Lactobacillus fermentum as a new inhibitor to control advanced glycation end-product formation during vinegar fermentation
2022-06-23QinLiLeiingLiHnjinZhuFnYngKeXioLinZhngMenglinZhngYongshengPengChoWngDongshengLiQinWuMengzhouZhou
Qin Li, Leiing Li, Hnjin Zhu, Fn Yng, Ke Xio, Lin Zhng, Menglin Zhng,Yongsheng Peng, Cho Wng, Dongsheng Li, Qin Wu,*, Mengzhou Zhou,*
a Key Laboratory of Fermentation Engineering (Ministry of Education), National "111" Center for Cellular Regulation and Molecular Pharmaceutics,Hubei Key Laboratory of Industrial Microbiology, Cooperative Innovation Center of Industry Fermentation (Ministry of Education & Hubei Province),Hubei University of Technology, Wuhan 430068, China
b Wuhan Product Quality Supervision and Inspection Institute, Wuhan 430000, China
Keywords:
Lactobacillus fermentum
Advanced glycation end-product
Vinegar fermentation
Antioxidant capacity
A B S T R A C T
The inhibitory activity of lactic acid bacteria (LAB) toward advanced glycation end-products (AGEs) during vinegar fermentation was studied, and its relationships with the substrate consumption, antioxidant capacity,total phenolic content, total flavonoid compounds, α-glucosidase, and α-amylase activity inhibition were evaluated. The vinegar was made from rice powder flour by liquid-state fermentation (LSF). The selected LAB strains were separately co-cultivated with S accharomyces cerevisiae and Acetobacter pasteurianus 1.41 in alcoholic and acetic acid fermentation, respectively. Among 3 strains, Lactobacillus fermentum showed the strongest inhibitory effect on the formation of total fluorescent AGEs and carboxymethyl lysine ( CML)/carboxyethyl lysine (CEL) in the fermentation process. The corresponding mechanisms included the acceleration of substrate consumption, improvement of antioxidant activities, and inhibition of α-glucosidase and α-amylase. In addition, the fluorescent AGEs and the CML/CEL were negatively correlated with the antioxidant activities, while the α-glucosidase and α-amylase activities were positively correlated with the total phenols and total flavonoids. Moreover, the variety of mainflavor compounds increased, including esters, alcohols, phenols and acids. The results of the study support the potential use of screened LAB strains to inhibit the formation of fluorescent AGEs, CML and CEL on fermented products and in the food processing industry, without associated risks to consumers.
1. Introduction
The Maillard reaction is the most important reaction in the food processing industry [1]. Although the reaction can produce some unique flavors, it also produces many potential chemical hazards.Generally, the most studied potential chemical hazards for Maillard reactions are AGEs, which can be divided into two categories:the fluorescent cross-linking compounds, such as pentosidine and crossline, and the non- fluorescent non-crosslinked compounds, such as carboxymethyl lysine (Nε-(carboxymethyl) lysine, CML) and carboxyethyl lysine (Nε-(carboxyethyl) lysine, CEL) [1-3]. Among these, CML is a major AGEs owing to its relatively high level bothin vivoand in food products [4]. Moreover, CML, the major marker of AGEs in many studies, is the most widely studied type of AGEs [5].To date, studies on the human system have demonstrated that about 10% of alimentary AGEs are absorbed, accumulated and recycled [1].They may accelerate aging and cause diabetic complications such as inflammation, nephropathy, protein denaturation and oxidative stress,posing a threat to public health [4,6]; therefore, there is a growing interest in the removal of AGEs.
Vinegar is consumed worldwide as a food condiment and has a long history of more than 3 000 years [7]. Traditional Chinese vinegar production processes include solid-state fermentation (SSF)and liquid-state fermentation (LSF) [8]. Compared with SSF, LSF is characterized by high yield, short fermentation time and low cost [9].Meanwhile, in the process of LSF and brewing, free amino groups and reducing sugars (starch and protein hydrolysis) react to form AGEs [10]. The most important AGEs in vinegar were CML and CEL, which had been identified [10]. Most of the inhibition methods for AGEs involve the use of exogenous inhibitors, such as catechin,phenolics and alkaloids. However, the high cost and the potential adverse effects of these inhibitors on the fermentation process make it difficult to apply them to industrial production. Some researchers have found that microorganisms can prevent AGEs production, which may be an alternative method to inhibit AGEs production during fermentation [11].
The production of vinegar through LSF involves two successive biochemical processes: alcoholic fermentation and acetic acid fermentation, where yeast and acetic acid bacteria (AAB) play vital roles [12]. Moreover, in a previous study, in the traditional solid-state fermentation vinegar, LAB populations dominated the bacterial community and played an important role in the microbial community,as they affected both the product quality and the microbial stability [13].A symbiotic system can exist among yeast, AAB and LAB [8,14].In addition, as an important strain in vinegar, LAB can improve the quality of finished products; however, the research on the effect of LAB on AGEs formation during vinegar fermentation is limited.
It would be of interest to highlight advances in the field of LSF vinegar fermented with LAB in inhibition of harmful substances.The aim of this study was to evaluate the inhibitory effects of LAB on AGEs generation in vinegar. Accordingly, the objectives were(1) to prepare the LSF vinegar fermented with the selected LAB strains; (2) to identify and quantitate the formations of fluorescent AGEs and non-fluorescent AGEs (e.g., CML/CEL) at different LSF stages (alcohol fermentation and acetic acid fermentation); and(3) to investigate the corresponding mechanisms, including substrate consumption, antioxidant capacity, and inhibition ofα-glucosidase andα-amylase. The systematic study on the effect of LAB addition on vinegar fermentation based on the mechanisms of AGEs inhibition will provide new insights into inhibitors and improve the quality of vinegar.
