Capsular polysaccarides of probiotics and their immunomodulatory roles
2022-06-23JingLiSaisaiFengLeileiYuJianxinZhaoFengweiTianWeiChenQixiaoZhai
Jing Li, Saisai Feng, Leilei Yu, Jianxin Zhao, Fengwei Tian,Wei Chen,c, Qixiao Zhai,*
a State Key Laboratory of Food Science and Technology, Jiangnan University, Wuxi 214122, China
b School of Food Science and Technology, Jiangnan University, Wuxi 214122, China
c National Engineering Research Center for Functional Food, Jiangnan University, Wuxi 214122, China
Keywords:
Capsular polysaccharides
Probiotics
Bacteroides
Immunomodulation
A B S T R A C T
Studies have determined the immunomodulatory activities of cell-surface polysaccharides of lactic acid bacteria (LAB) and Bacteroides; however, the mechanisms, synthesis, regulation, structure, and functional links have not been systematically discussed. We first introduce the structure of the capsular polysaccharides (CPSs)of commonly studied probiotics and Bacteroides. Wzx-Wzy dependent and ATP-binding cassette (ABC)transporter-dependent pathways are the two main biosynthesis and secretion of CPS pathways. The genes known to be associated with these two pathways are mainly those associated with priming glycosyltransferase (pGT);a variable number of genes encoding for different glycosyl transferases (GTs); Wzx/Wzy-encoding enzymes related to flippases and polymerases; and ABC-transporter genes. In addition, the effects of CPSs on host immunity as well as their related underlying mechanisms are described. Surface polysaccharides on probiotics can serve as a mask to aid in their escape from attacks from the host’s immune system. In turn, they also exhibit immunomodulatory activities, such as strengthening the functions of macrophages, promoting the maturation of antigen-presenting cells, and inducing regulatory T cells. All of these effects of cell-surface polysaccharides exhibit their significant protective properties in immunocompromised diseases, such as colitis,arthritis, and dermatitis. Finally, we focused on their structure and functional links.
1. Introduction
Bacterial capsule polysaccharides (CPSs), also known as“exopolysaccharides,” are mainly composed of long-chain polysaccharides with repeating unit structures, and have gained widespread attention for their various bioactivities. CPSs are known to have protective properties for bacterial cells, such as protection against dehydration, macrophages, bacteriophages, protozoa,antibiotics, and toxic compounds [1-4]. Recently, CPSs have been reported to promote the bacterial colonization and adhesion [2,5,6],inhibit the initial attachment and auto-aggregation of pathogens [7-9].CPSs of pathogenic bacteria have been demonstrated to help the bacteria escape phagocytosis, to shield them from the recognition and attack by the host’s immune system, and to ultimately cause diseases [10,11]. Notably, CPSs of meningococcus, pneumococcus,SalmonellaVi, andHaemophilus influenzatype bhave been used as immune adjuvants, and the conjugation of CPSs to proteins can improve the efficacy of vaccines [12]. In addition, CPSs of probiotics have been reported to possess antitumor, antiulcer, antimutagenic,cholesterol-lowering and immunomodulatory activities [13-17].
Given the significant roles of natural bacterial polysaccharides, we focused on the immunomodulatory roles of surface polysaccharides from probiotics andBacteroides, one of the main phyla in the gut and also a promising category of next-generation probiotics. CPSs have been reported to induce the proliferation of macrophages [18],enhance the antigen presentation ability of dendritic cells (DCs), and promote the generation of T regulatory (Treg) cells in the intestine.Especially, they can induce immune-tolerance in the intestine and protect against various sterile inflammatory disorders, including viral encephalitis and colitis [19-21].
In recent years, several reviews on CPSs produced by bacteria have been published, but they have mostly focused on the characterization of the chemical structure and industrial production of CPSs produced byLactobacillusorBifidobacteria[22-24]. Other reviews have summarized the immunomodulatory abilities of CPSs produced by lactic acid bacteria (LAB) orBacteroides[25-29]; however,few reviews have systematically discussed the monosaccharide composition, synthesis, regulation, structure, and immunomodulatory functional links of CPSs produced by LAB andBacteroides.
Based on these studies, here we discuss the molecular structure of CPSs of probiotics andBacteroides, their synthesis and regulation,the links between their structure, their immunomodulatory activities,and their molecular mechanisms. Finally, we also discussed the future applications and any conflicts between the results of present studies and the safety concerns to promote further investigations and clinical application of these CPSs.
