A New Cyrtodactylus Species (Reptila:Gekkonidae) from Nan Province,NorthernThailand
2022-06-25SiriwadeeCHOMDEJWaraneePRADITParinyaPAWANGKHANANTChakkrapongKUENSAENApichayaPHUPANBAIMaliNAIDUANGCHANPrompornPIBOONKorakotNGANVONGPANITZhiyongYUANYinpengZHANGJingCHEPhupingSUCHARITAKUL0andChatmongkonSUWANNAPOOM
Siriwadee CHOMDEJ,Waranee PRADIT,Parinya PAWANGKHANANT,Chakkrapong KUENSAEN,4,Apichaya PHUPANBAI,Mali NAIDUANGCHAN,Promporn PIBOON,Korakot NGANVONGPANIT,7,Zhiyong YUAN,Yinpeng ZHANG,Jing CHE,Phuping SUCHARITAKUL0 and Chatmongkon SUWANNAPOOM*
1 Department of Biology,Faculty of Science,Chiang Mai University,Chiang Mai 50200,Thailand
2 Research Center in Bioresources for Agriculture,Industry and Medicine,Department of Biology,Faculty of Science,Chiang Mai University,Chiang Mai 50200,Thailand
3 Division of Fishery,School of Agriculture and Natural Resources,University of Phayao,Phayao 56000,Thailand
4 International College of Digital Innovation,Chiang Mai University,Chiang Mai 50200,Thailand
5 Rabbit in the Moon Foundation,Ratchaburi 70180,Thailand
6 Animal Bone and Joint Research Laboratory,Department of Veterinary Biosciences and Public Health,Faculty of Veterinary Medicine,Chiang Mai University,Chiang Mai 50100,Thailand
7 Excellence Center in Veterinary Bioscience,Chiang Mai 50100,Thailand
8 Key Laboratory of Freshwater Fish Reproduction and Development,Ministry of Education,Southwest University,Chongqing 400715,China
9 State Key Laboratory of Genetic Resources and Evolution,and Center for Excellence in Animal Evolution and Genetics,Kunming Institute of Zoology,Chinese Academy of Sciences,Kunming 650223,Yunnan,China
10 School of Environment,Tsinghua University,Beijing 100084,China
Abstract Here,a new species of bent-toed gecko,Cyrtodactylus phukhaensis sp.nov.,is described from Doi Phu Kha,Nan province,Thailand based on molecular and morphological evidence.A phylogeny based on NADH dehydrogenase subunit 2 (ND2) and its flanking tRNAs places the new species in the chauquangensis group as a sister taxon to Cyrtodactylus wayakonei.The new species can be differentiated from other members of the chauquangensis group by having a unique combination of 7 or 8 supralabials,23-28 longitudinal rows of dorsal tubercles,8-10 infralabials,9 femoral pores,7 precloacal pores,40-47 ventral scales,and a lack of bands crossing the temporal area.In addition,results from a chromosome study of C.phukhaensis sp.nov.showed that the new species has a diploid chromosome number of 40 with a fundamental number of 46.The formula of the karyotype was as follows:2n (40)=2m+4sm+34t.Our findings suggest that further studies of Cyrtodactylus biodiversity in northern Thailand are needed.
Keywords bent-toed gecko,Cyrtodactylus wayakonei,Cyrtodactylus chauquangensis,karyotype,phylogeny,taxonomy
1.Introduction
Cyrtodactylus,commonly known as bent-toed geckos,is the most diverse genus of geckonids with more than 320 described species (Uetzet al.,2021),many of which have been discovered at a high rate in the past decade.Cyrtodactylusinhabit the karstic region,a complex ecosystem known as a hotspot for vertebrate endemism and persistence (Grismeret al.,2021a).Therefore,many species of bent-toed geckos are endemic,although they are widely distributed in India,Sri Lanka,the southern Himalaya region,Southeast Asia,Papua New Guinea,and some areas in northern Australia (Grismeret al.,2021a).Thus,changes to these unique and restricted habitats,such as those caused by wildfires,deforestation,and forest exploitation,can pose a high risk of extinction for the geckos.
Presently,Cyrtodactylusis the most diverse genus in Gekkota with a wide distribution,but each species typically has a restricted or unique habitat (Grismeret al.,2020c).Studies of their biodiversity,however,are lacking because of their syntopic distribution with other geckonids.This distribution leads to cryptic or complex species,which make it difficult to determine whether unidentified specimens are new taxa or morphological variations.Several studies onCyrtodactylushave confirmed the existence of cryptic species,which have since been reclassified as many new taxa using both morphological characterization and molecular tools (Grismeret al.,2012;Grismeret al.,2018;Nazarovet al.,2012;Sumonthaet al.,2012).Therefore,the identification ofCyrtodactylusis still required,especially in some unexplored areas,to advance our understanding of the taxonomy,distribution,and biodiversity ofCyrtodactylusspp.and facilitate their long-term conservation.
According to the distribution ofCyrtodactylus,Thailand is one area that harbors manyCyrtodactylusspp.;these are mostly found in western and southern areas,which contain more than half the species in Thailand (Uetzet al.,2021).However,a fewCyrtodactylusspp.have been found in the northern part of Thailand includingCyrtodactylus doisuthep,Cyrtodactylus dumnuii,Cyrtodactylus inthanon,Cyrtodactylus er ythrops,Cyrtodactylus maelanoi,andCyrtodactylus khelangensis,which were found in only three provinces (Chiang Mai,Mae Hong Son,and Lampang) (Baueret al.,2009;Baueret al.,2010;Grismeret al.,2020a;Kunyaet al.,2014;Kunyaet al.,2015;Pauwelset al.,2014b).This finding is inconsistent with those for many lineages ofCyrtodactylusin the surrounding countries,e.g.,Myanmar and Laos,which have continuous massif to those in northern Thailand with many limestone ecosystems that are suitable habitats.From several years of surveys in northern Thailand,an unknownCyrtodactylusspp.was identified that differed from any previously describedCyrtodactylusspp.Therefore,this species ofCyrtodactyluscollected from Nan province in northern Thailand was identified here as a new species based on its morphology,phylogeny,and karyotype.
