Factors Influencing Home Ranges of the Qinghai Toad-headed Lizard(Phrynocephalus vlangalii) on the Dangjin Mountain,Gansu
2022-06-25XiaolongZHAOWeiYUZeyuZHUYuxiaYANGandZhigaoZENG
Xiaolong ZHAO,Wei YU,Zeyu ZHU,Yuxia YANG and Zhigao ZENG
1 The Key Laboratory of Zoological Systematics and Application,School of Life Science,Institute of Life Science and Green Development,Hebei University,Baoding 071002,Hebei,China
2 Key Laboratory of Animal Ecology and Conservation,Institute of Zoology,Chinese Academy of Sciences,Beijing 100101,China
3 College of Wildlife and Protected Area,Northeast Forestry University,Harbin 150040,Heilongjiang,China
Abstract Home range is an important ecological parameter reflecting the suitability of animal habitats.To study the size and factors influencing the home ranges of the Qinghai toad-headed lizard(Phrynocephalus vlangalii) in different habitats,from July to September 2020,we radio-tagged and tracked 15 individuals in each of sites distributed at high (3600 m)and low (2600 m) altitudes on the Dangjin Mountain,Gansu Province,northwest China.We calculated home range size using the 100% minimum convex polygon method,and analyzed the influence of inherent lizard characteristics and external environmental factors.Our results revealed that for both high-and lowaltitude lizard populations,the sizes of home ranges were positively correlated with lizard body mass.Moreover,after eliminating the effect of body mass as a covariable,we established that the home ranges of high-altitude lizards (5255.1±1103.8 m2) were larger than those of the low-altitude lizards (2208.1±348.7 m2).Lizards in the high-altitude population were also characterized by longer daily suitable activity times and spent significantly more time in full sunlight than those in the low-altitude population.Furthermore,the food resources for lizards in low-altitude habitats were more abundant than those in high-altitude habitats.In conclusion,we established that P.vlangalii lizards inhabiting high-altitude sites had larger home ranges than conspecific lizards distributed at a lower altitude,which was associated not only with endogenous factors,such as body mass,but also with habitat-related environmental factors,such as the quality of thermal resources and availability of food.
Keywords home range,influence factor,plateau lizard,radio tracking
1.Introduction
For survival,animals need to locate habitats with suitable temperatures and resources necessary for foraging and reproduction,as well as enabling predator avoidance(Greenwoodet al.,1983).The area that an individual animal needs to maintain its daily activities and reproduce for a certain period of time is referred to as the home range (Rose,1982),which is an indicator closely related to the energy demand,behavior,and habitat conditions of an animal (Perry and Garland,2002).As an important ecological parameter,home range can reflect habitat suitability,and studies on home range can provide valuable insights with regard to the activity space and factors influencing animals,in addition to providing important information relating to available resources (Fair and Henke,1999).
Most studies on the home ranges of non-migratory animals have focused either on the influence of the intrinsic biological traits of individuals or the effects of environmental variables on the home range size of populations.By comparing and analyzing differences in the home ranges of individuals or groups,numerous previous studies have examined the relationships between intraspecific variations in home range size and the inherent traits of individuals such as age,sex,body mass,reproductive status,and diet (Garland,2002;Harestad and Bunnell,1979;Perry and Roth,2005;Webb and Shine,1997).Others have focused on the seasonal changes in home ranges,by assessing the effects of environmental factors such as climate,food resource availability,and habitat quality (Ariano-Sánchezet al.,2020;Corrialeet al.,2013;Hellicksonet al.,2008;Van Beestet al.,2011).However,relatively few studies have sought to evaluate the influence of geographical variation in home ranges with an aim to determine the integrated effects of both intrinsic traits and environmental factors (Morelletet al.,2013;Ruby and Dunham,1987;Stellatelliet al.,2016).Consequently,our current understanding of factors contributing to the inter-population variation in home range is still relatively limited.