2. Materials and methods
2.1 Materials and culture medium
Lactobacillus fermentum,L. casei,L. paracasei, andA. pasteurianusAS1.41 were obtained from the Research Center of Food Fermentation Engineering and Technology of Hubei, China.S. cerevisiae(dry yeast) was purchased from Angel Yeast Co.,Ltd. (Yichang, China). Rice flour was obtained from Anhui Qianlixiang Food Factory (Anhui, China).α-Amylase (13 U/mg)was obtained from porcine pancreas, andα-glucosidase (32.4 U/mg)from a yeast strain ofAspergillus nigerwas obtained from Sinopharm Chemical reagent factory (Shanghai, China). Methanol and formic acid (HPLC grade) were acquired from Fisher Scientific(Massachusetts, USA). All other reagents were of analytical grade and obtained from Sinopharm Chemical reagent factory(Shanghai, China).
MRS medium was used for the LAB-based fermentation. YG medium was used for the fermentation ofA. pasteurianus. The agar culture media were composed of 20 g/L agar and MRS medium or YG medium.
2.2 Preparation of vinegar
To study the inhibitory effect of LAB addition on AGEs at different fermentation stages for vinegar production, three LAB strains (L. fermentum,L. caseiandL. paracasei) were separately adopted in the alcohol fermentation and acetic acid fermentation stages (Fig. S4). Rice powder (1 kg) was prepared as raw materials for the vinegar production by adding water four times the rice powder weight to the raw materials. The mixture was heated to a homogeneous paste and then cooled to 80 °C; next, 0.2% high-temperatureα-amylase was added and left for 2 h. Afterward, the mixture was cooled to 50 °C,glucoamylase was added for hydrolysis, and the mixture was left for 5 h.The feed was incubated at 30 °C for 5 days withS. cerevisiae(2 g active dry yeast powder) to obtain rice wine. No further CO2was produced after the alcohol was fermented. The supernatant was collectedviacentrifugation at 5 000 ×gand held at 70 °C for 30 min.
The final ethanol concentration of rice wine obtained through the fermentation was 12.2%, which was then adjusted to 6% (V/V). The rice wine was incubated with 4%A. pasteurianusseed in a rotary shaker incubator (180 r/min) for 7 days at 30 °C. The supernatant was collected (7 000 ×g) and pasteurized (using a water bath at 70 °C for 30 min) at the end of vinegar fermentation. To avoid the influence of the culture medium, the microorganisms were collectedvia centrifugation at 8 000 ×gand held at 4 °C for 10 min.
The three LAB strains with a biomass of 4.0 × 109were co-cultivated withS. cerevisiaefor 5 days in the alcohol fermentation stage, and various indicators were measured. Additionally, the three LAB strains were co-cultivated withA. pasteurianusfor 7 days to determine the various indicators in the acetic acid fermentation stage,and the flavor and nutritional quality of the mature vinegar were determined. The samples were divided into four groups (sample without LAB: control; sample withL. fermentum:LF;sample withL. casei:LC; and sample withL. paracasei:LPA). All treatments were conducted in triplicate.
2.3 Inhibition of AGEs formation
The procedure for determining the AGEs formation inhibition was based on previous methods but with some modifications [15].The samples were obtained from the whole process of alcohol fermentation and acetic acid fermentation stages. Then, they were added into a 96-well microplate to quantitatively assess the formation of fluorescent AGEs using a spectrophotometer (Shimadzu RF-5301,Japan) at excitation and emission wavelengths of 370 and 440 nm,respectively. The LAB inhibition of the AGEs formation was calculated based on the following equation:

2.4 CML and CEL assay
The CML/CEL assay procedure was based on previous methods with some modifications [10]. To restore the emulsion, the vinegar samples (0.75 mL) were added to an equal volume of sodium borohydride (0.2 mol/L, pH 13-14), the mixture was left for 10 h at 4 °C, and the supernatant was collectedviacentrifugation (12 000 r/min,60 min, 4 °C). Then, the supernatant (0.5 mL) was passed through a pre-activated PCX solid-phase extraction column (pre-activation conditions: 3 mL of methanol and 3 mL of distilled water were sequentially added to the filter column core). The target compound was washed with 3 mL methanol and 3 mL ultrapure water and then eluted with methanol (5% ammonia). The methanol was then removed using a rotary evaporator and redissolved in 2 mL formic acid solution (0.1%). After the samples were filtered through organic filters (0.22 μm Whatman filters), they were analyzedviahigh-performance liquid chromatography-mass spectrometry(Agilent Technologies LC/Q-TOF, USA). The specific method of operation was performed according to a published method [1].The fragment atm/z84 was used for the quantification of CML(m/z205) and CEL (m/z219). The CML/CEL concentration was calculated based on a calibration curve created with known concentrations (10.00–45.00 μg/L).
2.5 Determination of antioxidant activities
The capacity to scavenge the 2,2-diphenyl picryl hydrazyl (DPPH,Sigma-Aldrich Co. LLC, St. Louis, MO, USA) free radical and hydroxyl radical (OH) were monitored according to the method by Chen et al. [16]. The DPPH radical scavenging activity was examined.First, 0.2 mmol/L DPPH (dissolved in ethanol) was mixed with the sample 1:1 (V/V), and then the mixture was left to react at room temperature in a dark room for 30 min; the mixture was centrifuged at 8 000 r/min for 10 min, and the absorbance (Ai) was measured at 517 nm. The control group was ethanol and DPPH· (Aj), and the blank group comprised ethanol and sample (Ak).