2.Materials and Methods
2.1.Sample collectionSamples were collected at Doi Phu Kha,Nan province,northern Thailand (Figure 1).Photographs of the specimens were taken to document their color pattern in life prior to euthanasia after which liver tissues were collected and stored in 95% ethanol.The specimens were then preserved in 10% buffered formalin and stored in 70%ethanol.Type specimens were subsequently deposited at Agriculture University of Phayao,Phayao,Thailand (AUP).The geographical coordinates and elevations of the sampling locality were recorded using a Garmin eTrex 10 device (WGS84 datum).

Figure 1 Distributions of the Cyrtodactylus chauquangensis group.Red star indicates the type locality of Cyrtodactylus phukhaensis sp.nov.in Nan Province,Thailand.Red dots represent the distributions of other Cyrtodactylus spp.including (1) C.zhenkangensis,(2) C.erythrops,(3) C.dumnuii,(4) C.doisuthep,(5) C.auribalteatus,(6) C.kunyai,(7) C.wayakonei,(8) C.vilaphongi,(9) C.spelaeus,(10) C.martini,(11)C.hekouensis,(12) C.sonlaensis,(13) C.otai,(14) C.puhuensis,(15) C.bobrovi,(16) C.huongsonensis,(17) C.cucphuongensis,(18) C.soni,and (19)C.chauquangensis.
2.2.Molecular studyTotal genomic DNA were extracted from liver tissues using a DNA extraction kit (RBC Bioscience,Taiwan,China).The NADH dehydrogenase subunit 2 (ND2)gene with partial flanking tRNA regions in mitochondrial DNA was amplified using PCR and the forward and reverse primers,L4437b (5’-AAGCAGTTGGGCCCATACC-3’) (Maceyet al.,1997) and H5937b (5’-AGGGTTCCGATATCTTTRTG-3’)(Maceyet al.,1999),respectively,in a total volume of 25 µL using 5x FIREPol® Master Mix (Solis BioDyne,Estonia).PCR reactions were completed under the following conditions:an initial denaturation at 95°C for 5 min and 35 cycles of denaturation at 95°C for 30 s,annealing at 50°C for 30 s,and extension at 72°C for 1 min followed by a final extension at 72°C for 5 min.The PCR products were loaded onto 2% agarose gel,stained with GelRed® (Biotium,USA),and visualized under UV illuminator.The successfully amplified PCR products were then purified using a NucleoSpin® Gel and PCR Cleanup kit (Machery-Nagel,Germany) before being sequenced in both directions (A T G C,Bangkok,Thailand).The obtained sequences were checked manually and assembled using BioEdit version 7.0.5.3.The new sequences were deposited in the GenBank database under the accession numbers MN871845,MN871846,and MZ439914-MZ439917 (Table 1).
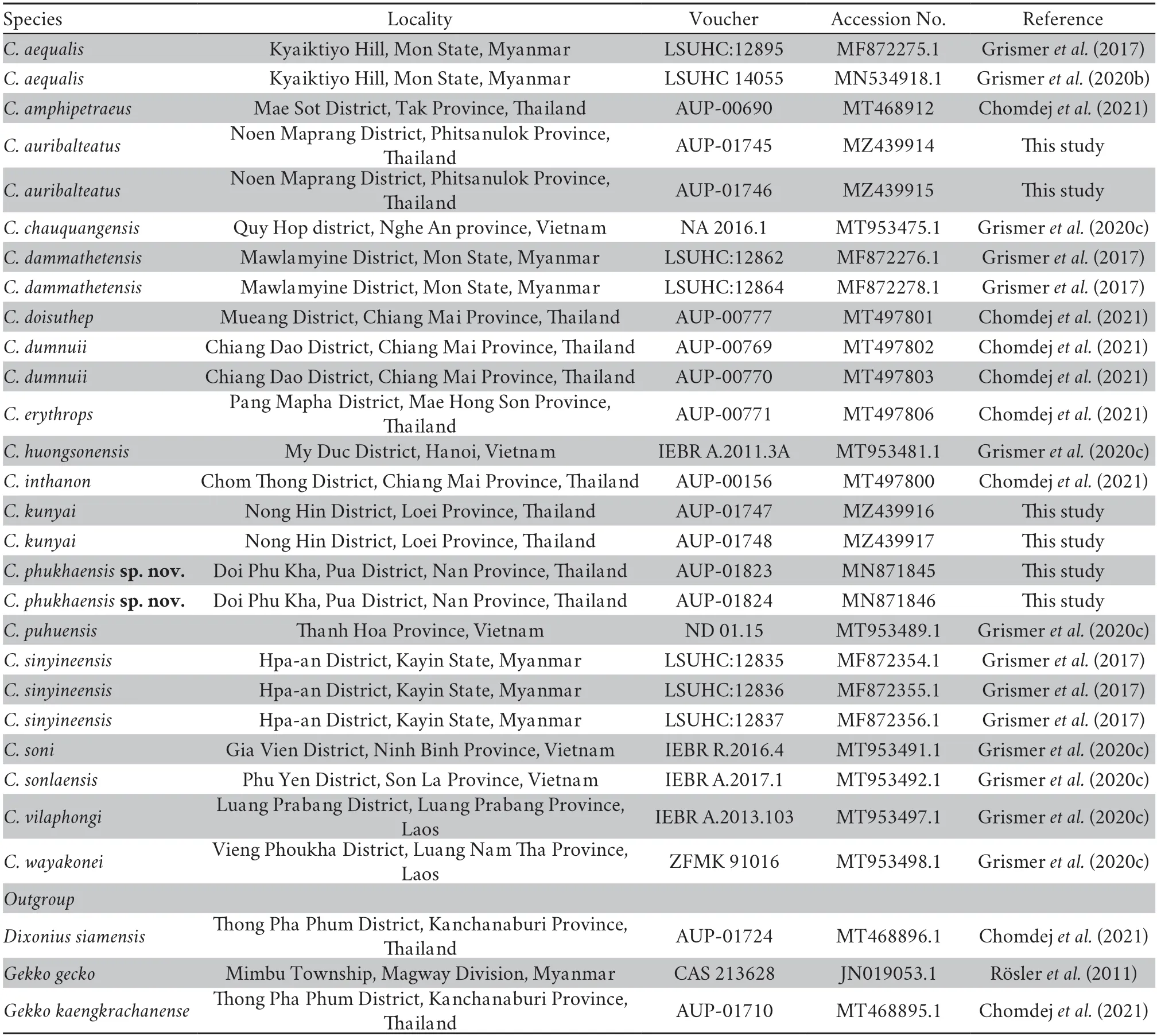
Table 1 List of specimens used in the phylogenetic analysis.