2. Molecular structures of CPSs
The external-most layer of the surface of many bacteria is covered by a type of polymeric compound composed of repeating units of monosaccharides. These bacteria include traditional probiotic lactic acid bacteria (LAB), several gut inhabitants such asBacteroides, and pathogens. These surface polymeric compounds can be tightly linked to the cell surface or loosely attached to the extracellular surface or secreted into the environment. Based on their chemical composition,these compounds can be classified as homopolysaccharides (HoPSs)or heteropolysaccharides (HePSs). HoPSs of LAB are mainly composed of glucans and fructans and may differ in their glycosidic bonds, branching, chain length, molecular weight (MW),and polymer structure [30]. HePSs of LAB are generally composed of glucose, galactose, rhamnose, mannose, arabinose, and xylose.Among monosaccharides, glucose is the most common and found in higher proportions than other monosaccharides [18].The MW of the HePSs of LAB ranges from 4 × 104Da to 6 × 106Da,and the polysaccharides of LAB that exist naturally can be either neutral or negatively charged [18,30]. Another type of important polysaccharide—zwitterionic polysaccharide (ZPS)—occurs particularly in someBacteroides, such asBifidobacterium fragilis, and has shown strong bioactivities. Table 1 and Fig. 1 list the compositions of the polysaccharides of LAB and the classic polysaccharide A (PSA) ofBacteroidesthat have been discovered in recent years.

Table 1Molar ratio of constituted monosaccharides of CPS in LAB.
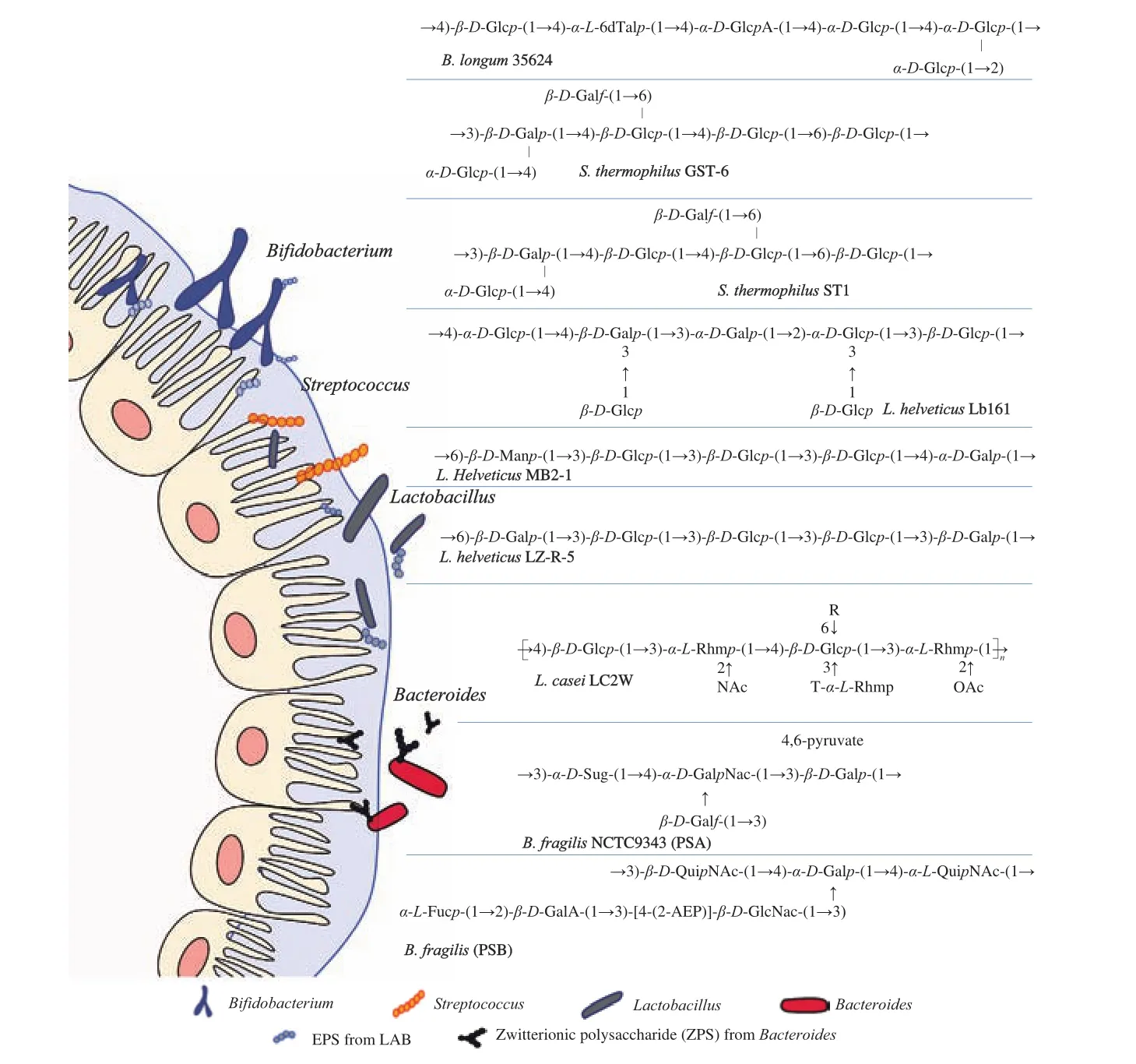
Fig. 1 CPS structure in LAB and Bacteroides. L. Helveticus Lb161 [30], L. casei LC2W [34,35], L. helveticus MB2-1 [44], L. helveticus LZ-R-5 [18],S. thermophilus ST1 [45], S. thermophilus GST-6 [46], B. longum 35624 [47], B. fragilis NCTC9343 (PSA, PSB) [48,49].