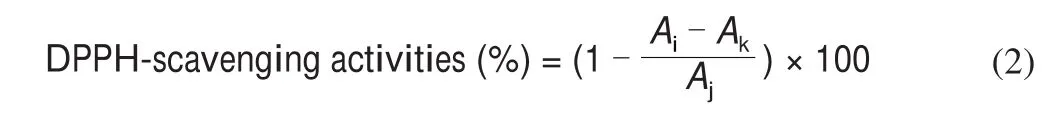
The OH· was generated in a mixture of 2-mL PBS (0.2 mol/L;pH 7.4), 1 mL ferrous sulfate solution (0.75%), 1 mL 1,10-phenanthrolinehydrate (0.75 mmol/L), and 1 mL hydrogen peroxide (0.12%,V/V). After the addition of 1 mL sample (L. plantarumR culture solution), the mixture was incubated in a water bath at 37 °C for 90 min. The absorbance of the mixture (As) was measured at 536 nm. The absorbance was of 1 mL distilled water instead of hydrogen peroxide, and the sample was recorded asAb, of which 1 mL distilled water instead of the sample was recorded asAp.

2.6 Determination of antioxidant enzyme activities
At the end of each fermentation stage, the superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) activities of the samples were assayed through the method by Chen et al. [16]. In the SOD enzyme activity assay, 2.35 mL Tris-HCl buffer (0.1 mol/L,pH 8.2) was incubated at 25 °C for 10 min; 1.8 mL H2O,0.2 mL sample, and 0.15 mL pyrogallol solution (4.5 mmol/L) were immediately mixed in a cuvette; and absorbance was measured at 325 nm immediately. Every 30 s, light absorption was tested for 4 min.SOD activities were expressed as follows:
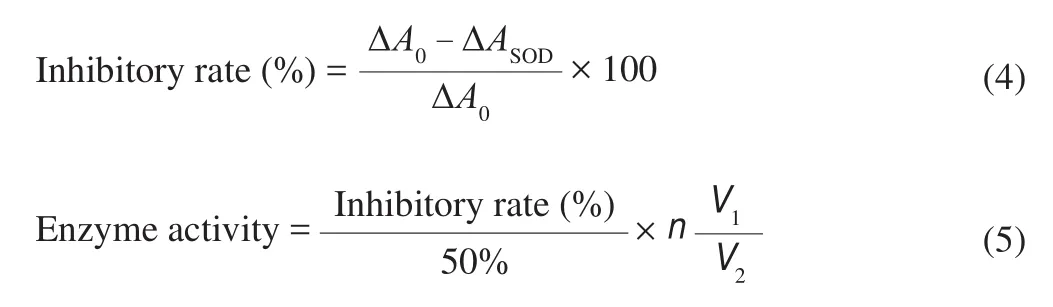
where ΔA0is the change of absorbance of the control group at 4 min, ΔASODis the change of absorbance of the test sample at 4 min,V1is the volume of reaction liquid,V2is the total volume of the sample, andnis the multiple of the diluted sample.
Additionally, in GSH-Px enzyme activity assay, 0.4 mL sample was mixed with 0.4 mL of 1.0 mmol/L GSH and then incubated at 37 °C for 5 min. H2O2(preheating at 37 °C) was then added into the mixture. This mixture was at 37 °C for 3 min. Then, 4 mL of metaphosphoric acid precipitant was added. The supernatant was collected by centrifugation (4 000 r/min, 10 min). Thereafter, 2 mL supernatant was mixed with 2.5 mL Na2HPO4(0.32 mol/L) and 0.5 mL DTNB. After 1 min, the absorbance of the mixture was measured at 420 nm (As). The control group included 0.4 mL of inactivated sample (A0). GSH standard curve was generated by 0, 0.2, 0.4, 0.8, and 1.0 μmol/L of GSH.
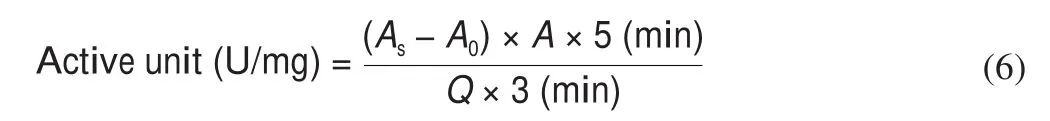
whereAis the slope of the calibration curve,Qis the weight of the sample,Asis the absorbance of the sample, andA0is the absorbance of the control.
2.7 Determination of titratable acidity (TA), total polyphenolic and total flavonoid content
The total TA in the acetic acid fermentation stage was expressed as the amount of lactic acid (%) required to titrate 0.1 mol/L NaOH to pH 8.3 [17].
The total phenolic content of the samples was determined using Folin-Ciocalteau reagent, with gallic acid as a standard [18].Then, 1 mL sample was diluted 50-fold. Afterward, 5 mL of 10% Folin-Ciocalteu reagent was added, 2 mL of 15% Na2CO3was added, and the volume was adjusted to 10 mL with distilled water. The mixture was stored in the dark for 1 h, and the absorbance was measured at 760 nm.
2.8 Determination of reducing sugar and lysine contents
The procedure for the determination of the reducing sugar and lysine contents was based on previous methods but with some modifications [19]. The reducing sugar content of the samples was determined using 3,5-dinitro-2-hydroxybenzoic acid (DNS),with glucose as a standard. First, 1 mL of distilled water and 1.5 mL of DNS solution were added to 1 mL of vinegar sample,and the system was properly mixed. The mixture was heated in boiling water for 5 min, cooled to room temperature and made up to 25 mL. The absorbance at 540 nm was determined using an ultraviolet spectrophotometer. The samples were analyzed using an automatic amino-acid analyzer (L-8900; Hitachi, Ltd.,Tokyo, Japan). The lysine content in the sample (mg/g) was calculated based on a standard sample. All experiments were conducted in triplicate.
