能使得NH3作为新一代能量载体极具发展潜力[13-15].然而,由于NH3具有较低的自燃性及火焰传播速度,其燃烧稳定性仍然存在挑战.目前,与高反应性燃料掺混燃烧是处理该问题的有效方案之一[16],如CH4/NH3二元燃料燃烧[17-18].此外,氨替代部分天然气应用于工业燃气轮机的理论可行性也得到了相应论证[3]. DME和NH3的有效利用对降低化石能源开采,实现“碳达峰”、“碳中和”发展目标具有重要意义.


1 计算方法
CH4/DME/空气、CH4/NH3/空气的层流预混火焰的传播是基于Cantera-2.5.1开源程序中的FreeFlame代码[26](类似于Chemkin-PRO中的PREMIX模块[27])实现的,其控制方程和边界条件的详细数学表达可以在文献[28]中找到.计算设置如下:计算域长度设置为0.2m,使用混合平均输运模型评估输运特性.为保证计算的准确性,曲率和梯度这两种自适应网格控制参数均设定为0.02,最终的解决方案中将超过800个网格点.
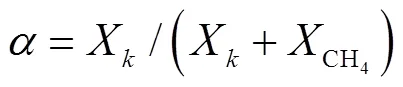
式中:为DME、NH3;为气体的摩尔分数.
当量比的计算如式(2)所示:
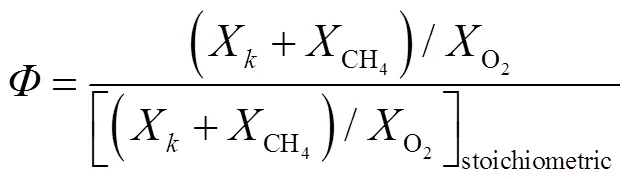
此外,在进行FreeFlame预混火焰传播的计算前,考虑以下假设[29]:①火焰为稳定的一维层流火焰,忽略了辐射传热和Dofour效应;②气体混合物符合理想气体混合模型;③“Soret效应”不显著;④外力作用可忽略不计;⑤整个计算域压力恒定,无明显波动.
由于层流预混火焰中黏性效应的贡献非常小,通常忽略不计[23-24],则多组分反应过程的局部熵产可描述为[24]

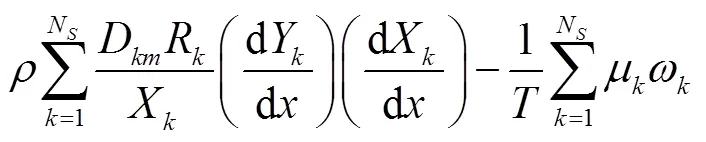
式中:gen-conduction、gen-diffusion和gen-reaction分别为由热传导、质量扩散和化学反应引起的局部熵产;R为第种组分的比气体常数(R=u/M,u=8.314J/(mol·K),M为摩尔质量);为局部温度;和分别为平均导热系数和密度;D、X、Y、μ和ω分别为混合物的平均扩散系数、第种组分的摩尔分数、质量分数、化学势和单位体积形成速率.
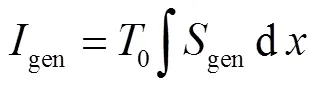

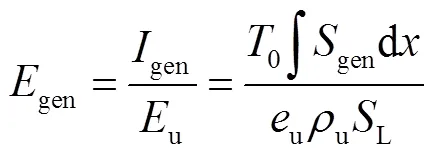
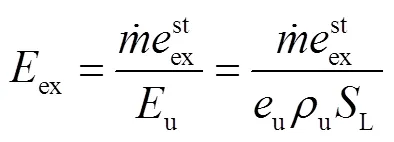


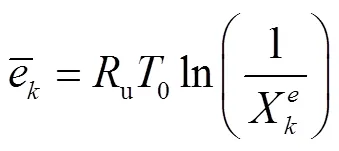


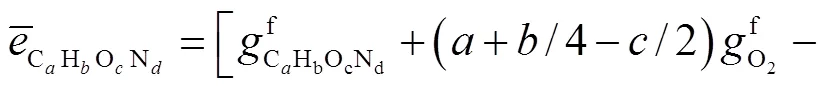
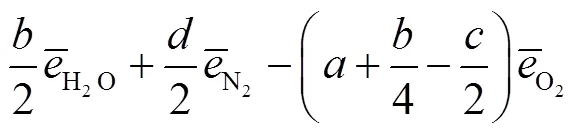

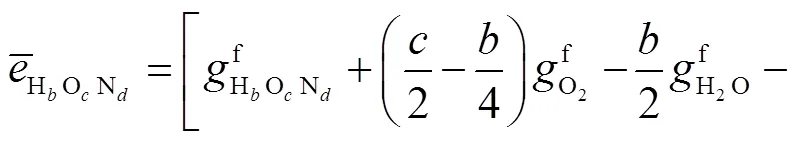
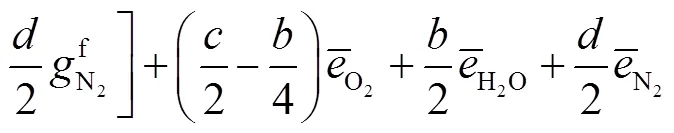