3. Synthesis and regulation mechanisms of CPSs
Genomic sequencing and gene functional studies have shown that the biosynthesis of extracellular or CPSs inLactobacillusandBifidobacteriumis directed by specific gene clusters [50-52].The diversity of these gene clusters and regulation of their expression finally leads to a variety of CPSs. In general, these bacterial polysaccharides are synthesized via the following four mechanisms: 1) the Wzx/Wzy-dependent pathway; 2) the ATP-binding cassette (ABC) transporter-dependent pathway; 3) the synthase-dependent pathway, and 4) extracellular synthesis using a single sucrose protein [53]. In the first three mechanisms, the stepwise elongation of the polymer strands is realized by the sequential reactions of various enzymes inside the cell. Notably,polysaccharides assembled through the Wzx/Wzy pathway typically employ a priming glycosyltransferase (pGT), one or more glycosyl transferases (GTs), a flippase, and a polymerase to produce an extracellular and highly diverse sugar.WzxandWzygenes are conserved in this pathway and usually occur within their exopolysaccharide operons.The polysaccharides assembled by the ABC transporter-dependent pathway are actually classical CPSs, and the final products remain connected to the cell surface. CPSs produced through this pathway carry a conserved glycolipid at the reducing terminus consisting of a phosphatidylglycerol and a poly-2-keto-3-deoxyoctanoic acid linker.The synthase-dependent pathway typically assembles HoPSs.
CPS biosynthesis gene clusters have been found to be abundant inL. casei,B. longum, andB. breve.Yasuda et al. [52]conducted gene survey analyses to determine the possible polysaccharide biosynthesis gene clusters in theL. caseiShirota genome. They found a unique polysaccharide biosynthesis gene cluster, which contains 10 genes lining up in the same direction and forming a relatively complete functional module [52]. The dTDP-rhamnose biosynthesis gene cluster was also identified downstream of the 10 genes. Song et al. [54]analyzed the genome sequence ofL. caseiLC2W and compared the differences in the CPS biosynthesis gene clusters between these twoL. caseistrains. They found that the 10 genes first reported in strain Shirota were also present in strain LC2W. In addition to the 10 previously identified genes, strain LC2W also has an invertible promoter and another 2 CPS-coding genes, which indicates an alterable possibility for CPS synthesis in response to environmental factors. Fanning et al. [51]found that the biosynthesis of extracellular polysaccharides (EPSs) on the surface ofB. breveUCC2003 is directed by either half of the bidirectional gene cluster, leading to the production of one of two possible EPSs. The alternate transcription of two opposing parts of the cluster appears to be the result of promoter reorientation.Tahoun et al. [50]reported that a putativeB. longum105-A CPS/EPS gene cluster consists of 24 putative genes encoding a pGT (cpsD), 7 glycosyltransferases, 4 CPS/EPS synthesis machinery proteins, and 3 dTDP-L-rhamnose synthesis enzymes. These enzymes form a complex system that is involved in the biogenesis of CPSs and/or EPSs. Another study compared the CPS biosynthesis gene clusters between 105-A and three otherB. longumstrains (35624,JCM1217, and NCC2705). The priming glycosyltransferase gene is present in all strains and NCC2705 shows a gene assembly method distinct from the other three strains [47]. All of these findings suggest gene diversification of the CPS biosynthesis cluster in different strains due to gene variations, including insertion, deletion, and accumulated mutations in this cluster.