2.9 Determination of α-amylase and α-glucosidase activity inhibition by LAB
In the mature vinegar, theα-glucosidase inhibition rate was determined based on the method by Zhang et al. [20]. In the 96-well plates, 50 μL of PBS solution (0.1 mol/L, pH 6.8) and 50 μL of a 2.5 mmol/Lp-nitrophenyl-α-D-glucopyranoside (PNPG) solution were mixed and incubated at 37 °C for 10 min. Then, 30 μL of a 0.4 U/mLα-glucosidase solution was added to the mixture, and the system was left at 37 °C for 30 min. Then, 50 μL of 1 mol/L Na2CO3solution was added to terminate the reaction. After the reaction was completed, the absorbance at 405 nm was measured.Phosphate-buffered saline solution (0.1 mol/L, pH 6.8) was used as a blank control for theα-glucosidase solution and the sample to be tested, acarbose was included as a positive control (C), and each group was set to three parallels.

whereAis a sample group containing a sample solution andα-glucosidase solution;Bis a blank group containing a sample solution with noα-glucosidase solution;Cis the control group, in which the sample solution contains acarbose andα-glucosidase solution;Dis a blank group in which the sample solution-free solution does not containα-glucosidase solution.
The inhibition rate ofα-amylase was determined with some modifications [10]. The 0.25 mL sample was mixed with an equal volume of 1 mg/mLα-amylase solution, and then the mixed solution was incubated at 37 °C for 10 min. Then, the reaction solution was added to 0.5 mL of 1.5% soluble starch solution at 37 °C. After 5 min of reaction, 1 mL of DNS solution was added to the mixture, and the mixture was placed in a boiling water bath for 5 min and then rapidly cooled to room temperature. The mixture was diluted 10-fold and allowed to stand for 30 min. Then, the absorbance at 540 nm was measured. Phosphate-buffered saline solution (0.1 mol/L, pH 6.8) was used as a blank control for the α-amylase solution and the sample to be tested. The calculation method was as above.
2.10 Determining the biomass in different rice vinegar groups
The biomass of mature vinegar was measured according to a previous method with some modifications [21]. First, 0.5 mL of the mixed sample was mixed with 4.5 mL of 0.87% normal saline. Then,1 mL of the suspension was pipetted and diluted to an appropriate concentration successively by gradients 10 times. The bacteria were cultured in selective MRS medium at 37 °C for 2 days to count LAB and cultured in YG with alcohol (2%) to count AAB.
2.11 Analysis of volatile flavor via headspace solid-phase microextraction-gas chromatography-mass spectrometry(SPME-GC-MS)
The headspace solid-phase microextraction method used for the extraction in mature vinegar was adapted from previous validated methods [22]. Analyses were conducted using an Agilent 6890 gas-chromatography system coupled to an Agilent 5975 inert quadrupole mass spectrometer. The fiber was purchased from Supelco(Bellefonte, USA) and coated with 50/30 μm of divinylbenzene/carboxen on polydimethylsiloxane fiber.
设计意图: 教师创设情境,引用生物入侵具体案例设置悬疑导入,来培养学生的学习兴趣,激发学生探究澳大利亚兔灾现象背后的种群数量增长规律的欲望。
Then, 2 mL of sample saturated in sodium chloride (1 g)was placed into a 30 mL headspace vial. Then, the samples were incubated at an agitation temperature of 50 °C. The volatile extraction was performed by exposing the fiber to the sample headspace (22 mm offiber depth) during 30 min and agitating at 250 r/min. For the desorption of compounds, the fiber was inserted and kept into the injector for 10 min at 240 °C in splitless mode and then at 220 °C for 4 min with a flow rate of 90 mL/min. The fiber was previously conditioned by inserting it into the GC-MS injector at 270 °C for 60 min.
2.12 Statistical analysis
One-way analysis of variance and Duncan’s multiple range test were performed to identify the differences between means using SPSS software version 20.0 (SPSS-IBM Chicago, IL, USA). Differences were considered statistically significant whenP< 0.05. HemI1.0 was used to analyze the difference and correlation of basic indicators, and the graphs were drawn using ORIGIN 2017. All the samples were measured in triplicate.
3. Results and discussion
3.1 Inhibitory effect of LAB on fluorescent AGE formation during vinegar fermentation
The traditional liquid fermentation vinegar model was employed to assess the inhibitory effect of LAB addition on the AGEs produced during protein glycation. As shown in Fig. 1, the fluorescence intensity of samples produced by three LAB strains during LSF had different trends. Only LFhad a positive correlation with time during the whole alcohol fermentation stage of the vinegar brewing procedure (Fig. 1A) and the inhibition rate of AGEs was approximately 15%, while the other groups had negative correlation trends. In addition, group LF could inhibit AGEs better than the control, and the inhibition rate of AGEs in group LF was higher than 15% (Fig. 1B).

Fig. 1 Inhibition effects of Lactobacillus on fluorescent AGEs formation in different groups of vinegar during different fermentation stages (A: alcohol fermentation, B: acetic fermentation). Bars of the same days with different letters indicate significant differences (P < 0.05).
These results showed that among the three groups, only group LF had significant inhibition activity on AGE formation during the two fermentation stages, while LC and LPA promoted the AGE formation during different fermentation stages.