2.3.Phylogenetic analysisPhylogenetic relationships were analyzed using both maximum likelihood (ML) and Bayesian inference (BI) methods.In our analysis,we used the new sequences obtained in this study,additional sequences (taken from GenBank) of currently knownCyrtodactylusdistributed close to the sampling site,and outgroups (Gekko gecko,Gekko kaengkrachanense,andDixonius siamensis;Table 1).The sequences were aligned using ClustalW,and then manually checked and trimmed in MEGA X version 10.2.2 (Kumaret al.,2018).The best fit substitution models for ML and BI were estimated using jModelTest 2.1.7 (Darribaet al.,2012;Posada,2008),which supported GTR+I+G for theND2gene and flanking tRNA regions as suggested by the Akaike information criterion.MrBayes version 3.2.7 (Ronquistet al.,2012) was used for BI analysis with a Markov chain Monte Carlo method involving 10 000 000 generations sampled every 1 000 generations.The first 2 500 trees were discarded as burn in and the BI tree was visualized in ITOL version 6.1.1 (http://itol.embl.de).ML analysis was performed using MEGA X version 10.2.2 (Kumaret al.,2018) with 1 000 bootstrap replicates.The confidence of the tree topology was assessed using nodes that had Bayesian posterior probabilities (BPP) >0.95,while bootstrap support (BS) values>95 were considered as strong support.Nodes with BPP and BS values of 0.90-0.94 were considered well supported.Mean uncorrected pairwise sequence divergence among and between species was calculated using MEGA X version 10.0.5 (Kumaret al.,2018).
2.4.Morphological studyMeasurements were taken with digital calipers to the nearest 0.1 mm.Bilateral scale counts were given as left/right.We followed the methodology of measurements and meristic counts described by Grismeret al.(2017).The following terms were abbreviated as shown in parentheses:snout-vent length (SVL),tail length (TL),tail width (TW),forearm length (FL),tibia length (TBL),axilla-togroin length (AG),head length (HL),head width (HW),head depth (HD),eye diameter (ED),eye to snout distance (ES),eyeto-nostril distance (EN),interorbital distance (IO),ear diameter(EL),internarial distance (IN),supralabials (SL),infralabials(IL),ventral scales (VS),subdigital lamellae on the fourth toe(ET4),femoral pores (FP),precloacal pores (PP),postprecloacal scales rows (PPS),body bands (BB),light bands on the tail (LB),and dark bands on the tail (DB).Morphological comparisons and analyses were based on specimen examination and data obtained from the existing literature (Baueret al.,2009;Baueret al.,2010;Kunyaet al.,2014;Leet al.,2016;Liu and Rao,2021;Luuet al.,2011;Nguyenet al.,2014;Nguyenet al.,2017;Nguyenet al.,2010;Pauwelset al.,2014a;Quanget al.,2007;Schneideret al.,2014;Sumonthaet al.,2010;Tri,2011;Zhanget al.,2021).
2.5.Chromosome preparation and stainingMetaphase chromosomes were prepared using the squash technique adapted from Praditet al.(2018).An intraperitoneal injection of colchicine solution (1 mg/mL) was administered at 15 µg/g body weight for 4 h.Subsequently,mitotic and meiotic cells were collected from the bone marrow of the fore (radius,ulna,and humerus bones) and hind limbs (fibula,tibia,and femur bones) as well as the testes.These cells were incubated in 0.075 M KCl solution for 30 min and then fixed in 3:1 methanol/acetic acid solution for three times.The cell spread was prepared by dropping 1 or 2 drops of cell suspension onto a precooled slide and allowing the suspension to air dry.The chromosome spreads were stained using 10% Giemsa solution for 15 min,and the well-spread chromosomes in metaphase were observed and photographed under a light microscope (Olympus Corporation,Tokyo,Japan).Karyotypes and ideograms were analyzed and constructed using KaryoType software (Altınorduet al.,2016).
3.Results
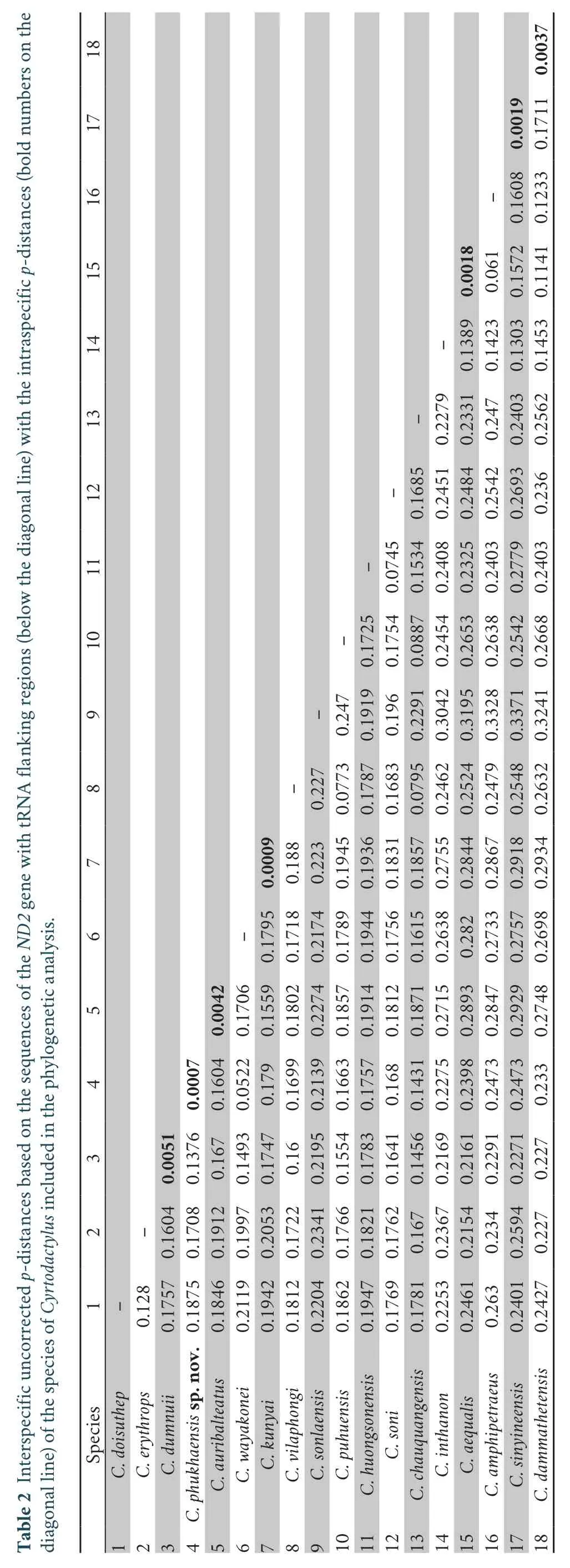
3.1.Molecular relationshipThe topology using theND2fragment and flanking tRNA regions derived from Bayesian analysis was highly similar to that from ML analysis.The genealogical relationships ofCyrtodactylusin this study were revealed to contain two clades (Figure 2);one consisted ofCyrtodactylusspp.from northern and western Thailand,including nearby regions in Mon and Karen,Myanmar,which included members of theCyrtodactylus sinyineensisgroup corresponding to a previous report by Grismeret al.(2021b);the other containedCyrtodactylusfrom northern and northeastern Thailand,including northern Laos and Vietnam.The newly discovered species,Cyrtodactylusphukhaensissp.nov.from Nan,northern Thailand,was nested in this clade with a clearly divergent lineage.The newly identified species represented a close sister taxon to a known species,C.wayakonei,with strong levels of nodal support (1.00/100 for BPP/BS values).This clade comprisedCyrtodactylusmembers of theC.chauquangensisgroup,as reported by Grismeret al.(2021b).