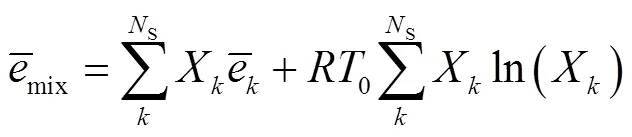
2 化学机理的选取与验证

国内外学者围绕CH4/DME、CH4/NH3热解与氧化特性开展了诸多讨论,形成了一系列热力学-化学动力学-输运机理,如表1所示.
表1 化学动力学机理
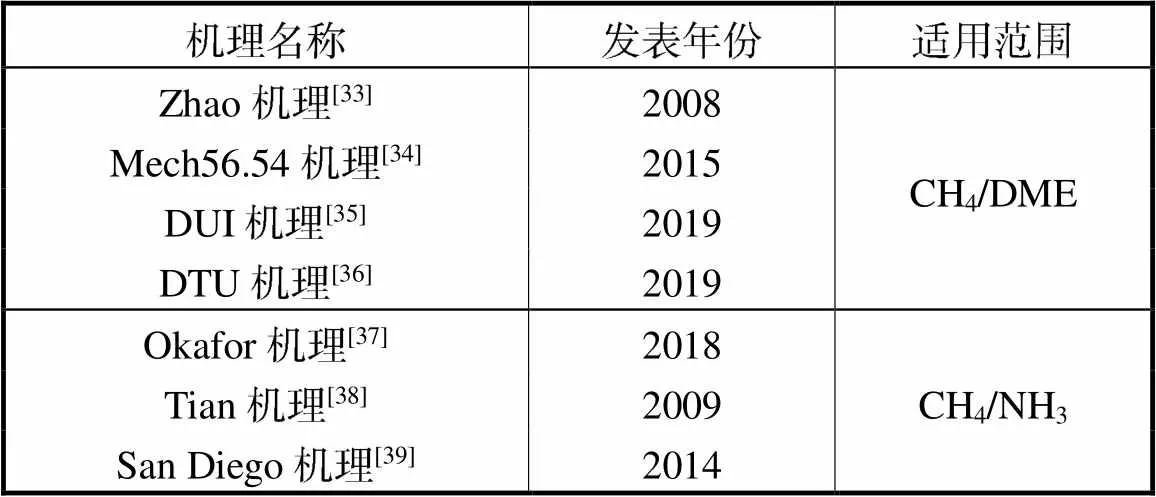
Tab.1 Chemical kinetics mechanism
其中Zhao机理与Okafor机理已在大量的实验数据验证中展现了良好的预测能力.以Zhao机理为基础机理同时添加Okafor机理中的NH3子机理,形成了78种组分、420步基元反应的合并机理(以下称为Zhao-Okafor机理).
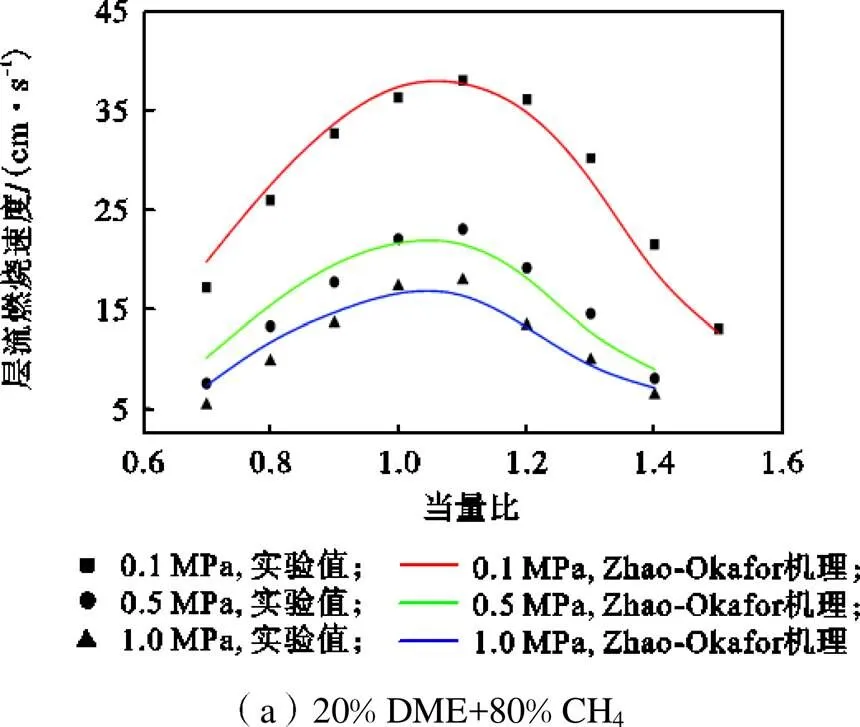
层流火焰速度体现了可燃气体的扩散性、反应性物理化学性质,是建立和验证化学动力学机理的基础数据.图1显示了Zhao-Okafor机理预测值与Lowry等[40]利用向外传播的球形火焰获取的CH4/DME实验值,以及Han等[41]采用热通量法获取的CH4/NH3实验值的对比.结果表明,Zhao-Okafor机理在不同燃料掺混比及初始压力条件下均展现了良好的预测能力.对于CH4/DME预混火焰,层流燃烧速度随着运行压力的升高单调下降,不同压力条件下层流燃烧速度均在=1.1附近达到峰值,Zhao-Okafor机理计算值与实验值的平均相对误差为9.6%,考虑到实验测量值的不确定度在2.5%~10.5%的范围,上述的差异性是可接受的.对于CH4/ NH3预混火焰,层流燃烧速度随着掺混比的增大单调下降,不同掺混燃料层流燃烧速度均在=1.05附近达到峰值.此外,Zhao-Okafor机理在贫燃侧的预测能力是优异的,相比于实验值的平均相对误差仅为2.7%.较大的误差源于富燃火焰侧,特别是高NH3掺混比燃料.Han等[41]也报道过类似的现象,并指出计算的误差主要与含氮反应速率参数的不准确性有关.总的来说,Zhao-Okafor机理良好再现了CH4/DME及CH4/NH3的层流燃烧速度变化趋势,且计算精度也是令人满意的.
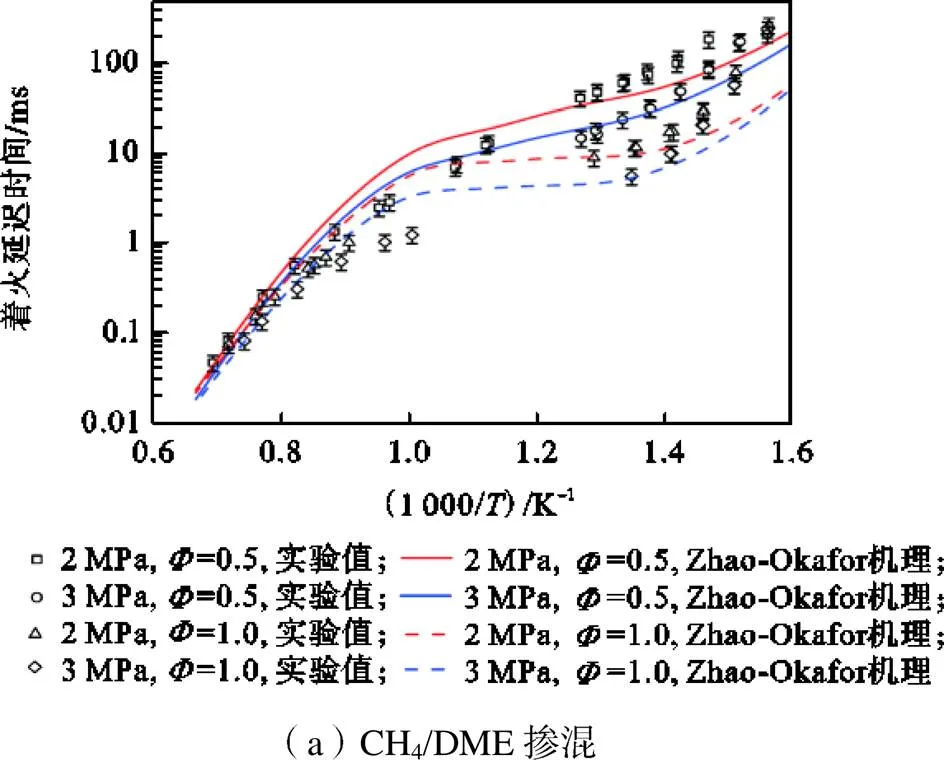
为进一步验证Zhao-Okafor机理的准确性,将Zhao-Okafor机理的预测结果与Burke等[8]利用快速压缩机(RCM)和激波管(ST)实验测量的甲烷/二甲醚燃料,以及Xiao等[42]利用ST实验测量的甲烷/氨气燃料着火延迟时间进行对比,结果如图2所示.可以看到,对于CH4/DME燃料,Zhao-Okafor机理能够准确捕捉到DME燃料复杂的负温度区间(NTC)行为,较大的误差源于<1000K温度区间,这是由于Zhao机理的固有缺陷,缺少对涉及CH3OCH2的反应速率参数压力敏感性关系的修正引起的[43].对于CH4/NH3燃料,在所有实验条件下,Zhao-Okafor机理均存在一定的过度预测,Dai等[44]与Li等[45]也都报道过类似的现象,这是因为目前最适合氨燃烧的化学动力学模型仍未被提出,对开发模型所需参数存在一定争论.但是,不同的模型在特定的条件下能够较为准确地预测氨火焰的燃烧速度、火焰结构和生成物浓度[46].总体而言,Zhao-Okafor机理能较好地预测着火延迟时间与当量比、压力和温度的关系.因此,本文将采用Zhao-Okafor机理开展热力学第二定律的计算分析.
3 结果分析
基于上述计算方法以及选取的化学机理,以纯CH4、80%CH4/20%DME、80%CH4/20%NH3火焰为例,计算了=1.0、=295K、=0.1MPa条件下,层流预混火焰各源项熵产率及温度随轴向距离的变化,如图3所示.热传导熵由温度和温度梯度决定,在火焰上游,较高的温度梯度克服了较低温度的影响,导致热传导熵产率峰值快速形成.在火焰下游,随着温度逐渐稳定,由组分摩尔分数梯度影响的质量扩散熵以及化学反应熵开始显著,相应熵产率峰值依次出现.此外,无论何种燃料类型,化学反应熵产率始终远高于热传导熵产率以及质量扩散熵产率,说明化学反应是主要的熵产源.