3.2 Results of CML/CEL determination in vinegar
Table 1 showed the inhibition rates of CML and CEL in vinegar for different treatment groups (Figs. S1C and S1D). In the alcohol fermentation stage, except for group LPA, the addition of LAB in other groups had an inhibitory effect on CML and CEL. Among them,group LF had the highest inhibition rates for CML and CEL, which were (16.28 ± 0.83)% and (14.32 ± 0.62)%, respectively. In the acetic acid fermentation stage, only group LF inhibited the formation of CML and CEL, and the inhibition rates were (17.07 ± 0.67)% and(14.29 ± 0.16)%, respectively. In addition, the remaining two strains of LAB promoted the formation of CML and CEL. At the end of the alcohol fermentation stage, the CML and CEL inhibition rates of group LC were (1.08 ± 0.32)% and (0.65 ± 0.35)%, respectively. The CML/CEL content of group LPA was higher than that of the control.At the end of the acetic acid fermentation stage, the contents of groups LC and LPA were higher than that of the control. Thus, the results showed that LF had the strongest inhibitory effect on CML and CEL at the end of the two fermentation stages. However, groups LC and LPA had no inhibitory effect, especially during the acetic acid fermentation stage, according to the fluorescent AGEs detected in vinegar samples during the different fermentation stages.
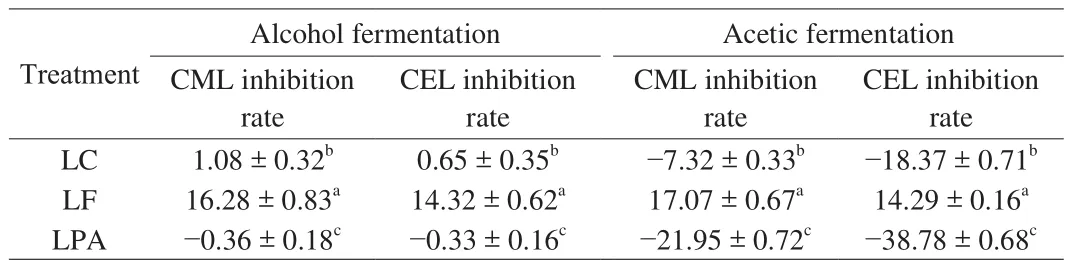
Table 1Inhibition rates (%) of CML and CEL in different groups of vinegar at the end of different fermentation stages.
3.3 Results of antioxidant activities in vinegar
Evidence from severalin vitroandin vivostudies has indicated that AGEs formation is closely related to oxidative stress and carbonyl stress [23]. Moreover, exogenously added substances with strong antioxidant capacity could effectively inhibit AGE formation [24].To study the differences in antioxidant capacity among the 4 different vinegar groups, two commonly used antioxidant capacity indicators DPPH· and OH· (Fig. 2) and two enzymes related to antioxidant capacity SOD and GSH-Px were selected (Fig. 2). The results showed that during the alcohol fermentation stage, the DPPH· scavenging rate of group LPA was significantly higher than those of the other groups, and those of groups LC and LF were higher than that of the control at the end of this stage (Fig. 2A). The OH· scavenging rate showed an overall downward trend, while the scavenging rate of group LF was always the highest; moreover, at the end of this stage,group LPA and the control exhibited the lowest scavenging rates,and there was no significant difference between them (Fig. 2B). In the acetic acid fermentation stage, there was no significant difference between the DPPH· scavenging rates of the different groups, which remained above 75% in the whole process in the first 5 days. The DPPH· scavenging rate of group LPA was the highest, and there was no significant difference between the scavenging rates of groups LC,LF, and the control at the end of the stage (Fig. 2E). Moreover, the addition of LAB made the OH· scavenging rate significantly higher than that of the control group on day 1. Although consistent with the alcohol fermentation stage, the OH· scavenging rate showed a downward trend overall. At the end of the fermentation stage, the scavenging rates of group LF was higher than those of the other groups, the OH· scavenging rate of group LC and the control were higher than those of group LPA, which had the lowest scavenging rate (Fig. 2F). Therefore, group LPA had the lowest DPPH·scavenging rate for the whole fermentation process. Moreover, the OH· scavenging rate of group LF and the control showed a strong ability. Recent studies have shown that the AGEs formation can be inhibited by several natural compounds with strong antioxidant activity [25]. Antioxidant enzymes are regarded as important enzymatic antioxidant defense systems in LAB [26]. SOD and GSH-Px are intracellular antioxidant enzymes that protect against oxidative stress [27]. The SOD and GSH-Px contents in the 4 groups of vinegar were determined (Fig. 2). At the end of alcohol fermentation, the SOD and GSH-Px of group LF were the highest: 1.92 and 11.52 U/mL,respectively (Fig. 2C). Generally, the enzyme activities of the LAB treatments were higher than that of the control group. In addition,similar results were obtained for the acetic acid fermentation stage.At this stage, group LF had the highest enzyme activities, but the SOD activity of group LC was lower than that of the control, and the GSH-Px activity of group LPA was lower than that of the control (Fig. 2G).Therefore, the addition of LF could increase the enzyme activities at any stage of the vinegar fermentation. Moreover, LAB, as a natural antioxidant, promoted the antioxidant capacity of the vinegar [28].Moreover, studies have shown that a strong antioxidant capacity can inhibit the formations of fluorescent AGEs and CML/CEL [29].Thus, the above results revealed that an underlying correlation existed between the inhibitions of fluorescent AGEs and CML/CEL and the antioxidant activities ofLactobacillusisolates.