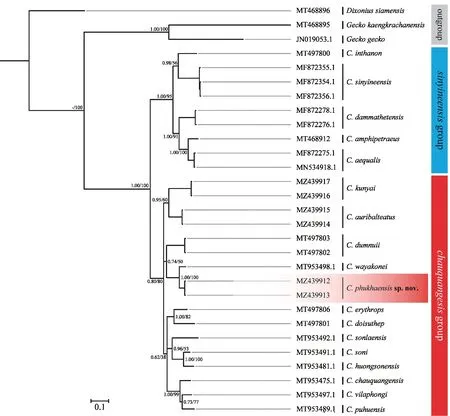
Figure 2 Bayesian inference phylogenetic tree of the mitochondrial ND2 gene with flanking tRNAs from the species of Cyrtodactylus in the chauquangensis and sinyineensis groups.Support values at the nodes represented the Bayesian posterior probability and maximum likelihood bootstrap support values from the same dataset.
3.2.Genetic divergenceThe uncorrectedp-distances in theND2gene with flanking tRNA regions among and within theCyrtodactylusspp.examined in this study are shown in Table 2.The observed interspecific distance withinCyrtodactylusvaried from 5.22% betweenC.phukhaensissp.nov.andC.wayakoneito 33.71% betweenCyrtodactylus sonlaensisandC.sinyineensis.The genetic difference amongC.phukhaensissp.nov.and otherCyrtodactylusspecies was 5.22%-24.73%.The species most closely related toC.phukhaensissp.nov.wereC.wayakonei(5.22%) andC.dumnuii(13.76%).Intraspecific genetic variations in theND2gene with flanking tRNA regions was observed to be very low (0.00% inC.phukhaensissp.nov.,Cyrtodactylus auribalteatus,Cyrtodactylus kunyai,Cyrtodactylus aequalis,C.sinyineensis,andCyrtodactylus dammathetensisto 0.01% inC.dumnuii).The newly identified species,C.phukhaensissp.nov.,was clearly distinct from other known species ofCyrtodactylus.
3.3.Morphological analyses
Taxonomic accounts
C yrtodact ylus phukhaensissp.nov.Chomdej,Pradit,Pawangkhanant,Naiduangchan,and Suwannapoom
Phukha bent-toed gecko (Figures 3-7)
HolotypeAUP-01823 (Figures 3 and 4),adult male from Doi Phu Kha,Pua District,Nan Province,Thailand,(19°10.40’N;101°06.44’E;1 700 m a.s.l.),collected on October 25,2018 by Parinya PAWANGKHANANT and Mali NAIDUANGCHAN.
ParatypesAUP-01824 and AUP-01825 (Figure 5),two adult females;collected between October 25 and 26,2018 by Parinya PAWANGKHANANT and Mali NAIDUANGCHAN from Doi Phu Kha,Pua District,Nan Province,Thailand (19°10.40’N;101°06.44’E;1 700 m a.s.l.).

Figure 3 Dorsal view of live Cyrtodactylus phukhaensis sp.nov.from Doi Phu Kha,Pua and Bo Kluea District,Nan Province,northern Thailand.

Figure 4 Holotype of adult male Cyrtodactylus phukhaensis sp.nov.(AUP-01823).(A) Lateral view of head;(B) dorsal view of top of head;(C) precloacal region;(D) precloacal-femoral series showing scalation and pore arrangements;(E) gular region showing mental,postmental,chin scale arrangement,and pair of chinshields under dentary;(F) plantar view of feet showing subdigital lamellae under left hindfeet toes.

Figure 5 Dorsal view of paratypes of Cyrtodactylus phukhaensis sp.nov.in preservative.

Figure 6 Habitat of Cyrtodactylus phukhaensis sp.nov.at the Doi Phu Kha,Nan Province,Thailand,i.e.,the type locality.

Figure 7 The specimen observed on a large boulder near the stream in the habitat.
EtymologyThe specific epithet,phukhaensis,is derived from the place name of Doi Phu Kha where the type series was collected.