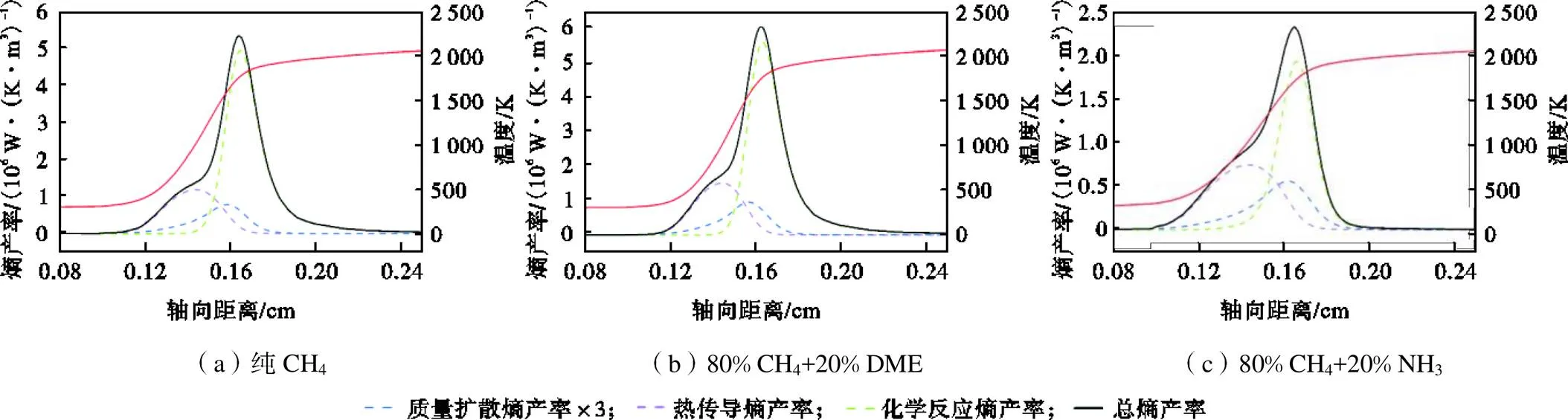
图3 在Φ=1.0、T=295K、p=0.1MPa条件下,不同层流预混火焰的熵产率和温度分布
3.1 不同燃料掺混比对损率的影响
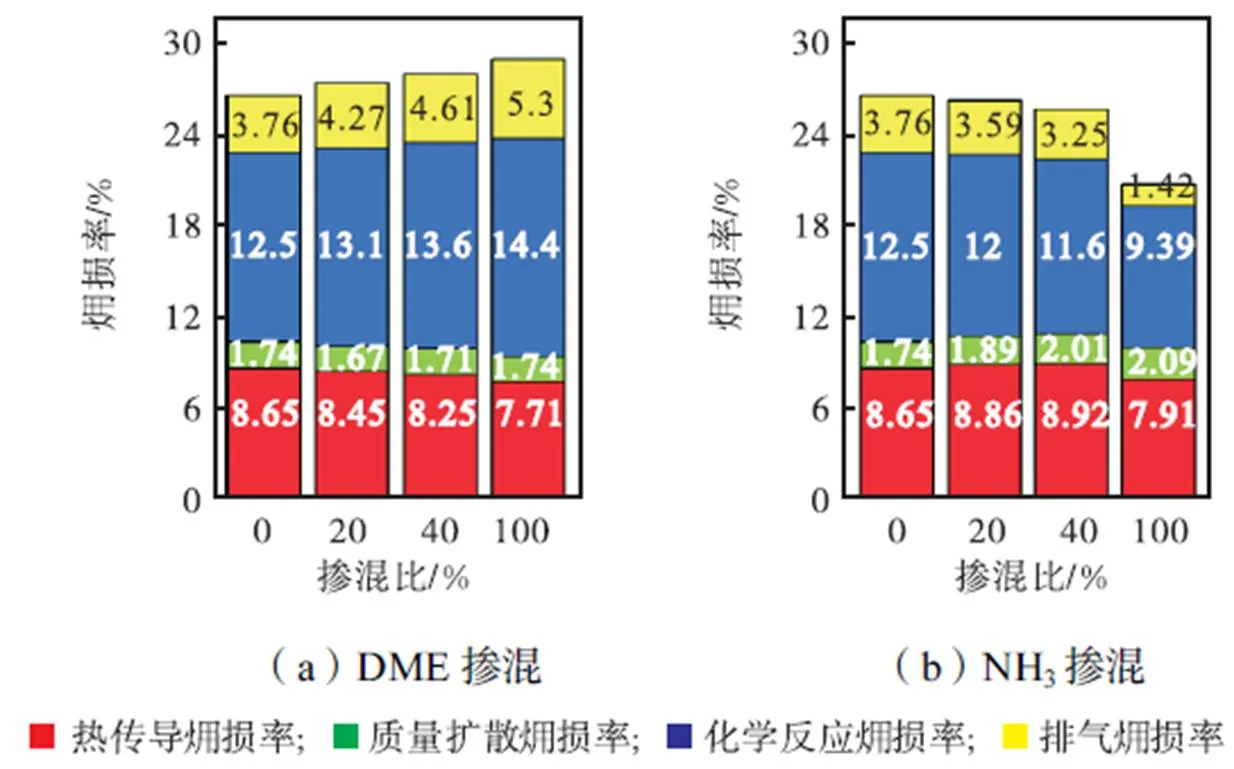
图4 在Φ=1.0、T=295K、p=0.1MPa条件下,不同DME、NH3掺混比对各源项损率的影响