Compared withLactobacillusandBifidobacterium, the regulatory mechanisms of CPSs inBacteroideshave been widely studied.B. fragilisNCTC9343, encoding at least 8 distinct CPSs, can express specific CPS genes under specific environmental conditions, which is highly associated with the bacteria’s immunogenicity [54]. These 8 CPSs, named PSA, PSB, PSC, PSD, PSE, PSF, PSG, and PSH,have distinct structures, with PSA being the most abundantly and frequently expressed CPS. Seven of the 8 CPS synthesis loci contain a promoter sequence.B. fragilismay express any combination of CPSs simultaneously, thus generating 256 possible surface profiles [55,56].Similarly, inB. thetaiotaomicron(B. theta), a substantial proportion of the genome is dedicated to the production of CPSs and contains the loci of for the synthesis of 7 CPSs, 4 of which have invertible promoter regions [57,58].B. thetahas the potential to switch the expression of its CPS loci and dynamically changing its CPSsin vivoto adapt to changing environmental conditions. For example,it has been reported that the production of immunoglobulin A (IgA)downregulates the expression of CPS4 ofB. theta in vivo, which dominantly expresses CPS4, and switches to other CPSs [59].Dynamic changes in CPS expression ofB. thetawere also observed in both high-fiber and low-fiber diets, as well as in adaptive immunodeficient mice [59]. CPS biosynthesis is controlled by several mechanisms, such as invertible promoters, NusG-like antitermination factors, and trans-locus inhibitors. DNA invertases mediate DNA inversions, resulting in phase-variable expression of each of the CPSs. DNA invertases consist of two distinct families—tyrosine site-specific recombinases (Tsrs) and serine site-specific recombinases (Ssr).It appears that all 7 of the invertible polysaccharide promoter regions ofB. fragilisare mediated by Mpi, a single member of the Ssr family [60]. The first two conserved genes of each CPS biosynthesis locus ofB. fragilisencodes the proteins UpxY and UpxZ (where x is a–h), and the genes downstream of these two Upx genes encode enzymes involved in CPS synthesis. UpxY has a conserved KOW motif, which directs the synthesis of proteins belonging to the NusG-like (NusGSP) family, and the NusGSPfamily has been found to function in the operon-specific transcriptional antitermination [61].The second gene of each of these loci encodes an UpxZ protein,which inhibits the synthesis of HePSs. These unique proteins interfere with the ability of UpxY proteins encoded by other polysaccharide biosynthesis loci to function in the transcriptional antitermination of their respective operons, thereby inhibiting the concomitant transcription of multiple polysaccharide biosynthesis loci within a cell [56].
The PSA ofB. fragilisNCTC9343 is a capsular ZPS, and the crucial positive charge of ZPSs is conferred by the same amino sugar—acetamido-amino-2,4,6-trideoxygalactose (AATGal). In the operon of PSA ofB. fragilisNCTC9343, the transcriptional regulatorsupaY,upaZ,wcfR, andwcfS, the key genes involved in the biosynthesis and attachment of the AATGal amino sugar, are conserved. Based on these four genes, Neff et al. [62]presumed that a ZPS producer,B. cellulosilyticusDSM 14838, would have an alleviating effect on colitis, similar to that ofB. fragilisNCTC9343.Table 2 has summarized some key genes and regulation motifs relating to CPS synthesis.