Lizards are often used as model animals for ecological studies on reptiles,as they are predominantly diurnal,conspicuous,and occur in high abundance (Hueyet al.,1983;Vitt and Pianka,1995).Moreover,their respective home ranges are largely unaffected by social behavior,in which they rarely participate (Somma,1990),and there are often significant inter-and intraspecies differences with respect to spatial use patterns (Martins,2014).Such differences accordingly present opportunities to examine the multifaceted effects of changes in home range size,and lizards can thus be considered ideal animals for investigating the changes in home ranges and identifying factors that contribute to different patterns of home range usage.Although numerous studies have examined the different features of lizard home ranges (Fair and Henke,1999;Hibbittset al.,2021;Perry and Garland,2002;Verwaijen and Damme,2008;Vesyet al.,2021),few have focused on the home ranges of lizards in China.
The species that have been studied include the Qinghai toad-headed lizard (Phrynocephalus vlangalii),a species endemic to the Qinghai-Tibetan Plateau region of China.Using the mark-recapture method in 2001-2002,Wanget al.(2004)investigated the home range ofP.vlangaliiin Zoige,Sichuan Province,and established that the home range sizes of this lizard were positively correlated with body mass,and that males had notably larger home ranges than did females.However,the factors affecting the size of lizard home ranges are complex and multifaceted (Stellatelliet al.,2016).Similar to Wanget al.(2004),numerous researchers have demonstrated that the home ranges of lizards are closely associated with intrinsic factors,such as gender and body size,suggesting that home range is positively correlated with body size (Escuderoet al.,2020),and that male lizards tend to have larger home ranges than do females (Perry and Garland,2002).Differences in environmental factors,such as climate,temperature,microenvironmental thermal quality,food resources at different spatial (Patterson and Blouin-Demers,2020;Stellatelliet al.,2016;Wasiolkaet al.,2010) and temporal (Ariano-Sánchezet al.,2020) scales,and thermoregulatory behaviors (Grant,1990) and thermoregulation sites (Hagen and Bull,2011),may also have a pronounced influence on home range size.
In this study,we sought to assess how these multifaceted factors affect the home range of ectothermic Qinghai toadheaded lizards.Specifically,we adopted a radio-tracking approach to determine the home range size of these lizards in different habitats at high (3600 m) and low (2600 m)altitudes on the Dangjin Mountain,Gansu Province,northwest China,and examined the effects of ecological factors on the home range,mainly with respect to body mass,sex,microenvironmental thermal quality,food resource availability,and thermal regulatory behavior.
2.Materials and methods
2.1.Study site and speciesThe study was conducted at sites located at two contrasting elevations on the Dangjin Mountain in Aksai Kazak Autonomous County,Jiuquan City,Gansu Province,China.The high-altitude site (39°18′47′N,94°15′35′′E)lies in an area of alpine steppe at an altitude of 3600 m near the peak of the mountain,whereas the low-altitude site (39°24′50′′N,94°14′10′E) is a comparatively warm steppe area located at an altitude of 2600 m at the mountain’s northern foot.The Qinghai toad-headed lizard inhabiting these sites is a viviparous species endemic to China,which is widely distributed across the Qinghai-Tibetan Plateau at elevations ranging from 2600 to 4500 m (Zhaoet al.,1999).
2.2.Capture and tracking of lizards and observations of thermoregulatory behaviorThe collection and handling of lizards conducted in this study were approved by the Animal Ethics Committee of the Institute of Zoology,Chinese Academy of Sciences (IOZ14001).In July 2020,we captured 15 lizard individuals (8 males and 7 non-gravid females) at each of the high-and low-altitude sites,for which we measured body mass(BM) using electronic scales (KFS-A6;Kaifeng) to 0.01 g and snout-vent length (SVL) using a digital vernier caliper (150 mm;Qifeng) to 0.01 mm.Sex was determined based on the presence of enlarged post-anal scales in adult males.Having obtained the necessary measurements,radio transmitters (151-153 MHz:PicoPip Ag379;Lotek Wireless Inc) weighing 0.4 g (<6% of body mass) were attached to the backs of the captured lizards.The radio-tagged lizards were subsequently released into a 200 m × 200 m plot within the respective native habitats(lizards originally inhabiting these plots that were not used for radio transmitter tracking were removed) and tracked for 2 weeks.