Fig. 2 Antioxidant activities in different groups of vinegar during different fermentation stages (A-D: alcohol fermentation; E-H: acetic fermentation).
3.4 Results of TA, total phenolic and total flavonoid in vinegar
Studies have shown that the formation of AGEs is inhibited by many factors, such as acidic conditions, total phenolic and total flavonoid [18,30,31]. The TA were shown in Fig. 3. In the acetic acid fermentation process, there was no significant difference in the acidity of mature vinegar between the LAB-intervention groups, and the TA of the LAB-intervention groups was significantly higher than that of the control group; thus, the LAB-intervention groups could increase the TA of vinegar.

Fig. 3 TA in different groups of mature vinegar. Bars of the same days with different letters indicated significant differences (P < 0.05).
Studies have shown that a higher total phenolic content indicates a stronger antioxidant capacity [30]. Both in the alcohol fermentation stage and the acetic acid fermentation stage, the polyphenol content showed an upward trend with increasing fermentation time (Fig. 2).Meanwhile, the total phenolic content of the LAB-intervention groups was significantly higher than that of the control group. In the alcohol fermentation stage, there was no significant difference in the total phenolic content in the vinegar between the LAB addition groups, but group LPA and the control showed no significant difference in the phenolic content on day 2 (Fig. 2D). In addition, the total phenolic content of group LPA was significantly higher than those of the other groups at the late stage of acetic acid fermentation (Fig. 2H).

Table 2Total flavonoid content (μg RE/mL) in different groups of vinegar at the end of different fermentation stages.
The results showed that the addition of LAB might increase the total phenol and flavonoid content in vinegar, and this is consistent with the result of Chen et al. [28]. Some studies have shown that the AGEs formation could be inhibited by several natural compounds with antioxidant activity, such as flavonoids, polyphenols, and anthocyanins [25,30-32]. Thus, the increased content of the total phenol and flavonoid may be related to the inhibition of AGEs formation in the present study.
3.5 Results of reducing sugar and lysine content in vinegars
The formation of AGEs occurs through a nonenzymatic reaction between carbonyl groups of reducing sugars [31]. The reducing sugar and lysine are the important substrates for CML/CEL formation and are also important participants in the Maillard reaction. On day 1 of the alcohol fermentation stage, there was no significant difference between the reducing sugar contents of the LAB-intervention groups, and the control group had the highest reducing sugar content,0.716 mg/mL. In addition, the reducing sugar content of group LF was significantly lower than those of the other groups. Moreover,the reducing sugar content of group LPA was the highest in the late stage of alcohol fermentation (Fig. 4A). A similar result was observed for the acetic acid fermentation stage; the reducing sugar content of group LF (only 0.085 g/100 mL) was significantly lower than those of the other groups. Furthermore, on day 4 of fermentation, the reducing sugar was completely consumed. On the last day, the reducing sugar content of group LC was higher than those of the other groups, whose difference in the reducing sugar content was not significant (Fig. 4B).These results showed that the addition of LAB, especially strains LF and LPA, could accelerate the reducing sugar consumption,which might inhibit the formation of CML/CEL. By controlling the content of components such as lysine, reducing sugar, and lipids of CML in food materials, the amount of CML produced in the product might be effectively suppressed [33]. However, the reducing sugar consumption ability of group LC was lower than that of the control group, which may be the reason group LC promoted the formations of AGEs, including CML and CEL.

Fig. 4 Reducing sugar content in different groups of vinegar during different fermentation stages (A: alcohol fermentation, B: acetic fermentation). Bars of the same days with different letters indicated significant differences (P < 0.05).
The lysine content during the acetic acid fermentation stage was detected. The results were presented in Table 3. There was no significant difference between the lysine contents of the 4 vinegar groups fermented for 7 days. The addition of LAB could not accelerate the consumption of lysine; therefore, the inhibition of CML and CEL may not be achieved by the consumption of the lysine substrate.

Table 3Lysine content (mg/L) in different groups of vinegar during acetic fermentation.
3.6 Results of α-glucosidase and α-amylase
α-Amylase andα-glucosidase are the main digestive enzymes in the process of dietary starch hydrolysis. The inhibition ofα-amylase andα-glucosidase activities is one of the most effective methods for postprandial glycemic control and diabetes care. Our results indicated that LAB could inhibit the activity ofα-glucosidase andα-amylase (Table 4).Among the LAB-intervention groups, group LF exhibited the highestα-glucosidase inhibition rate (19.23%) and group LPA exhibited the highestα-amylase inhibition rate (15.14%). Group LC exhibited the lowestα-glucosidase andα-amylase inhibition rates: 9.65% and 10.32%, respectively.α-Glucosidase andα-amylase are important digestive enzymes controlling starch hydrolysis to produce glucose,which is the vital substrate of glycation reaction [34,35]. Therefore,the addition of LAB might inhibit the formation of CML and CEL by inhibitingα-glucosidase andα-amylase activities to control the production of substrates in vinegar. In addition, evidence from severalin vitroandin vivostudies has indicated that polyphenols have an inhibitory effect on starch digestion and adsorption by inhibiting the digestive enzymes (α-amylase andα-glucosidase) [36].

Table 4Inhibition effects (%) of Lactobacillus on enzyme activities in different groups of mature vinegar.