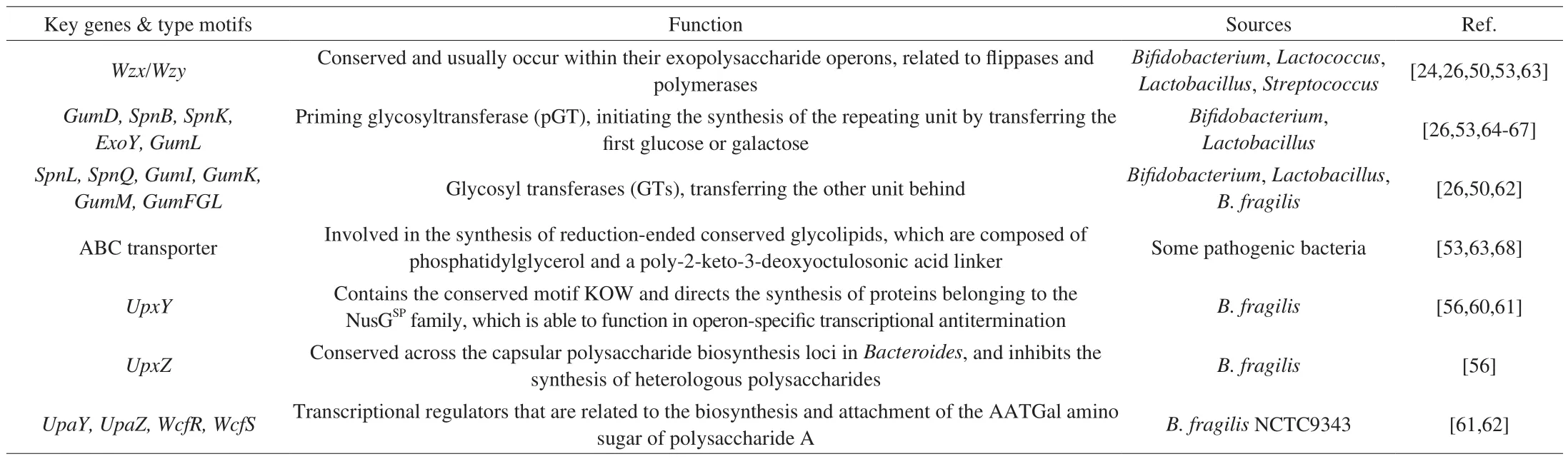
Table 2Key genes and regulation motifs relating to CPS synthesis.
4. Roles in host immunomodulation
4.1 Evidence in in vitro and in vivo models
Variousin vitromodels have been used to evaluate the immunomodulatory effect of microbial EPSs. The commonly used cell lines and cultures include RAW cell line, bone marrow-derived dendritic cells (BMDCs), peripheral blood mononuclear cells (PBMCs),mesenteric lymph node (MLN) cells or splenocytes. They focused on different functions of these immune cells, such as the maturation of macrophages and DCs, the cytokine secretion profiles of PBMC and MLN. It has been shown that specific EPSs isolated from LAB, such asL. helveticusLZ-R-5 andStreptococcus thermophilesAR333, increase the proliferation of macrophages and enhance phagocytosis, acid phosphatase activity, nitric oxide (NO) production, and cytokine production in RAW264.7 [18,70]. In addition, the coculture of EPSs purified fromB. longumBCRC 1464 using macrophage cells (J774A.1 cell line) promotes the proliferation of macrophages, increases the release of interleukin (IL)-10, and reduces the secretion of tumor-necrosis factor (TNF)-α induced by lipopolysaccharides [7]. However, it has also been reported that some EPSs, such as those fromB. longum105-A, confer resistance of LAB from being internalized by murine macrophages [50]. EPSs fromL. plantarumcan upregulate the expression of MHC II and CD86 of DCs, which enhances their antigen-presentation ability [71].EPSs fromL. ParaplantarumBGCG11 strain can stimulate a significant increase in the IL-10 and IL-1β expression of PBMCs [72].Similarly, those fromB. longum35624 were found to induce relatively low levels of cytokine secretion from human DCs (i.e., PBMCs and monocyte-derived DCs [MDDCs]), including IL-12p70, interferon(IFN)-γ, IL-17, TNF-α, and IL-6, compared with the isogenic exopolysaccharide-negative mutant derivative [73]. After coculturingB. breveUCC2003 with isogenic EPS+and EPS-with splenocytes from naïve mice, EPS+was observed to further significantly reduce the levels of the proinflammatory cytokines IFN-γ, TNF-α, and IL-12 than EPS-[51]. In another study, the incubation of the PSA ofB. fragiliswith CD11c+BMDCs and naïve splenic CD4+T cells led to a dose-dependent increase in the proliferation of T cells and the secretion IFN-γ from Th1 cells [74].