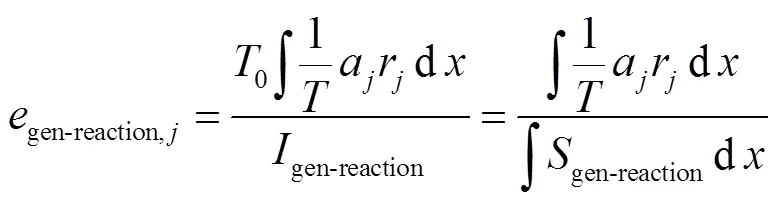
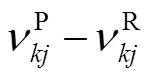
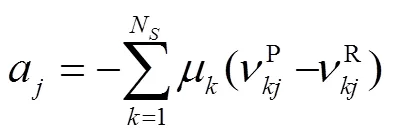

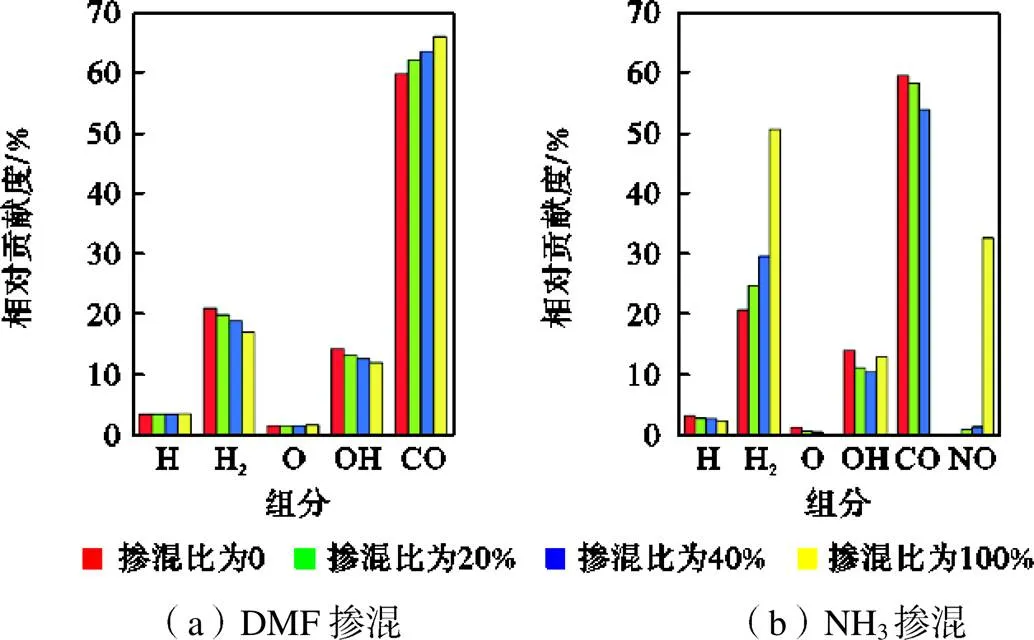
图5 在Φ=1.0、T=295K、p=0.1MPa条件下,不同DME、NH3掺混比时排气混合物中各组分对排气损率的相对贡献度
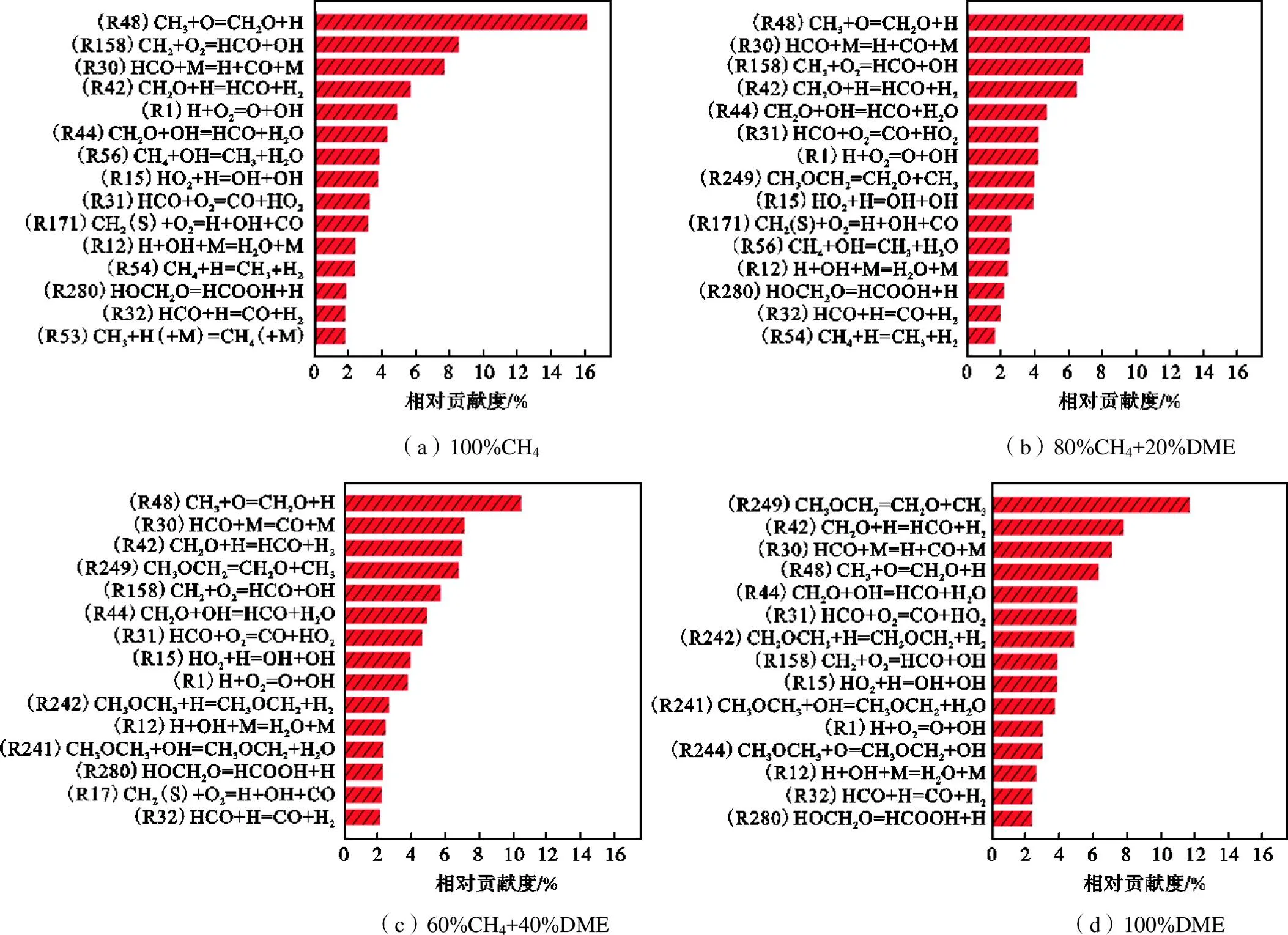
图6 在F=1.0、T=295K、p=0.1MPa条件下,添加DME时,对化学反应损贡献最大的基元反应
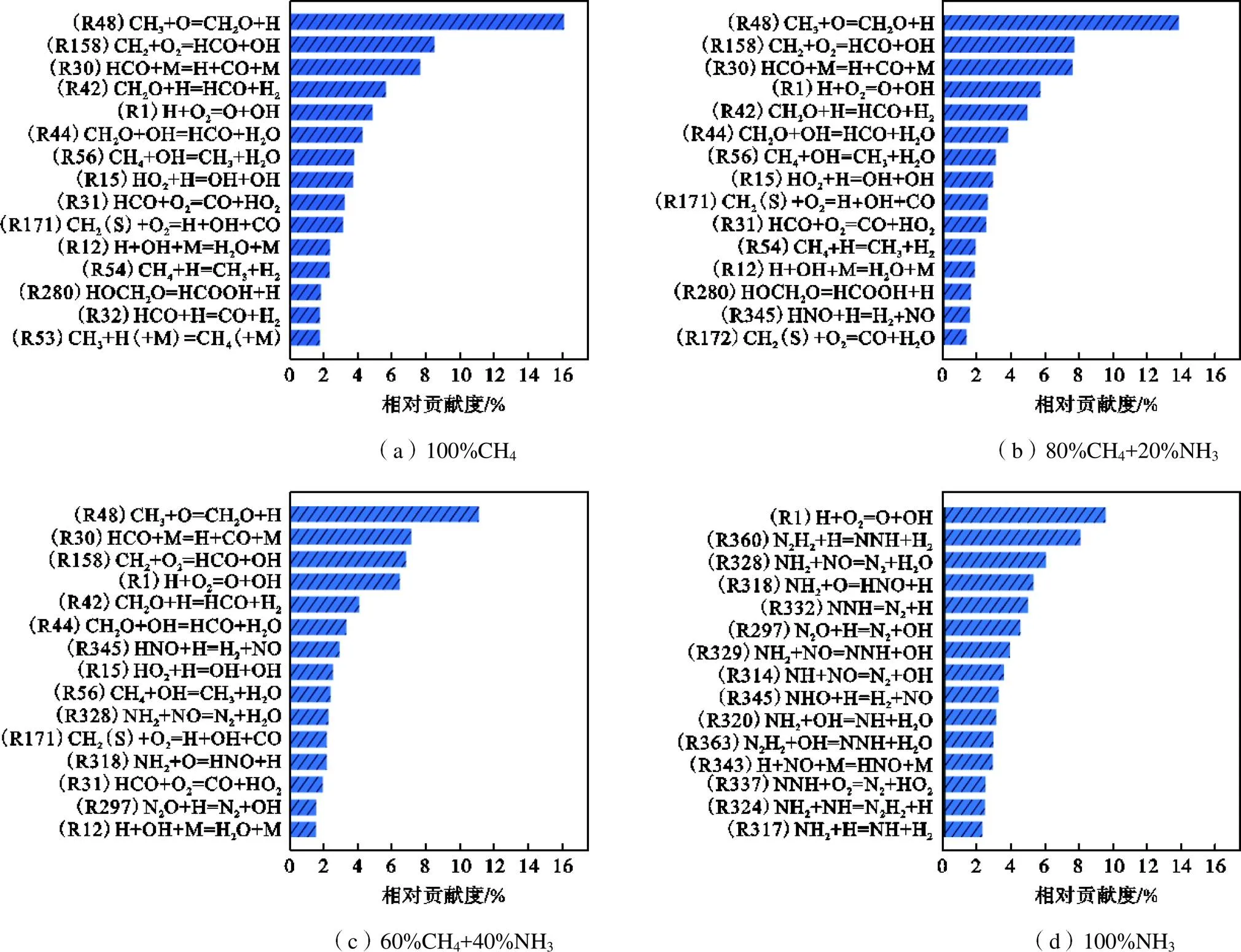
图7 在F=1.0、T=295K、p=0.1MPa条件下,添加NH3时对化学反应损贡献最大的基元反应
3.2 不同温度对损率的影响