3.7 Results of biomass in vinegar
The plate-count method can accurately indicate the microbial bacteria content in food, which can be converted into the number of bacteria in the sample according to the dilution factor. Moreover, the number of bacteria in the sample can effectively show the interaction of microorganisms. The experimental results were presented in Table 5.At the end of the acetic acid fermentation stage, the group of LF withA. pasteurianusshowed the best performance, as the number of LAB and AAB colonies were 6 × 107and 1 × 107CFU/mL,respectively. The addition of LF significantly promoted the AAB growth compared with the control group (1 × 107CFU/mL AAB only),while the other two LAB strains suppressed the AAB growth.The LAB and AAB of the LC co-culture group were grown at concentrations of 3 × 106and 7 × 106CFU/mL, respectively, which were both lower than those of group LF. These results revealed that LF could achieve good symbiotic effect with AAB in vinegar, while strains of LC and LPA were in a competitive relationship with AAB.In addition, the reducing sugar content of group LF was low compared to groups LPA and LC, which is consistent with the biomass results of LAB and AAB. Therefore, the consumption of reducing sugar, the key substance for AGEs formation, might result in different inhibition effects of LF, LC, and LPA on the formations of AGEs, including CML and CEL.

Table 5LAB and AAB biomass (CFU/mL) in different groups of mature vinegar.
3.8 Analysis of volatile flavor via headspace SPME-GC-MS
Through GC-MS-based flavor profiling, an OPLS-DA model was constructed to explore the detailed information about marker compounds contributing to the data differences between samples of vinegars. The OPLS-DA biplot displayed similarities and dissimilarities between different fermented vinegars, which showed that they were clearly distinguishable (Fig. 5). According to the results of multivariate statistical analysis, 16 compounds withP-values less than 0.05 and variable importance in the projection (VIP) values larger than 1.0 were selected as potential candidates for discriminant volatile compounds (Fig. S2). In addition, the principal component analysis (PCA) plot showed the same results that reveal dissimilarities among the different samples with respect to the flavor profile, and the results indicated that appreciable differences existed among the samples. Group LF was well distinguished from other groups.Several studies have proved that the addition of LAB promoted the flavor characteristics of fermentation production [37,38]; thus,LAB strains are closely associated with flavor formation. Moreover,the addition of LAB, especially LF, increased the different flavor compounds of mature vinegar (Fig. S3). The results showed that the different flavor compounds of group LF included esters, alcohols,phenols, and acids (acetic acid, 2-methylbutyl acetate, phenethyl alcohol, 3,5-dimethylbenzaldehyde, phenethyl acetate, 2,4-di-tertbutylphenol, palmitic acid and stearic acid). Group LF possessed the most different flavor compounds and provided an important contribution to the product flavor (Fig. S3C). In addition, group LC had few flavor compounds, such as acetic acid and phenylethyl alcohol (Fig. S3B). Some studies have shown that alcohols and esters enhance the fragrance of fermented juice [39]. Therefore,the addition of LAB, especially LF, increased the abundance of flavor substances.
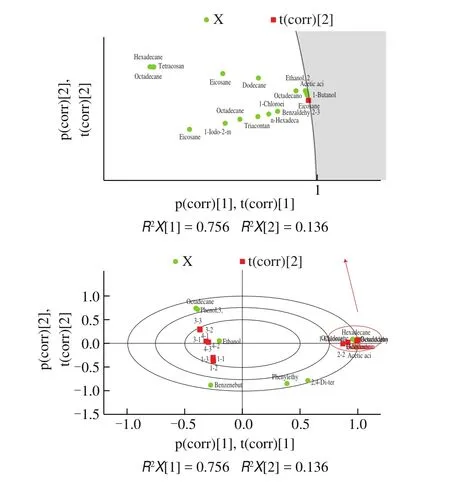
Fig. 5 Biplot using OPLS-DA obtained from GC-MS data of vinegar in different groups. S-plot (samples) and loadings plot (variables) were displayed simultaneously using correlation scaling, showing the relationship between samples and variables. The green circle indicates the flavor substances, the red hexagon indicates groups (LC: 1-1, 1-2, and 1-3; LF: 2-1, 2-2, and 2-3;Control: 3-1, 3-2, and 3-3; LPA: 4-1, 4-2, and 4-3).
3.9 Correlation analysis
Correlation analyses were adopted to further illustrate the relationships between antioxidant capacity, substrate, and AGEs (CML and CEL). Here, the Pearson correlation coefficient was calculated. The CML and CEL contents were negatively correlated with the OH· scavenging rate (Fig. 6). The SOD and GSH-Px could inhibit the formation of CML and CEL by excellent free radical scavenging activity. Previous studies have shown that antioxidants with strong antioxidative capacity affect the AGEs formation [40].In our study, the addition of LAB could inhibit the formation of non-crosslinked and non-fluorescent AGEs (CML and CEL). In addition, the reducing sugar content was significantly positively correlated with the CML and CEL content. The consumption of reducing sugars, which are substrates of the Maillard reaction, could inhibit the CML and CEL formation to a certain extent. Moreover, the total phenols, total flavonoids and TA were significantly negatively correlated withα-glucosidase andα-amylase. Some studies have shown that total flavonoids and total polyphenols can inhibit the enzymatic activities ofα-glucosidase andα-amylase and also inhibit AGEs formation by inhibiting the starch digestibility to reduce the substrates of the Maillard reaction [41,42]. In addition, the increase in the TA not only inhibited theα-glucosidase andα-amylase activities but also inhibited the glycosylation reaction [43]. However, in this study, the consumption of lysine, another substrate of AGEs, had no significant correlation with the AGEs formation. Therefore, the increase in the antioxidant capacity, the consumption of reducing sugar substrate, and the total phenol and flavonoid contents were the main factors responsible for the inhibition of the formation of AGEs,especially CML and CEL.