In addition to thein vitrocell models, the immunomodulatory effects of CPSs have been demonstrated on both homeostatic (healthy)and immunocompromised animal models. For example, mice treated with CPS+compared with CPS-B. breveexhibited no immune response because of reduced adaptive B-cell numbers and diminished secretion of cytokines and antibodies [51]. The CPSs ofL. caseicould induce and promote the differentiation of CD4+T cells of Peyer’s patches into Th17 cells in healthy mice [36]. In addition,CPSs produced byB. longum35624 alleviated the weight loss and disease symptoms in the T cell transfer colitis model and prevented an increase in the percentage of IFN-γ+lymphocytes in the lungs in an ovalbumin respiratory allergy model [73]. Similarly, CPSs fromB.adolescentisIF-03 induce lower levels of IL-1, IL-6 and TNF-α and higher levels of IL-10 in DSS-induced colitic mice [75]. Moreover,the treatment with CPSs increases the proportion of Treg/Th17 cells when compared with the model mice [75]. The bacterial cell surfaceβ-glucan/galactan (CSGG) ofB.bifidumadministered to germ-free mice was shown to induce thede novogeneration of pTreg cells in the colon of those mice, as well as suppress intestinal inflammation in the T cell transfer colitis model [19]. In addition, Mazmanian et al. [74]found that the purified PSA ofB. fragiliscould restore CD4+T cell proportions among splenic lymphocytes to conventional levels and could drive a Th1-mediated response to help maintain Th1/Th2 balance in germ-free mice. Purified PSA ofB. fragiliscan protect animals withH. hepaticus-induced experimental colitis by suppressing the production of pro-inflammatory IL-17 by intestinal immune cells and increasing IL-10-producing CD4+T cells [21]. PSA treatment also resulted in a 5%-10% increase in the amount of Foxp3+cells in the CD4+CD25+cells of MLNs in mice with trinitrobenzene sulfonic acid-induced colitis, which significantly alleviated the severity of the disease [76]. A recent study has shown that oral treatment with PSA can protect mice from fatal herpes simplex encephalitis by inducing IL-10 secretion from regulatory CD4+and CD8+T cells [20].The anti-stimulatory CPSs ofB. thetawere also found to activate polyclonal T cells more weakly than the pro-stimulatory CPSs in germ-free mice [77]. What’s more, EPS fromL. rhamnosusATCC 7469 andL. acidophilus20079 showed some anti-tumor effects in dimethylhydrazine (DMH)-induced colorectal carcinogenesis of rat.The downregulation of nuclear factor-κB (NF-κB) and STAT3 has been reported in these studies [78-80].
Although variousin vivoandin vitromodels have been used to evaluate the immunomodulatory effects of polysaccharides, the mechanisms of and additional functional predictions about these bacterial polysaccharides need further investigation. Basically, CPSs or EPSs on bacteria’s surface can help them escape attack by host immunity. CPSs can also promote the invasion and pathogenicity of pathogens. For many probiotics and symbiotic bacteria in the gut,CPSs can shield the cell-surface antigens, promote their inhibition in the gut, and guide immune tolerance in the host. Interestingly, the CPSs of these specific strains can also function in the host’s health and disease, such as providing anti-inflammatory responses, enhancing the functions of immune cells and promoting their maturation, and promoting the development of T cells, DCs, or other immune cells.
4.2 Molecular pathways
The innate recognition of the glycan of microorganisms is mediated by several classes of pattern-recognition receptors, including mainly lectin receptors and Toll-like receptors (TLRs) (Fig. 2). TLRs were found on the surface or endosomal membranes of different cell types, such as macrophages and DCs, and on nonimmune cells,such as epithelial cells, hepatocytes, or adipocytes. It has been demonstrated that both the CSGG ofB.bifidumand PSA ofB. fragilisare the ligands of TLR-2 [19,81]. Verma et al. [19]reported that the CSGG ofB.bifiduminduces the generation of regulatory DCs in a TLR-2-dependent pathway, which finally facilitates the production of Foxp3+IL-10highIFN-γlowTreg cells. Erturk-Hasdemir et al. [82]demonstrated that PSA fromB. fragiliscan bind to two receptors,TLR-2/1 heterodimer and Dectin-1 on APCs (macrophages and DC), and then activate the downstream phosphoinositol 3-kinase(PI3K) signaling pathways (Fig. 2). Finally, phosphorylated Akt inhibits the activation of NF-κB and promotes cAMP response element-binding protein (CREB)-dependent gene transcription to exert anti-inflammatory effects [82].