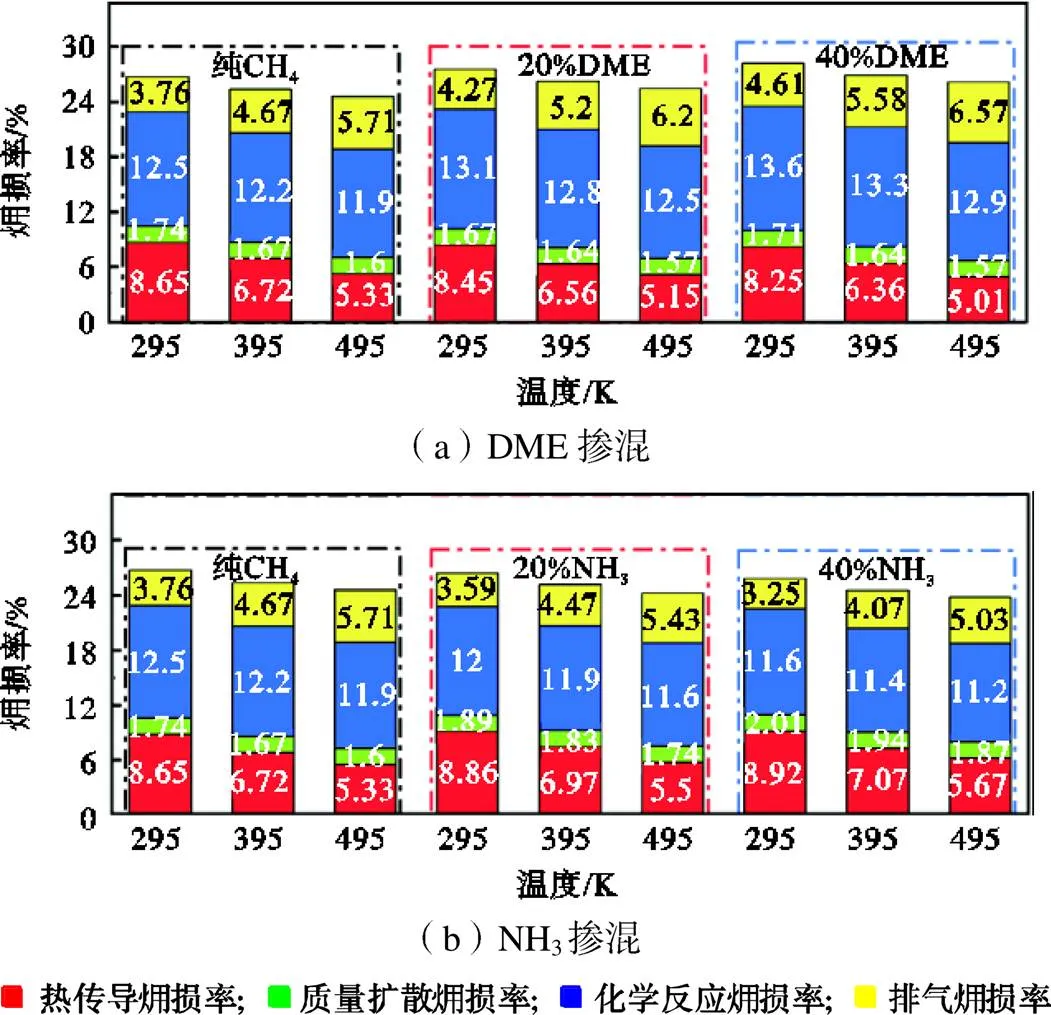
图8 在F=1.0、p=0.1MPa条件下,不同温度对各源项损率的影响
3.3 不同压力对损率的影响


图9 在F=1.0、T=395K条件下,不同压力对各源项损率的影响


3.4 不同当量比对损率的影响


图10 在T=395K、p=2MPa条件下,不同当量比对各源项损率的影响
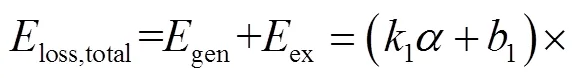
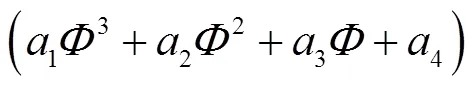

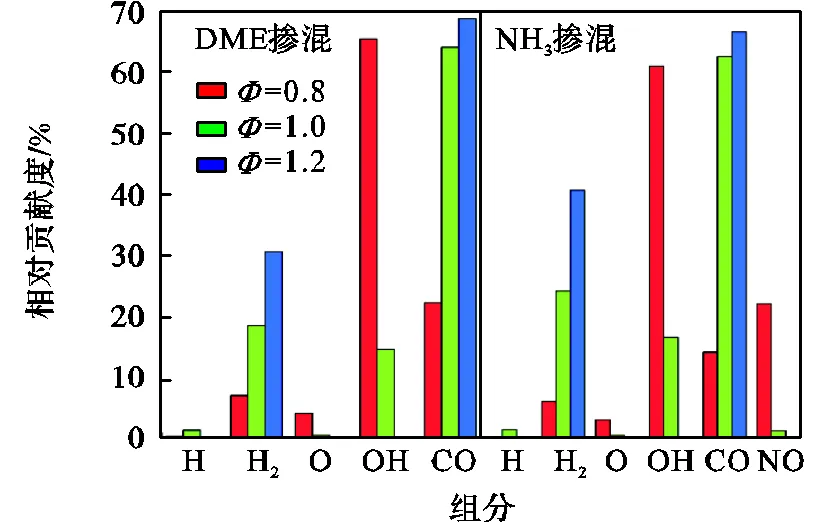
图11 在T=395K、p=2MPa条件下,不同当量比时排气混合物中各组分对排气损率的相对贡献度
表3 响应面函数拟合参数