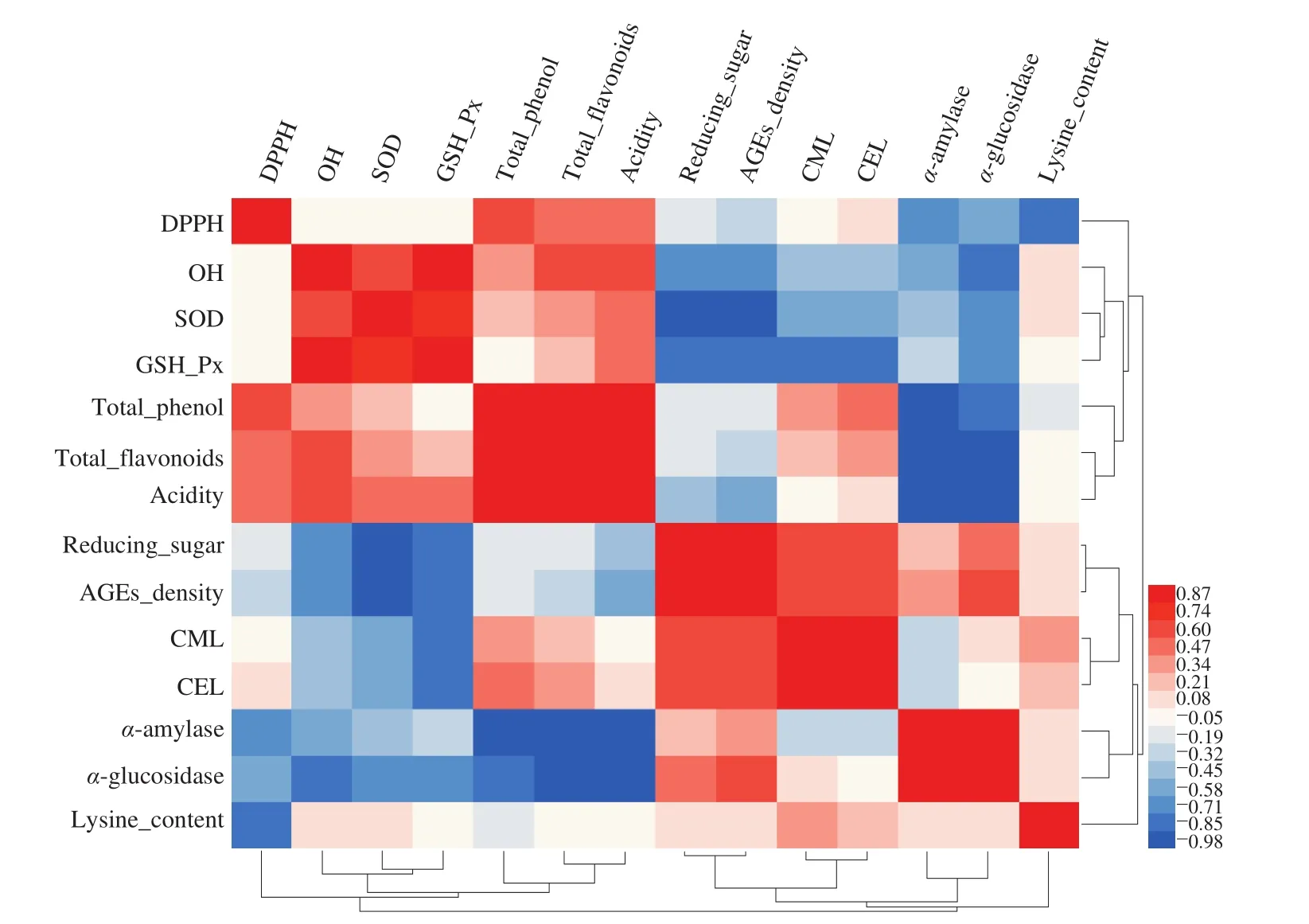
Fig. 6 Spearman’s rank correlation analysis to study antioxidant capacity, substrate, and AGEs in mature vinegar.
4. Conclusion
In this study, LAB strains are proposed as a new inhibitor.The LAB inhibited the formations of fluorescent AGEs and non-fluorescent AGEs (e.g., CML and CEL), mainly by promoting synergistic effects of LAB and AAB; enhancing the antioxidant activity of vinegar; increasing the contents of total flavonoids, total polyphenols, and titratable acids; accelerating the consumption of reducing sugar; and inhibiting theα-glucosidase andα-amylase activities. However, the different LAB strains showed the different effects. The sample group with LF not only had the strongest inhibitory effect on fluorescent AGEs, CML and CEL but also had the most abundant variety of flavor metabolites, including esters, acetic acid and phenethyl alcohol. This research may contribute to the future advancement of LAB-based strategies for AGEs control.
conflict of interest
The authors declare that they have no conflicts of interest.
Acknowledgement
The authors wish to acknowledge the National Natural Science Foundation of China (31601455), National Natural Science Foundation of China (32001705) and the Science and Technology Innovation Project of Hubei Grain Bureau (2017/58).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.1016/j.fshw.2022.04.031.
猜你喜欢
杂志排行
食品科学与人类健康(英文)的其它文章
- Hypoglycemic natural products with in vivo activities and their mechanisms: a review
- Bacteroides utilization for dietary polysaccharides and their beneficial effects on gut health
- Capsular polysaccarides of probiotics and their immunomodulatory roles
- Natural compounds may contribute in preventing SARS-CoV-2 infection: a narrative review
- A comprehensive review on the effects of green tea and its components on the immune function
- A review on current and future advancements for commercialized microalgae species