To determine the locations of the lizards,we used radiotracking technology comprising a portable receiver (LA12-Q)with a three-element antenna (AVM Instrument Company,Ltd),and for each individual,we recorded information for one or two locations on each sunny day using a highaccuracy GPS receiver (MG858S;UniStrong).Having located the lizards,we continued to observe their thermoregulatory behavior from a distance greater than 2 m without disturbing their activities,and recorded the time they spent in five microenvironments (full sun,filtered sun,full shade,cave entrance,and cave interior).Each lizard was observed continuously for 20-min intervals,once every hour during the daytime (09:00-18:00),and the percentage of time the lizards spent in different microhabitats during each observation period was calculated.At the end of each observation,we measured the body temperature of lizards by capturing the individuals and inserting the probe of a UT325 electronic thermal meter(Shenzhen Meter Instruments) into the cloaca,after which they were released in situ.
2.3.Operative temperatures of lizards in different microenvironmentsOperative temperature (Te) refers to the temperature of a stationary object similar in size,shape,and radiation characteristics to animals under the same environmental conditions,which is considered more biologically significant than air or substrate temperature (Seebacher and Shine,2004).From July 25 to September 31,2020,we used 30 iButton data loggers (DS1921;MAXIM Integrated Products Ltd.)embedded in a copper model (sealed pipes with a diameter of 15 mm and length of 70 mm) to record operative temperatures(Tes) in three microenvironments (full sun,filtered sun,and full shade) at the high-and low-altitude sites (Liet al.,2017).Following the protocol described by Hertzet al.(1993),the temperature data recorded using the iButton loggers were calibrated against a dead lizard under a heat lamp (temperature ranging from 18 to 48 ℃,n=49).We accordingly established that there was a significant linear relationship between lizard body temperatures (Tbs) and logger-recorded temperatures(slope=1.18,intercept=-0.86,R2=0.99,P< 0.001),and we used the averageTes values calibrated byTbs values to quantify the operative thermal microenvironment at each study site.
2.4.Optimal active period of lizards within habitatsWe averaged the lowest and highestTbs values of all lizards in each of the populations,and considered the range between the lowest and highest body temperatures as the suitable activity temperature range for the lizard populations.For both highand low-altitude habitats,we obtained fluctuating curves during the daytime (09:00-18:00) using the averageTes recorded at hourly intervals,and then estimated the optimal active period of lizards in each habitat by matching the suitable activity temperature range with the averageTes curve during the day.
2.5.Available food resources of lizardsFrom July to September 2020,we installed eight sets of Malaise traps and yellow trays in each of the high-and low-altitude sites.Captured insects were collected at weekly intervals,preserved in 75% alcohol,and taken to the laboratory for identification,based on the appearance of their external morphological features.Based on the findings of a previous study on the feeding habits of Qinghai toad-headed lizards (Baoet al.,1998),we only considered and counted small insects in the orders Diptera,Coleoptera,Hymenoptera,and Lepidoptera.
2.6.Statistical analysesAll data were analyzed using IBM SPSS Statistics 20 software,with the level of significance being set at α=0.05.All values are presented as the means ±standard error (SE).Dependent variables were assessed for normality and homogeneity of variance using the Shapiro-Wilk and Levene tests,respectively.ArcMap 10.3 was used to calculate the home range sizes of lizards according to the 100%minimum convex polygon method (MCP) (Biebouw,2009).The relationship between home range size and body size (BM and SVL) of lizards inhabiting the high-and low-altitude sites were analyzed by simple linear regression,and a generalized linear model was used to analyze the differences in home range between the high-and low-altitude lizards [home range sizes were Ln(x) transformed,and we eliminated the influence of body size by removing BM as a covariable].The Mann-WhitneyUtest was used to analyze the sex-related differences in home range size,the altitudinal differences in lizard utilization of microenvironments,and the number of insects as available lizard food resources.