Tab.3 Fitting parameters of response surface function
注:2表示拟合优度.
表4 正交工况
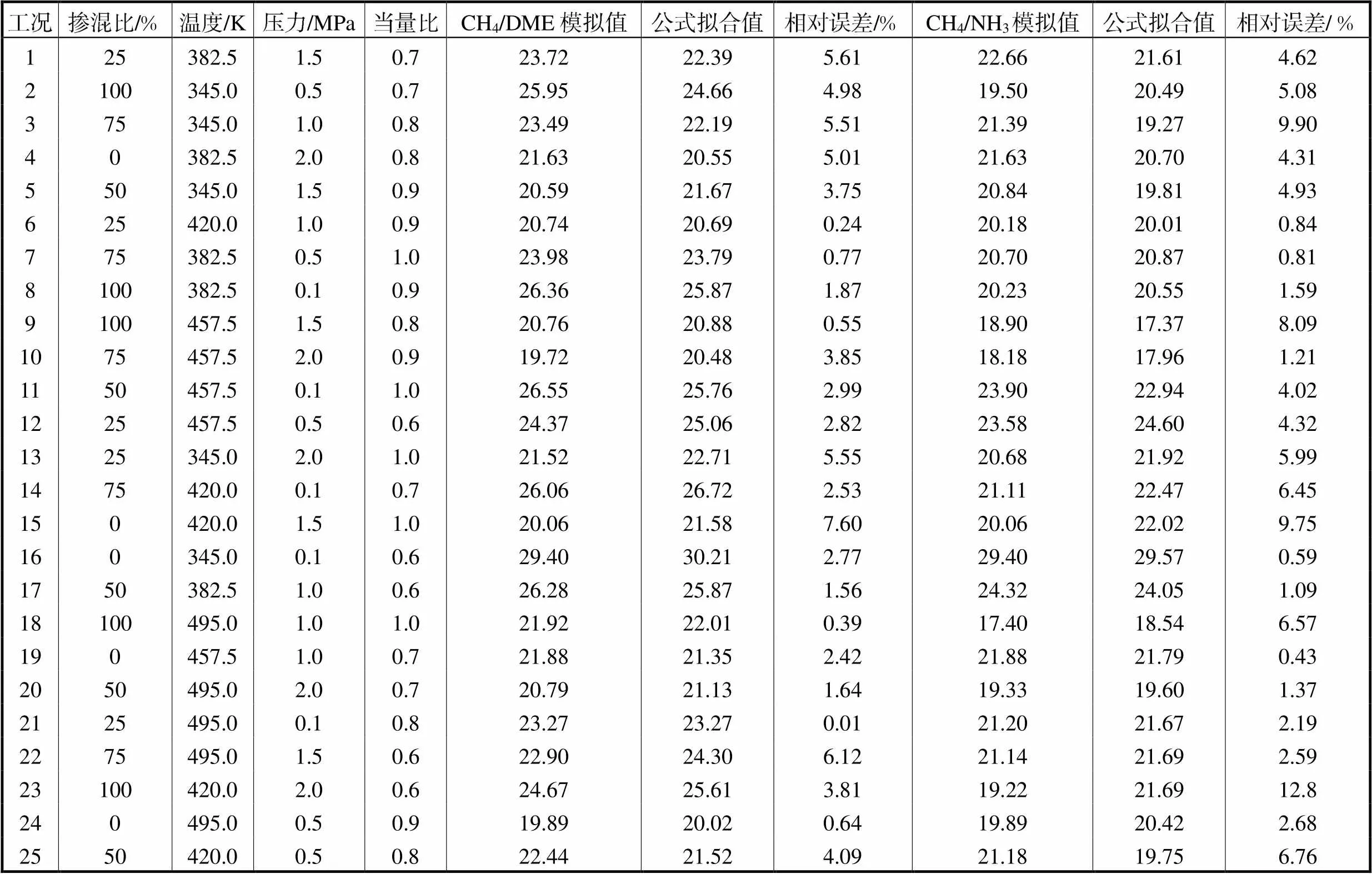
Tab.4 Orthogonal working conditions
4 结 论