DiagnosisCyrtodactylus phukhaensissp.nov.differs from all other species in theC.chauguangensisgroup by having a combination of 8 or 9 supralabials;8-10 infralabials;36-44 paravertebral tubercles;23-28 longitudinal rows of dorsal tubercles;40-47 ventral scales;7 expanded subdigital lamellae on the fourth toe,11 or 12 unmodified subdigital lamellae on the fourth toe,and 18 or 19 total subdigital lamellae on the fourth toe;27-34 enlarged femoral scales;10-12 pore-bearing femoral scales in males;8-11 enlarged precloacal scales;7 pore-bearing precloacal scales in males;3 rows of enlarged postprecloacal scales;4-7 broken to hour glass-shaped dorsal body bands;4-8 light-colored caudal bands (n=2);3-8 darkcolored caudal bands (n=2);raised and strongly keeled dorsal tubercles that extend beyond the base of the tail;enlarged femoral and precloacal scales that are nearly the same size and continuous;pore-bearing femoral and precloacal scales that are not continuous;medial subcaudals 2-3 times wider than long and extending onto the lateral side of the tail;green irises;a nuchal loop lacking an anterior azygous notch and bearing a jagged posterior border;dorsal bands bearing paravertebral elements that are generally equal in width to the interspaces,bear lightened centers,and are edged with white tubercles;dark markings in the dorsal interspaces;light caudal bands in adults bearing dark-colored markings;light-colored caudal bands that do not encircle the tail,and a mature regenerated tail that is not spotted (Table 3).
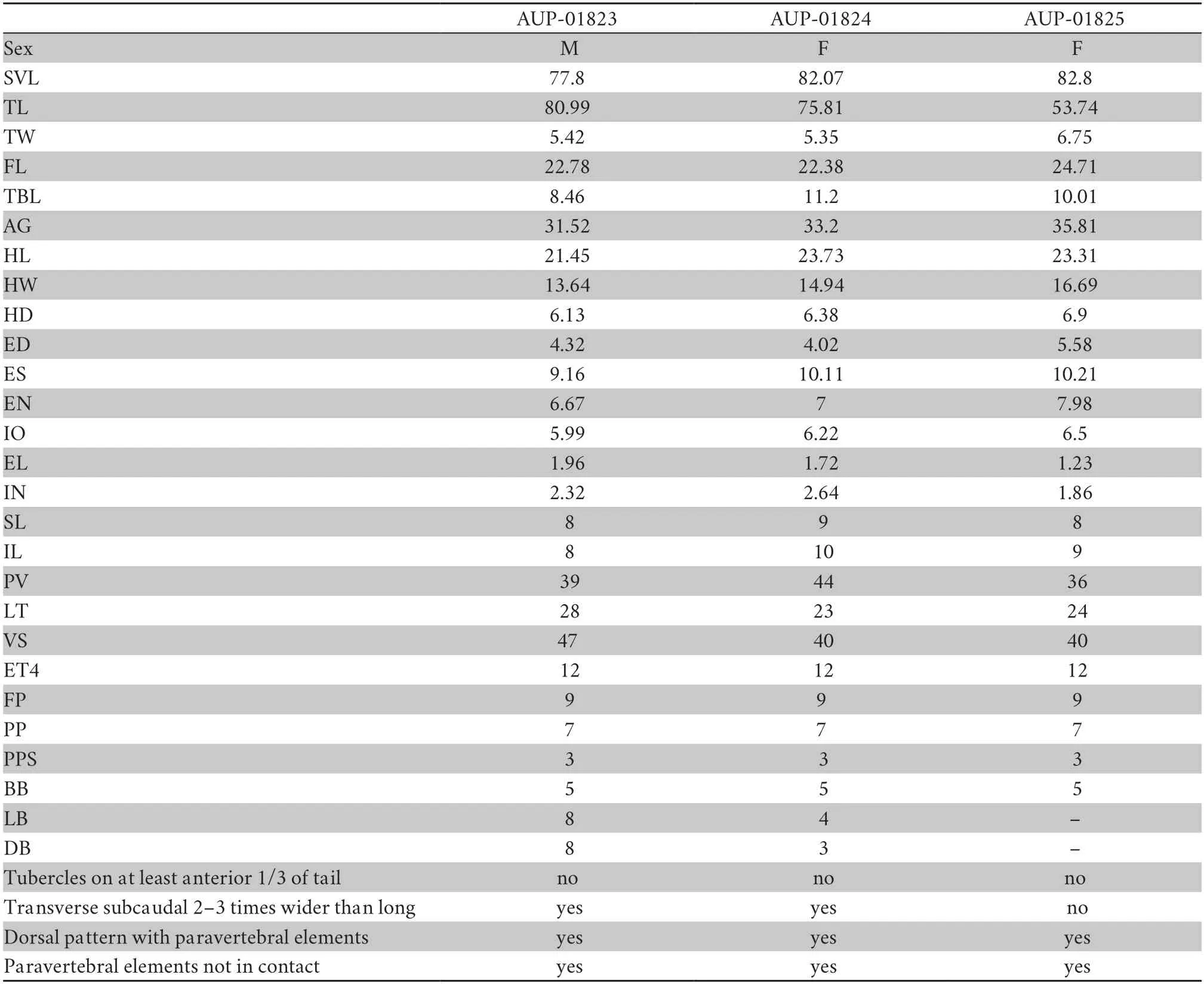
Table 3 Measurements (mm),meristic data,and color pattern for the type series of Cyrtodactylus phukhaensis sp.nov.