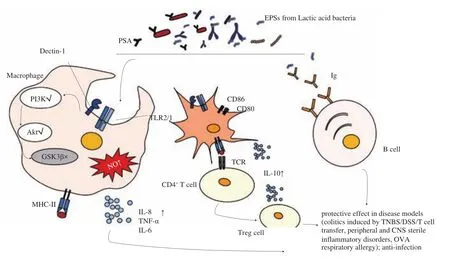
Fig. 2 Functional mechanisms of CPS in host health and disease. Both the TLRs and C-type lectin receptors (CLRs) are types of pattern recognition receptors (PRRs) present on the surface of macrophages and DCs, where they can sense the microbial CPSs and trigger the activation of downstream signals via their intracellular domains, followed by regulation of gene expression, and finally, launching a response to CPS stimulation that changes their functions. On macrophages, recognition by PRRs leads to increased macrophage proliferation, enhanced phagocytosis and acid phosphatase activity, and the production of NO and cytokines (IL-8, IL-1β, IL-6 and TNF). On DCs, PRR stimulation can increase the expression of cell-surface proteins, such as MHC II, CD80, and CD86, each of which is associated with its antigen-presentation functions. In addition, they may produce lead to the production of cytokines such as IL-10.CPS-threatened DCs reach the lymph nodes where they encounter T cells and promote their activation and differentiation. Innate-like B cells, known as B1 cells that mainly produce IgM antibodies, can also respond to some common bacterial CPSs; however, limited research has focused on this type of B cell as well as its role in host health and disease [19,73,75,82,87,88].
In general, the downstream of the TLR-2 pathway is believed to be the initiation of myeloid differentiation primary response gene 88 (MyD88). This leads to the activation of transcription factors NF-κB,which is associated with the expression of inflammatory cytokines [83].However, Sandra Santos et al. reported another downstream of TLR-2 signaling, including the PI3K-dependent phosphorylation of Akt, phosphatidylinositol(3,4,5) P3 (PIP3) generation and macrophage polarization [84]. In the PI3K pathway, glycogen synthase kinase-3β (GSK3β), a key kinase, is phosphorylated and inactivated through phosphorylation by Akt. GSK3β inhibits CREB DNA binding activity [85].Thus, the inactivation of GSK3β cannot promote the binding of NF-κB to CREB binding protein. Instead, it cause transcription of CREB binding protein-dependent anti-inflammatory genes [82].
Although most CPSs cannot be presented by the MHC complex and do not activate follicular B2 cells, they have been classified as type 2 T cell-independent (TI-2) antigens. It is worth mentioning that TI-2 antigens with their repetitive structures can directly activate mature B cells (B1 or marginal zone B cell subsets) by cross-linking specific B cell antigen receptors [86].
There is limited literature on the mechanisms of polysaccharides in probiotics andBacteroides. How these supplemented bacterial polysaccharides are presented or recognized in the gut environment or how they enter the circulation system and consequently affect the systemic and peripheral immunity remains to be investigated.
4.3 Structure and functional links
The monosaccharide composition, chemical modifications, and polymers of EPSs collectively determine their MW and charged or not, all of which have been recognized as the most critical properties by which to determine their immunomodulatory effects. Some researchers have postulated that polysaccharides of LAB having a negative charge and/or a light MW are able to act as mild stimulators of different immune cells, whereas neutral and heavy MW (HMW,approximately 106Da) polymers have a suppressive profile or can attenuate an excessive response, thus helping the producing bacteria to evade the immune response of the host [25]. Polysaccharides isolated fromL. delbrueckiissp.bulgaricusOLL1073R-1 are modified by a phosphate group and exhibit mitogenic activities, such as stimulating lymphocyte proliferation and inducing IFN-γ production [89]. Acidic EPS103 fromL. plantarumJLAU103 has been found to enhance the phagocytic activity of RAW264.7 macrophages and promote the release of IL-6, TNF-α, and NO of RAW264.7 macrophages [90].Moreover, EPS fromPediococcus pentosaceusKFT18 containing uronic acid (mannuronic acid and glucuronic acid) has been reported to stimulate macrophages and primary splenocytes to induce immune responses and improve the cyclophosphamide-induced immunosuppression in mice [91]. EPS fromL. plantarumNCU116 containing glucuronic acid has been reported to induce the upregulation of STAT3 in Caco-2 cells [42]. In addition,HMW-EPS fromL. rhamnosusRW-9595M (mucous variant)was shown to induce little or no TNF-α and IL-6 secretion from macrophages, whereasL. rhamnosusATCC9595 with a highly similar genetic background induced high levels of TNF-α, IL-6, and IL-12 and showed decreased IL-10 production, a property that decreases with an increase in the degree of EPS hydrolysis [92]. The EPSs from strain RW-9595M are composed of units of 3Rhaα-3Glcβ-3[Gal4,6(R)Pyα-2]Rhaα-3Rhaα-3Rhaα-2Glcα, where Rha refers to rhamnose and Py corresponds to pyruvate acetal; it appears that this structure is unique toL. rhamnosus[93]. In addition, EPSs purified from 3B. animalissubsp.lactisstrains, A1 (parental) and 2 bile acid-adapted derivative strains A1dOx and A1dOxR, elicited different cytokine patterns. EPSs from A1dOxR elicited lower production of cytokines in human PBMCs, induced significantly lower levels of pro-inflammatory cytokines (IL-6 and IL-8) in Caco2 cells, and promoted the balance of Treg/Th17 when compared with EPSs isolated from the other two strains [25]. Interestingly, only EPSs isolated from A1dOxR contain a HMW (3.5 × 106Da) fraction,and the other two fractions—middle weight (3.0 × 104Da) and low weight (4.9 × 103Da) EPSs—exist in all three strains [94,95].