[1] Bargiacchi E,Antonelli M,Desideri U. A comparative assessment of Power-to-Fuel production pathways[J].,2019,183:1253-1265.
[2] Aliyu M,Nemitallah M A,Said S A,et al. Characteristics of H2-enriched CH4-O2diffusion flames in a swirl-stabilized gas turbine combustor:Experimental and numerical study[J].,2016,41(44):20418-20432.
[3] Ichikawa A,Naito Y,Hayakawa A,et al. Burning velocity and flame structure of CH4/NH3/air turbulent premixed flames at high pressure[J].,2019,44(13):6991-6999.
[4] Rivera-Tinoco R,Farran M,Bouallou C,et al. Investigation of power-to-methanol processes coupling electrolytic hydrogen production and catalytic CO2reduction[J].,2016,41(8):4546-4559.
[5] Kang Y,Lu T,Lu X,et al. Study on combustion characteristics of dimethyl ether under the moderate or intense low-oxygen dilution condition[J].,2016,108:549-565.
[6] Chen W,Hsu C,Wang X. Thermodynamic approach and comparison of two-step and single step DME (dimethyl ether)syntheses with carbon dioxide utilization[J].,2016,109:326-340.
[7] Wang Y,Liu H,Ke X,et al. Kinetic modeling study of homogeneous ignition of dimethyl ether/hydrogen and dimethyl ether/methane [J].,2017,119:373-386.
[8] Burke U,Somers K P,O Toole P,et al. An ignition delay and kinetic modeling study of methane,dimethyl ether,and their mixtures at high pressures [J].,2015,162(2):315-330.
[9] 亢银虎. 二甲醚火焰燃烧特性及工程应用的研究[D]. 重庆:重庆大学,2015.
Kang Yinhu. Study on Combustion Characteristics of Dimethyl Ether Flame and Engineering Application[D]. Chongqing:Chongqing University,2015(in Chinese).
[10] 亢银虎,卢啸风,严 谨,等. 基于多尺度模拟的二甲醚MILD火焰NO排放机理[J]. 燃烧科学与技术,2018,24(4):345-353.
Kang Yinhu,Lu Xiaofeng,Yan Jin,et al. Multi-dimensional study on NOemission mechanisms of DME-MILD flame[J].,2018,24(4):345-353(in Chinese).
[11] Jin T,Wu Y,Wang X,et al. Ignition dynamics of DME/methane-air reactive mixing layer under reactivity controlled compression ignition conditions:Effects of cool flames[J].,2019,249:343-354.
[12] Ezoji H,Shafaghat R,Jahanian O. Numerical simulation of dimethyl ether/natural gas blend fuel HCCI combustion to investigate the effects of operational parameters on combustion and emissions[J].,2019,135(3):1775-1785.
[13] 周 梅,楚育纯,王兆林,等. 氨-丙烷混合燃料降碳燃烧的排放特性[J]. 燃烧科学与技术,2020,26(3):257-264.
Zhou Mei,Chu Yuchun,Wang Zhaolin,et al. Emis-sion characteristics of NH3-C3H8-air mixture for carbon reduction combustion[J].,2020,26(3):257-264(in Chinese).
[14] Nakamura H,Hasegawa S. Combustion and ignition characteristics of ammonia/air mixtures in a micro flow reactor with a controlled temperature profile[J].,2017,36(3):4217-4226.
[15] 边志坚,王金华,赵浩然,等. 氨/氢气湍流预混火焰传播特性实验研究[J]. 燃烧科学与技术,2020,26(6):551-557.
Bian Zhijian,Wang Jinhua,Zhao Haoran,et al. Experimental study on turbulent premixed flame propagation characteristics of ammonia/hydrogen mixtures[J].,2020,26(6):551-557(in Chinese).
[16] Zhang J,Zhong A,Huang Z,et al. Second-law ther-modynamic analysis in premixed flames of ammonia and hydrogen binary fuels[J].,2019,141(7):071007.
[17] Dai L,Gersen S,Glarborg P,et al. Autoignition studies of NH3/CH4mixtures at high pressure[J].,2020,218:19-26.
[18] 张 君. NH3/CH4燃烧室燃烧特性及污染物排放研究[D]. 大连:大连海事大学,2019.
Zhang Jun. Study on Combustion Characteristics and Emissions of NH3/CH4[D]. Dalian:Dalian Maritime University,2019(in Chinese).
[19] Zhang J,Huang Z,Han D. Effects of mechanism re-duction on the exergy losses analysis in n-heptane autoignition processes [J].,2020,21(9):1764-1777.
[20] Caton J A. Exergy destruction during the combustion process as functions of operating and design parameters for a spark-ignition engine[J].,2012,36(3):368-384.
[21] Chen S,Li J,Han H,et al. Effects of hydrogen addition on entropy generation in ultra-lean counter-flow methane-air premixed combustion [J].,2010,35(8):3891-3902.
[22] Chen S,Mi J,Liu H,et al. First and second thermodynamic-law analyses of hydrogen-air counter-flow diffusion combustion in various combustion modes[J].,2012,37(6):5234-5245.
[23] Nishida K,Takagi T,Kinoshita S. Analysis of entropy generation and exergy loss during combustion[J].,2002,29(1):869-874.
[24] Acampora L,Marra F S. Second law thermodynamic analysis of syngas premixed flames[J].,2020,45(21):12185-12202.
[25] Liu D,Wang H,Zhang Y. On the entropy generation and exergy loss of laminar premixed flame under engine-relevant conditions[J].,2021,283:119245.
[26] Goodwin D G,Speth R L,Moffat H K,et al. Cantera:An object-oriented software toolkit for chemi-cal kinetics,Thermodynamics,and Transport Proc-esses(Version 2. 5. 1)[EB/OL]. https://doi.org/10.5281/ zenodo.4527812,Zenodo,2021.