3.Results
3.1.Home range size of lizardsAs determined using the MCP approach,the average home range size of the 15 high-altitude lizards was 5255.1±1103.8 m2(range 363-13461 m2),whereas that of the 15 low-altitude lizards was 2208.1±348.7 m2(range 380-4144 m2).As depicted in Figure 1,clear overlaps were observed among the home ranges of different individuals at both sites.Lizards inhabiting the high-altitude site (BM:8.33±0.32 g,SVL:56.45±0.71 mm) were found to be significantly larger (BM:F1,29=65.63,P <0.001,SVL:F1,29=23.13,P <0.001) than those in the low-altitude site (BM:5.42±0.16 g,SVL:51.78±0.66 mm).After eliminating the effects of BM as a covariable,the home ranges of lizards in the high-altitude habitat were found to be larger than those in the low-altitude habitat (F1,29=16.570,P <0.001)(Figure 1).

Figure 1 Home ranges of Phrynocephalus vlangalii in high-altitude (A) and low-altitude (B) habitats on the Dangjin Mountain,Gansu Province,China.The dashed and solid lines denote male and female home ranges,respectively.
We found that the size of lizard home ranges was significantly linearly correlated with lizard BM (high-altitude:r2=0 .583,F1,14=18.201,P=0.001;low-altitude:r2=0 .412,F1,14=9.112,P=0.010) (Figure 2),but not with SVL (high-altitude:r2=0.139,F1,14=2.097,P=0.171;low-altitude:r2=0.106,F1,14=1.539,P=0.237).However,no significant differences were observed in the home range sizes of male and female lizards at either site(high-altitude:U=27,P=0.955;low-altitude:U=29,P=1.000).
3.2.Active period and thermal resource utilization of lizardsThroughout the course of monitoring,the minimum and maximum mean body temperatures of the 15 radio-tagged high-altitude lizards during the active period (09:00-18:00)were 28.70±0.54 ℃ and 37.04±0.29 ℃,respectively.On the basis of a comparison of the daily changes in the averageTes,we established that the optimal period of activity spanned the 6 h between 11:30 and 17:30 (Figure 3A).Comparatively,the minimum and maximum body temperatures of the 15 radiotagged low-altitude lizards during the daily active period were 30.37±0.43 ℃ and 37.25±0.45 ℃,respectively,and these lizards were optimally active for approximately 3.5 h split between two periods (10:40-12:40 and 16:30-18:00) (Figure 3B).Consequently,Qinghai toad-headed lizards inhabiting the high-altitude site appear to have a longer active period in a suitable thermal environment than those at the low-altitude site.
With respect to thermal resource utilization in different microenvironments,we found that the active time that highaltitude lizards spent in full sunlight was significantly greater than that of low-altitude lizards (U=14910,P< 0.001).In contrast,lizards inhabiting the low-altitude site tended to spend more time in filtered sun and the entrances to cave than highaltitude lizards (filtered sun:U=10206.5,P< 0.001;entrance of cave:U=10727.5,P=0.016) (Figure 4).
3.3.Available food resources of lizardsIn terms of food source availability,we found that in both high-and lowaltitude habitats,dipteran species were the predominant insect prey,with the abundance of these insects being significantly greater in low-altitude than in high-altitude habitats (U=5.00,P=0.003).Similarly,the abundances of other small insects were observed to be significantly greater in low-altitude than in high-altitude sites (Hymenoptera:U=7.50,P=0.007;Lepidoptera:U=4.50,P=0.002).however,coleopteran beetles tended to be rarer in low-altitude than in high-altitude habitats(U=80.50,P< 0.001) (Figure 5).These findings thus indicate that lizards inhabiting low-altitude sites on the Dangjin mountain have a potentially richer supply of food resources than those found in high-altitude habitats.

Figure 2 Relationship between home range size and the body mass of Phrynocephalus vlangalii at high and low altitudes on the Dangjin Mountain,Gansu Province,China.L-A-P:lowaltitude population;H-A-P:high-altitude population.