Description of holotypeAdult male,SVL=77.8 mm (Figure 4);head moderate in length (HL/SVL=0.28),wide (HW/HL=0.66) and flat (HD/HL=0.28),distinct from neck,triangular in dorsal profile;lores inflated,prefrontal region concave,canthus rostralis rounded;snout,elongated (ES/HL=0.39),rounded in dorsal profile,broad in lateral profile;eye,large (ED/HL=0.25);ear opening,oval (EL/HL=0.10);eye-to-ear distance,greater than eye diameter;rostral,rectangular,partially divided dorsally,bordered posteriorly by supranasals and one internasal,laterally by first supralabials;external nares,bordered anteriorly by rostral,dorsally by supranasals,posteriorly by smaller postnasals,and ventrally by first supralabials;9 (R,L)rectangular supralabials,extending to below eye midpoint;7(R,L) infralabials,tapering posteriorly to commissure of jaw;scales of rostrum and lores,slightly raised,larger than granular scales on top of head and occiput;scales on top of head and occiput,intermixed with small tubercles;dorsal superciliaries,weakly pointed and directed posteriorly;mental triangular,bordered laterally by first infralabials and posteriorly by large left and right trapezoidal postmentals,which contact medially for 50% of their length posterior to mental;one row of enlarged chinshields,outermost row bordering first five infralabials;gular and throat scales,granular,grading posteriorly intolarger,subimbricate pectoral and ventral scales.Body relatively short (AG/SVL=0.42) with well-defined ventrolateral folds;dorsal scales,small,raised,and interspersed with large,raised,semi-regularly arranged,strongly keeled tubercles;tubercles,extending from top of head to base of tail just beyond the postcloacal swelling;tubercles on nape,smaller than those on the body;35 paravertebral tubercles;approximately 18 longitudinal rows of dorsal tubercles;28 flat,subimbricate,ventral scales larger than dorsal scales;11 enlarged precloacal scales;8 porebearing precloacal scales,separated on the midline by 1 poreless scale;3 rows of large,postprecloacal scales;and no deep precloacal groove or depression.Forelimbs moderate in stature,relatively short (FL/SVL=0.17);forearm scales,slightly raised,juxtaposed,larger than those on body,intermixed with large tubercles;palmar scales,slightly raised,juxtaposed;digits,welldeveloped,relatively long,inflected at basal interphalangeal joints;digits narrow distal to inflections;widened proximal subdigital lamellae extending onto palm;slight webbing at base of digit;claws,well-developed,sheathed by a dorsal and ventral scale at base;hind limbs more robust than forelimbs,moderate in length (TBL/SVL=0.15),covered dorsally by small,raised,juxtaposed scales intermixed with large pointed tubercles and bearing flat,slightly larger imbricate scales anteriorly;ventral femoral scales flat,imbricate,much larger than dorsals;one row of 13 or 14 (R,L) enlarged femoral scales and enlarged precloacal scales,continuous;enlarged femoral scales,nearly equal in size;small postfemoral scales form an abrupt union with larger,flat,ventral scales on posteroventral margin of thigh;6 (R,L) pore-bearing femoral scales,not continuous with pore-bearing precloacal scales;subtibial scales,flat,imbricate;plantar scales,raised;digits,relatively long,well-developed,inflected at basal,interphalangeal joints;7 (R,L) transversely expanded subdigital lamellae on fourth toe proximal to joint inflection,not extended to sole;11 (R,L) unmodified subdigital lamellae,distal to inflection;claws well-developed,base of claw sheathed by a dorsal and ventral scale.Tail complete,
original,gracile in proportions,115.1 mm in length,9.9 mm in width at base,tapering to a point (TL/SVL=1.42);dorsal scales of tail flat,forming indistinct whorls;median row of transversely expanded subcaudal scales,three times as wide as long,extending onto lateral subcaudal region;three enlarged postcloacal tubercles at base of tail on hemipenal swellings;postcloacal scales large and flat.
VariationParatypes are similar to holotypes in terms of the morphological characteristics,measurements,and scalations provided in Table 3.Despite a limited number of specimens,few variations were found in the three geckos.Labrials varied in relation to supralabials 8-9 and infralabials 8-10 (Figure 5).
DistributionCyrtodactylus phukhaensissp.nov.is known only from the type locality at Doi Phu Kha,Pua and Bo Kluea District,Nan Province,northern Thailand.
Ecology and natural historyThe type series was collected at night between 19:00 and 23:00 in the Montane forest at elevations of 1 250-1 715 m (Figure 6).Specimens were found on large rocks and large trees about 1.0-2.5 m above the stream and uphill.In addition,one specimen was observed on a large boulder near a stream (Figure 7).
Only one adult male (holotype) was collected at night from the large boulder near the stream,which was covered with lower herbaceous plants.The male was sitting upside down on the boulder 1.5 m above the stream.Another specimen observed at the summit of the national park was observed on a large Sapindaceae (Acersp.) tree.One of the paratypes (AUP-01825)was observed to predate an unknown orthopteran.Other herpetofauna species were observed as follows:Pseudocalotes microlepis(Agamidae),Dopasia gracilis(Anguiidae),Elaphe taeniuracf.mocquardi(Colubridae),Ovophis monticola(Viperidae),andRhacophorus kio(Rhacophoridae).
ComparisonsSpecies ofCyrtodactylusare known to be endemic to their narrow distributions because of geoisolation;thus,the comparisons in this study were mainly between the new species and those from theC.chauquangensisgroup in its neighborhood,typicallyCyrtodactylusspp.from northern and northeastern Thailand,northern Laos,and Vietnam (Figure 1).
Based on phylogeny,the new species ofCyrtodactylusis a sister toC.wayakonei(p-distance=5.22%,Table 2 and Figure 2).Morphologically,C.phukhaensissp.nov.differed fromC.wayakoneiin the following ways:the number of supralabials (C.phukhaensissp.nov.8 or 9 vs.C.wayakonei7 or 8);the number of body tubercle rows (C.phukhaensissp.nov.23-28 vs.C wayakonei17-19);the condition of femoral pores (distinct onC.phukhaensissp.nov.vs.absent onC.wayakonei);and the number of precloacal pores (C.phukhaensissp.nov.7 vs.C.wayakonei6-8).Moreover,C.phukhaensissp.nov.can also be distinguished from other neighboringC.chauquangensisgroup species by having the following:8 or 9 supralabials (vs.C.auribalteatus10 or 11,C.chauquangensis9 or 10,C.doisuthep10-12,andC.huongsonensis10-13);8-10 infralabials (vs.C.chauquangensis9-11 andC.huongsonensis10-12);the absence of a band crossing the temporal area (present onC.auribalteatus,C.chauquangensis,C.doisuthep,C.erythrops,C.huongensis,C.kunyai,C.puhuensis,C.sonlaensis,andC.vilaphongi);23-28 rows of trunk tubercles (vs.C.doisuthep19-20,C.dumnuii18-22,C.huongsonensis14-16,C.soni10-13,C.sonlaensis13-15,andC.vilaphongi15 or 16);5 narrow body bands (n=3) (vs.4 bands onC.auribalteatus,irregular broad bands onC.chauquangensis,6 or 7 bands onC.doisuthep,and 6 or 7 bands onC.dumnuii);40-47 ventral scales (vs.C.auribalteatus38-40,C.chauquangensis36-38,C.doisuthep29-35,C.erythrops28,C.kunyai34,C.puhuensis36,andC.vilaphongi34-36);9 femoral pores (vs.C.auribalteatus4 or 5,C.chauquangensis0,C.doisuthep0,C.dumnuii6,C.erythrops9 or 10 on each side of thigh,C.huongsonensis15-17,C.kunyai10-12,C.puhuensis0,C.soni12-16,andC.sonlaensis28-30);and 7 precloacal pores (vs.C.auribalteatus6,C.dumnuii5 or 6,C.erythrops9,C.huongsonensis6-8,C.kunyai3,C puhuensis5,andC.sonlaensis8).In addition,C.phukhaensissp.nov.is superficially similar toC.martiniin having reticulations on the head but it differs fromC.martiniby being substantially larger in size (SVL:77.8-82.8 mm vs.64.4-96.2 mm),having more precloacal pores (7 vs.4),fewer supralabials (8 or 9 vs.9-11).Furthermore,C.phukhaensissp.nov.is distinguishable fromC.zhenkangensisin having clear reticulations on the head and different body coloration in life(dark chocolate brown vs.light-brown);it also differs fromC.zhenkangensisby being significantly smaller in size (SVL:77.8-82.8 mm vs.87.4 mm),having fewer precloacal pores (7 vs.8),and possessing fewer transverse bands on the dorsum of the body (4 or 5 vs.8 or 9).Cyrtodactylus phukhaensissp.nov.differs fromC.hekouensisin having reticulations on the head,dark body color,fewer precloacal pores (7 vs.33-39),and fewer ventral scale rows (40-47 vs.68-72) as well as by being smaller in size (SVL:77.8-82.8 mm vs.92.3 mm).