[27] Kee R J,Grcar J F,Smooke M D,et al. PREMIX: a Fortran Program for Modeling Steady Laminar One-Dimensional Premixed Flames[R]. Sandia National Laboratories Report,1985:SAND85-8249.
[28] Kee R J,Coltrin M E,Glarborg P.:[M]. Hoboken,New Jersey:John Wiley and Sons,Inc.,2005.
[29] Williams F A.[M]. Boca Raton:CRC Press,1985.
[30] Bejan Adrian.[M]. Hoboken,New Jersey:John Wiley & Sons,Inc.,2016.
[31] Yan F,Su W. Numerical study on exergy losses of n-heptane constant-volume combustion by detailed chemi-cal kinetics[J].,2014,28 (10):6635-6643.
[32] Mohebbi M,Reyhanian M,Ghofrani I,et al. Availability analysis on combustion of n-heptane and isooctane blends in a reactivity controlled compression ignition engine[J].,2018,232(11):1501-1515.
[33] Zhao Z,Chaos M,Kazakov A,et al. Thermal decomposition reaction and a comprehensive kinetic model of dimethyl ether[J].,2008,40(1):1-18.
[34] Burke U,Somers K P,O Toole P,et al. An ignition delay and kinetic modeling study of methane,dimethyl ether,and their mixtures at high pressures[J].,2015,162(2):315-330.
[35] Bhattacharya A,Basu S. An investigation into the heat release and emissions from counterflow diffusion flames of methane/dimethyl ether/hydrogen blends in air[J].,2019,44(39):22328-22346.
[36] Hashemi H,Christensen J M,Glarborg P. High-pressure pyrolysis and oxidation of DME and DME/CH4[J].,2019,205:80-92.
[37] Okafor E C,Naito Y,Colson S,et al. Experimental and numerical study of the laminar burning velocity of CH4-NH3-air premixed flames[J].,2018,187:185-198.
[38] Tian Z,Li Y,Zhang L,et al. An experimental and kinetic modeling study of premixed NH3/CH4/O2/Ar flames at low pressure[J].,2009,156(7):1413-1426.
[39] Mechanical and Aerospace Engineering(Combustion Research),The UCSD. Chemical-kinetic mechanisms for combustion applications[EB/OL]. http://web.eng. ucsd.edu/mae/groups/combustion/mechanism.html,Uni-versity of California at San Diego,2014.
[40] Lowry W B,Serinyel Z,Krejci M C,et al. Effect of methane-dimethyl ether fuel blends on flame stability,laminar flame speed,and Markstein length[J].,2011,33(1):929-937.
[41] Han X,Wang Z,Costa M,et al. Experimental and kinetic modeling study of laminar burning velocities of NH3/air,NH3/H2/air,NH3/CO/air and NH3/CH4/air premixed flames[J].,2019,206:214-226.
[42] Xiao H,Lai S,Valera-Medina A,et al. Experimental and modeling study on ignition delay of ammonia/meth-ane fuels[J].,2020,44(8):6939-6449.
[43] Lu M,Fu Z,Yuan X,et al. Study of the reduced kinetic mechanism of methane/dimethyl ether combus-tion[J].,2021,303(1):121308.
[44] Dai L,Gersen S,Glarborg P,et al. Autoignition studies of NH3/CH4mixtures at high pressure[J].,2020,218:19-26.
[45] Li R,Konnov A A,He G,et al. Chemical mechanism development and reduction for combustion of NH3/H2/CH4mixtures[J].,2019,257:116059.
[46] 陈达南,李 军,黄宏宇,等. 氨燃烧及反应机理研究进展[J]. 化学通报,2020,83(6):508-515.
Chen Da’nan,Li Jun,Huang Hongyu,et al. Progress in ammonia combustion and reaction mechanism[J].,2020,83(6):508-515(in Chinese).
Analysis of Exergy Losses in Laminar Premixed Flames of Dimethyl Ether, Ammonia and Methane Blends
Sun Guojun,Fu Zhongguang,Lu Maoqi
(School of Energy,Power and Mechanical Engineering,North China Electric Power University,Beijing 102206,China)
To elucidate the energy-mass conversion characteristics during the combustion of alternative fuels and to explore the principle of improving the efficiency of combustion process,the exergy loss characteristics of CH4/air laminar premixed flames blended with dimethylether(DME) and ammonia(NH3) were analyzed separately by the second law of thermodynamics. The results show that the performance of NH3is better than that of DME over a wide range of blending ratio;the conduction loss drives the total exergy loss to decrease with increasing temperature;the increase of operating pressure contributes to the reduction of the chemical and exhaust gas exergy loss rates;the total exergy loss rate decreases first and then increases as the equivalent ratio increases,reaching a minimal value near the stoichiometric ratio. In addition,a response surface function for the total exergy loss rate was constructed with a wide range of operating parameters,which is expected to be useful for the design optimization and operation of CH4/DME and CH4/NH3fuel combustion systems.
exergy loss;laminar premixed flame;dimethyl ether;ammonia;chemical kinetics
O64
A
1006-8740(2022)03-0271-12
2021-09-12.
北京市自然科学基金资助项目(3162030).
孙国军(1997— ),男,硕士研究生,sgj@ncepu.edu.cn.
付忠广,男,博士,教授,fzg@ncepu.edu.cn.
10.11715/rskxjs.R202109008
(责任编辑:隋韶颖)