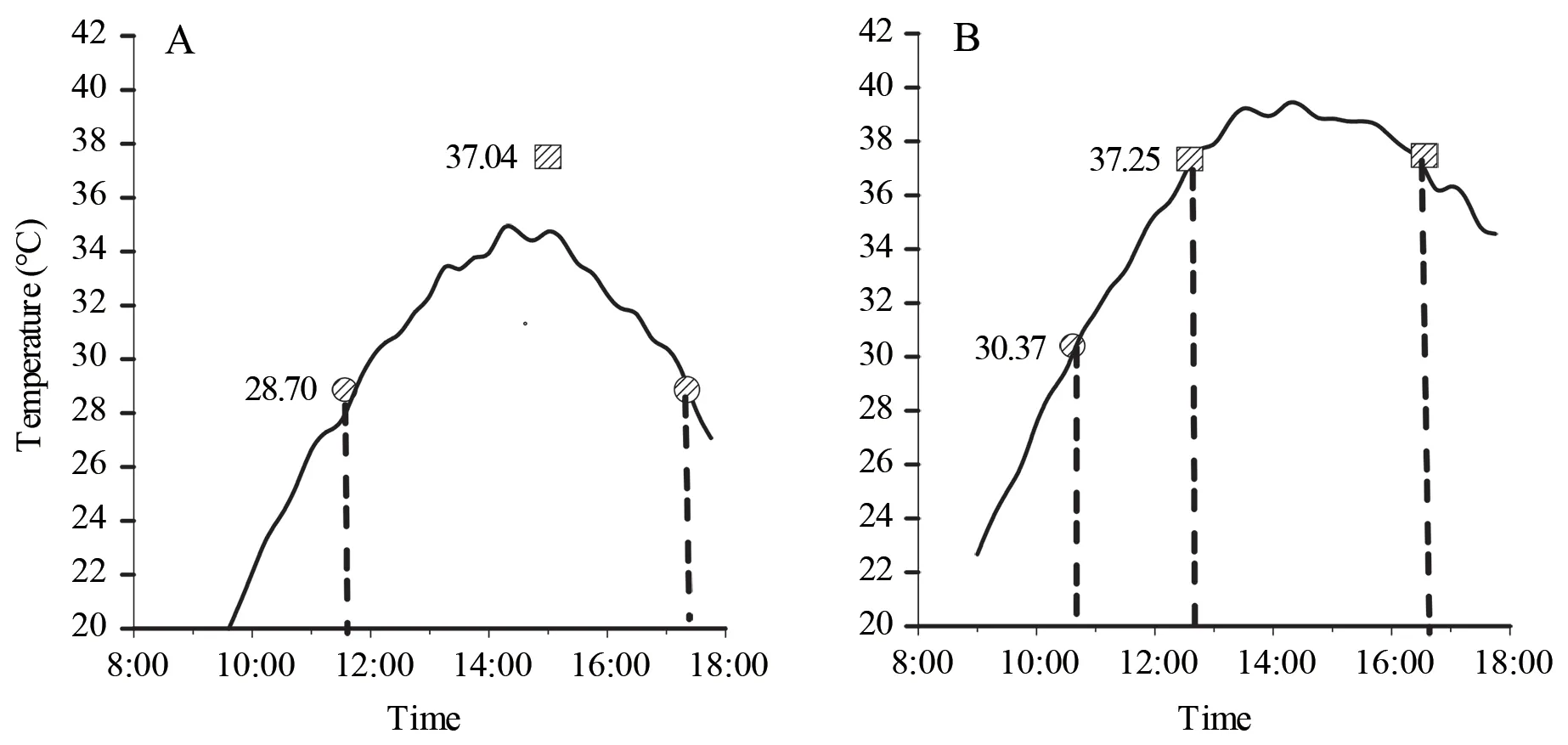
Figure 3 Diurnal variation in the average operative temperatures of Phrynocephalus vlangalii inhabiting high-altitude (A) and low-altitude(B) sites on the Dangjin Mountain,Gansu Province,China.Circles and squares represent the lowest and highest body temperature of lizards,respectively.