3.4.Karyotype analysisThe diploid chromosome numbers ofC.phukhaensissp.nov.were 2n=40,and the fundamental number (NF) was 46.The karyotype ofC.phukhaensissp.nov.was composed of 2 metacentric,4 submetacentric,and 34 telocentric chromosomes (Figure 8).The karyotype formula was as follows:2n(40)=2m+4sm+34t.There was no sex difference between the male and female karyotypes.The ideogram ofC.phukhaensissp.nov.is shown in Figure 9 with the standard values given in Table 4.

Figure 8 Conventional metaphase plates and karyotypes of male (A) and female (B) Cyrtodactylus phukhaensis sp.nov.(scale bar=10 μm).

Figure 9 Ideogram of Cyrtodactylus phukhaensis sp.nov.according to conventional staining.
4.Discussion
C yrtodact ylus phukhaensissp.nov.was placed in theCyrtodactylus chauquangensisgroup because the type locality was in the area of distribution previously mentioned by Grismeret al.(2021b),i.e.,ranging from northern Thailand and Laos to northeastern Vietnam and Yunnan Province in China.However,the low genetic divergence betweenC.phukhaensissp.nov.andC.wayakonei(5.22%) was surprising given that the distance between the type locality of these species is approximately 170 km,which is far greater than the distance betweenC.phukhensissp.nov.andC.vilaphongiorCyrtodactylus spelaeus(both approximately 125 km).This may be attributable to the adjacent mountain range between the Luang Prabang mountain range,on which Doi Phu Kha is located,and the mountains in Luang Nam Tha Province.This should be investigated further with the inclusion of information on the newCyrtodactylusspp.found on this mountain range.Thus,there is a need for field work on this endemic species in this region including the many hidden areas in northern Thailand.
The chromosomal data onC.phukhaensissp.nov.is shown in Table 5 with data from previous reports on the genus from Thailand and Malaysia.The chromosome number forC.phukhaensissp.nov.,2n=40,was in agreement with those from otherCyrtodactylusspecies,which ranged from 32 to 48 (King,1987),showing that chromosomal diversification existed within the genus (Otaet al.,1992).In particular,the chromosomal numbers ofC.phukhaensissp.nov.were similar to those ofC.kunyaiandCyrtodactylus jarujini(2n=40),one of the most frequent numbers found amongCyrtodactylusspp.,i.e.,2n=40 or 2n=42 (Thongnetret al.,2021).The obtained karyological characteristics ofC.phukhaensissp.nov.included many acrocentric chromosomes that gradually decreased in size (telocentric) and a few metacentric or submetacentric chromosomes.These features are consistent with the karyotype evolution of other gekkonids and agree with the hypothesis of rearrangement from ancestral karyotypes via Robertsonian fissions,fusions,and pericentric inversions (Gorman,1973).However,additional studies of cytogenetic approaches are required to clarify the chromosomal characteristics of not onlyC.phukhaensissp.nov.but also other species fromCyrtodactylus.