4.Discussion
In this study,we established that Qinghai toad-headed lizards inhabiting high-and low-altitude sites on the Dangjin Mountain have relatively large home ranges,with average sizes of 5255.1 and 2208.1 m2,respectively,as measured over a 2-week period using the MCP technique.These are considerably larger than the average annual home ranges of the adult males(63.71 m2) and females (8.31 m2) of this species estimated by Wanget al.(2004) in Zoige,Sichuan.There are several plausible explanations that might account for these disparate findings.Firstly,the home ranges of lizards in Zoige were determined using the mark-recapture technique,and the number of recaptures were fewer (up to eight times) than those in the present study,which might be reflected in the estimates of home range size.Secondly,there may be regional differences in population density and food availability between the two study areas,and these factors would be expected to have notable influences on the home range size of lizards (Ruby and Dunham,1987).Thirdly,some of the radio-tagged lizards were captured from areas beyond the demarcated sample plots,indicating that they might need to move farther distances to locate suitable caves and microhabitats,thereby expanding the home range size.In addition,unlike the observations reported by Wanget al.(2004),we were unable to detect any notable differences in the home range of male and female Qinghai toad-headed lizards.This disparity could be attributable to the fact that we tracked non-gravid females in the present study,and thus their mobility would not have been impaired to the same extent that might be expected in pregnant females.
A conspicuous feature of our estimates of home range size was the notable difference among individuals,with sizes ranging from 363 to 13461 m2.Such marked individual variation is a typical characteristic of lizards of the same species occupying the same habitat,as exemplified by the home ranges of female and maleAnolis carolinensis,which can vary in size from 16.06 to 1537.67 m2and from 18.15 to 846.21 m2,respectively(Weberet al.,2021).Similarly,significant individual differences in the monthly home range size (291-14690 m2) have been reported for the Texas horned lizard (Phrynosoma cornutum),as determined using radio-telemetry (Fair and Henke,1999).As indicated previously,such marked differences could be ascribed to the dispersal movements of lizards.In addition,the endurance capacity of lizard locomotion may have a permissive effect on the potential extent of home ranges (Singleton and Garland,2018).A further important factor influencing the size of lizard home ranges is body size,and in the present study,we detected a positive correlation between the home range size of the plateau lizards and their body mass (Figure 2).In this regard,the body size of lizards is closely associated with food or energy requirements,and thus it might be predicted that larger individuals would need a more extensive foraging range in order to obtain sufficient more nutritional resources to sustain growth (Christian and Waldschmidt,1984;Tamburelloet al.,2015).
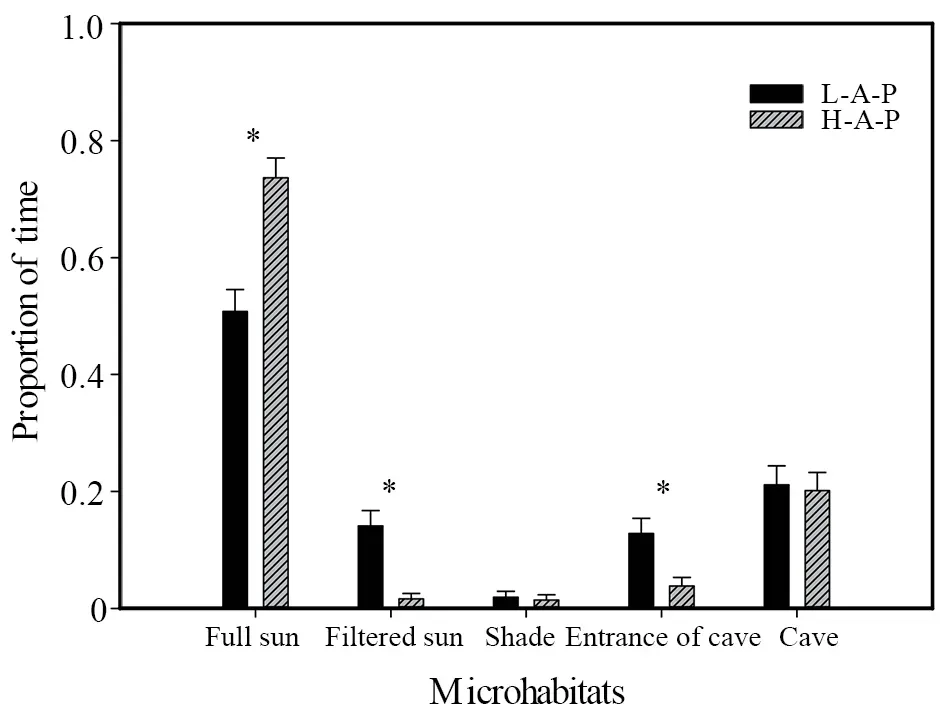
Figure 4 Differences in the time spent by Phrynocephalus vlangalii utilizing different microenvironments at different altitudes on the Dangjin Mountain,Gansu Province,China.L-A-P:low-altitude population;H-A-P:high-altitude population.Asterisks indicate significant altitudinal differences(Mann-Whitney U test,α=0.05).
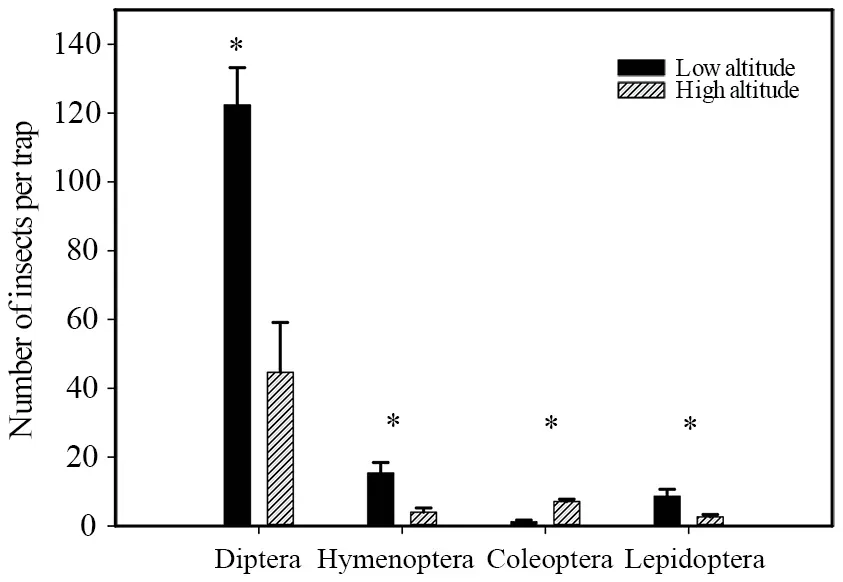
Figure 5 Differences in potential food resources of Phrynocephalus vlangalii at different altitudes on the Dangjin Mountain,Gansu Province,China.Asterisks indicate significant altitudinal differences (Mann-Whitney U test,α=0.05).
As mentioned previously,we found that Qinghai toadheaded lizards inhabiting the high-altitude site on the Dangjin Mountain were characterized by significantly larger home range sizes than those in the low-altitude site (Figure 1).Highaltitude individuals with a larger body size than low-altitude individuals are more likely to maintain active predation during the daytime and expand their foraging ranges in order to obtain sufficient food resources (Verwaijen and Damme,2008),which may thus account for the significantly larger home ranges of the high-altitude lizards.Large body size is considered a beneficial trait for high-altitude lizards,as the correspondingly large fat reserves would presumably enhance overwintering survival (Civantos and Forsman,2000).Interestingly,however,we found that the home ranges of high-altitude lizards were still larger than those of low-altitude lizards after eliminating the effect of body mass as a covariable.Consequently,we believe that certain habitat-related environmental factors also play prominent roles in determining home range sizes.