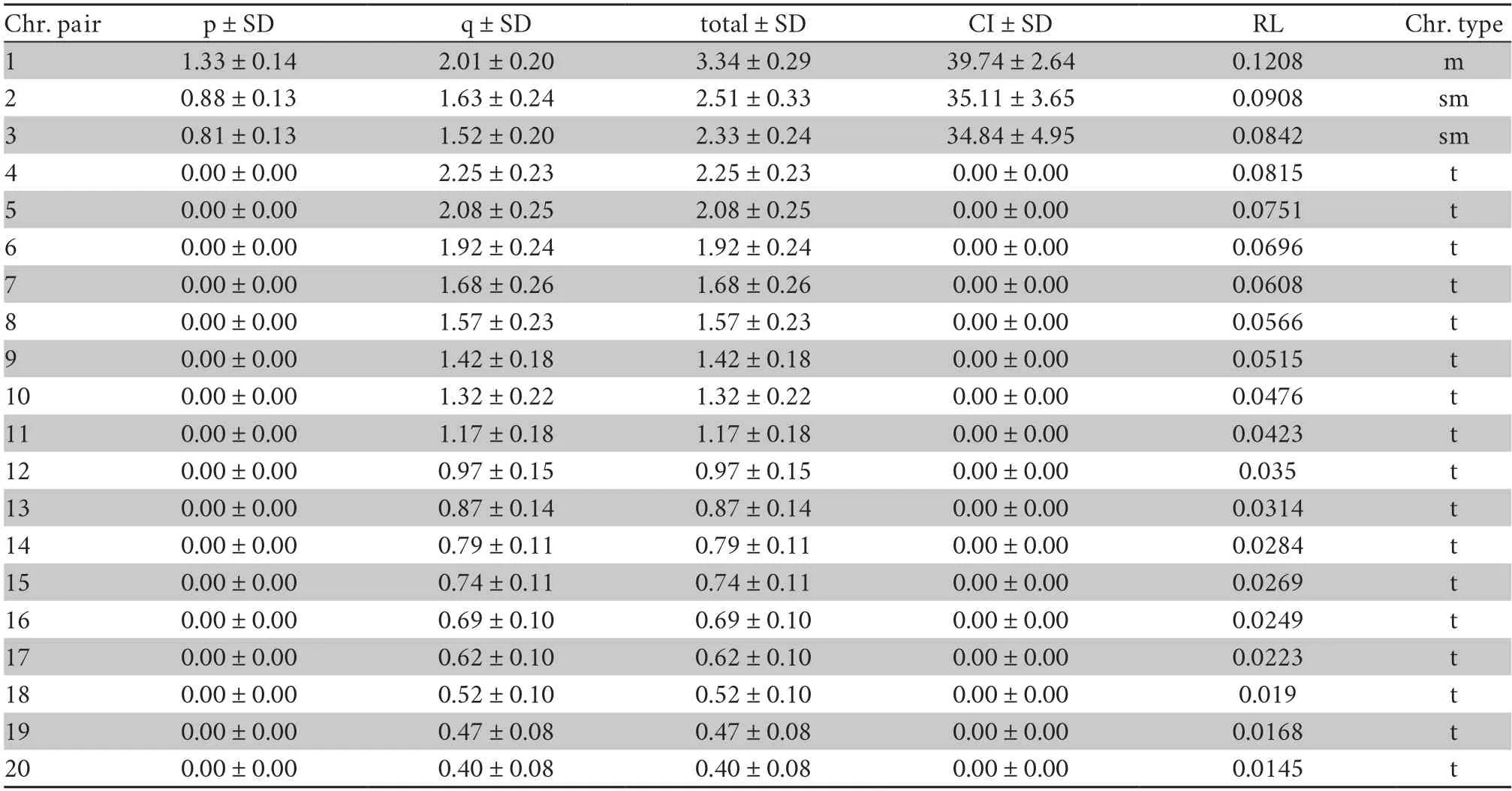
Table 4 Mean length of short (p) and long (q) chromosome arm,total length of chromosome (total),centromeric index (CI) with standard deviation (SD),and relative length (RL) from metaphase of male and female Cyrtodactylus phukhaensis sp.nov.
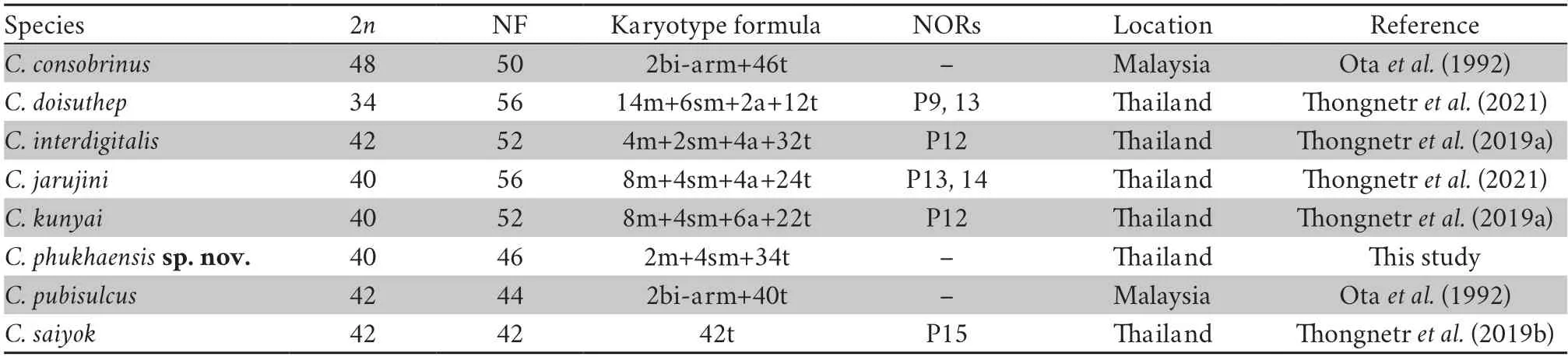
Table 5 Review of karyotype studies for the genus Cyrtodactylus.
AcknowledgementsThis research was financially supported by the National Research Council of Thailand(167951),the Thailand Science Research and Innovation (TSRI)(DBG6180025) and Chiang Mai University to Siriwadee CHOMDEJ.The work was partially supported by the Thailand Science Research and Innovation fund and the University of Phayao (FF65-UoE003) to Chatmongkon SUWANNAPOOM,the International Partnership Program of Chinese Academy of Sciences (CAS) (152453KYSB20170033),and Southeast Asia Biodiversity Research Institute,CAS (Y4ZK111B01:2017CASSEABRIQG002) to Jing CHE,and Excellence Center in Veterinary Bioscience to Korakot NGANVONGPANIT.This study was carried out with permission by the Institutional Ethical Committee of Animal Experimentation of the University of Phayao,Phayao,Thailand (certificate number UPAE59-01-04-0022 issued to Chatmongkon SUWANNAPOOM)and were strictly complacent with the ethical conditions of the Thailand Animal Welfare Act.Field work,including collection of animals in the field and specimen exportation,was authorized by the Institute of Animals for Scientific Purpose Development (IAD),Bangkok,Thailand (permit number U1-04995-2559,issued to Siriwadee CHOMDEJ).
杂志排行
Asian Herpetological Research的其它文章
- Factors Influencing Home Ranges of the Qinghai Toad-headed Lizard(Phrynocephalus vlangalii) on the Dangjin Mountain,Gansu
- Behaviours in Attachment-Detachment Cycles of Geckos in Response to Inclines and Locomotion Orientations
- Spatial Patterns and Drivers of Chinese Lizard Richness among Multiple Scales
- A New Species of Nidirana (Anura,Ranidae) from Southern Guangxi,China
- A New Species of the Asian Leaf Litter Toad Genus Leptobrachella (Amphibia,Anura,Megophryidae) from Chongqing City,Southwest China