Population density and food resource availability are often used to explain the observed variations in home range size (Ruby and Dunham,1987),and in this regard,we found that the low-altitude site examined in the present study would provide a greater abundance of food resources forP.vlangaliithan would the high-altitude habitat (Figure 5).In our assessments of home range size,at both high and low elevations,we released the same number of lizards into the demarcated study plots,the original lizards in which had either been fitted with radio transmitters or removed.Consequently,although these released lizards may have dispersed within and beyond the boundaries of the study plots,the effects of population density on home range establishment can be considered to have been largely eliminated.Thus,we can assume that the greater abundance of food resources at the low-altitude site enabled the lizards therein to find sufficient food within a smaller range than those inhabiting high-altitude site,and accordingly explain why the high-altitude lizards had larger home ranges than the low-altitude lizards after eliminating the influence of differences in body size (Figure 1).Stellatelliet al.(2016) arrived at a similar conclusion based on their study of the small sand lizard (Liolaemus multimaculatus)in Argentina,in which they found that the home range sizes of non-forest populations in habitats characterized by a low abundance of food resources were significantly larger than those of forest species with abundant food resources.In this regard,our findings tend to be consistent with tenets of the optimal foraging theory,which state that organisms can adjust their home range in accordance with the availability of local food resources.Accordingly,it can be predicted that in response to a greater availability of food,lizards can reduce home range size in order to lessen the risk of predation (Verwaijen and Damme,2008).
In addition to food resources,we also established that habitat thermal resource quality is among the important factors influencing the home range size of ectotherms such as lizards.The physiological and behavioral activities of ectotherms are largely dependent on the ambient temperature (Buckley,2008),with unsuitable thermal habitats potentially limiting the scope of such activities (Sound and Veith,2000).In this context,it is generally assumed that warmer environments are more beneficial for activity.Conversely,however,our observations would tend to indicate that excessively warm thermal environments can be detrimental for lizard activity.Our comparison of averageTes values obtained for lizards revealed that although the low-altitude habitats were warmer than those at a higher altitude,at certain times throughout the day,notably in the early afternoon,the low-altitude habitats tended overheat,with averageTes values reaching temperatures up to 40 ℃ (Figure 3),which are considered unsuitable for ectotherm lizards.In addition,by monitoring the thermoregulatory behavior of lizards,we discovered that during the course of daily activities,lizards at low altitudes spent a greater amount of time in cooler shaded sites than did lizards at high altitudes,thereby minimizing the risk of overheating.These observations are consistent with Kearney’s view (2009) that excessive temperatures force lizards to spend more time in the shade,otherwise their body temperature will reach the heat tolerance range and can prove fatal.Our findings similarly revealed that the activities of low-altitude lizards were notably restricted during a prolonged afternoon period extending from 12:45 to 16:30 (Figure 3),during which it is necessary to periodically retreat to suitably shaded spots to reduce body temperatures.In contrast,lizards at high altitudes are less constrained by unsuitably high habitat temperatures,as indicated by our observations that these lizards are characterized by longer active periods in a suitable thermal environment than are those inhabiting low-altitude sites (Figure 4).Consequently,the more conducive habitat thermal resources at higher altitudes will enable the lizards inhabiting these sites to exploit a larger area as a home range.
In conclusion,we established that the home ranges ofP.vlangaliiinhabiting a high-altitude site were significantly larger than those of conspecific lizards found in a site at lower altitude,which was not only associated the inherent characteristics (body mass) of individuals but also the levels of habitat resources (thermal and food resources).Moreover,our observations revealed that there are significant differences in the home ranges ofP.vlangaliiat the individual level in the same habitats and at the population level in different habitats,which can be attributed to the combined effects of different factors.The findings of this study will contribute to enhancing our comprehension of the effects of different factors in determining the home ranges of ectotherms.
AcknowledgementsWe thank Weiguo DU for his advice regarding experimental design and manuscript preparation.We would also like to thank Kun WANG,Haoyu LIU,and Yibin BA for their assistance in the field or laboratory.This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA20050201),and the National Natural Science Fund of China (31861143023).
杂志排行
Asian Herpetological Research的其它文章
- A New Species of the Asian Leaf Litter Toad Genus Leptobrachella (Amphibia,Anura,Megophryidae) from Chongqing City,Southwest China
- A New Cyrtodactylus Species (Reptila:Gekkonidae) from Nan Province,NorthernThailand
- A New Species of Nidirana (Anura,Ranidae) from Southern Guangxi,China
- Spatial Patterns and Drivers of Chinese Lizard Richness among Multiple Scales
- Behaviours in Attachment-Detachment Cycles of Geckos in Response to Inclines and Locomotion Orientations