As T cell-independent antigens, most CPSs cannot be processed and presented by MHC molecules. ZPSs fromB. fragilisappear to be a special example of how carbohydrates induce immune responses.ZPSs, containing both a positively and a negatively charged motif in each repeating unit, have been shown to be processed and presented by the MHC II pathway [96]. ZPSs are taken into the endosome of the APCs, where their molecular size is reduced by NO. Moreover,ZPSs follow the same MHC II vesicular pathway as conventional protein antigens before being presented to the αβ TCR through the MHC II molecule. Mazmanian et al. reviewed that the specific activation of T cells by the zwitterionic PSA ofB. fragilisis as follows: first, similar to protein antigens, PSAs travel through the endocytic pathway after internalization by APCs, and then co-localize with the endosomal markers lysosomal-associated membrane protein 1 (LAMP1) and HLA-DM in a process that requires the polymerization of actin and microtubules and the acidification of endosomes; second, the HMW molecules of PSA are processed into smaller fragments after internalization into endosomes, which is a chemical reaction that involves oxidation; third, TCRs are required for responses to PSA, and a tertiary complex between PSA, an MHC class II molecule, and a TCR might mediate the activity of PSAs; last,PSA molecules bind to purified MHC class II molecules in a manner that requires the peptide-exchange factor HLA-DM [21,28]. However,Erturk-Hasdemir et al. [82]recently found that the anti-inflammatory role of PSA depends on its anchor to cell surface lipids. The structural analysis ofB. fragilisPSA showed that PSA contains a hydrophobic lipid anchor, which belongs to lipid A family with great structural variability. They also proposed that the lipid part of PSA activates the TLR-2/1 while the carbohydrate portion of PSA binds to Dectin-1, both of which initiates the activation of PI3K. When the APCs were stimulated with the delipidated PSA, the immuno-stimulatory effects were diminished and the production IL-10 by CD4+T cells was not sufficient.
Some studies have suggested that the composition of monosaccharides and their proportion to polysaccharides may be related to their immunomodulatory effects. The HMW-EPS A1dOxR mentioned above and the polysaccharide RW-9595M fromL. rhamnosushave a similar immunosuppressive action [92]and both contain > 50% rhamnose repeating units [93,94]. In addition, a recent study has shown that the galactose content of EPSs (galactose >rhamnose > glucose) synthesized byL. reuterienhances their anti-inflammatory effects on macrophages [97]. These findings indicate that the immunomodulatory functions of the polysaccharides with negative charge or zwitterion and MW of the polysaccharides have been clearly studied; however, the monosaccharide composition and proportion of polysaccharides and their immunosuppressive activities have been not sufficiently reported.
5. Outlooks
Polysaccharides synthesized by probiotics andBacteroidesare highly diverse and their applications are gradually expanding into the medical and pharmaceutical fields because of their potential immunomodulatory abilities. The immunomodulatory effects of most polysaccharides, such as the alleviation of colitis and viral encephalitis, have been demonstrated in animal models; however, the structure and functional links as well as the underlying mechanisms remain to be determined. Therefore, it is essential to establish rapid screening methods and correlations between the structure, physical properties, and health benefits of polysaccharides. In addition, the safety issue of these polysaccharides is another critical question to be answered before they can be widely used as functional food or medicine. Recently, ZPSs inBacteroideshave been recognized as effective polysaccharide compounds by which to treat or alleviate various inflammation-associated diseases [20,21]. However, PSA molecules ofBacteroideswere previously shown to be pathogenic when injected into the abdominal cavity of mice [98]. When microbial polysaccharides with anti-inflammatory effects are developed as drugs, the risk to patients with poor intestinal barrier function should be considered. Therefore, the safety of microbial polysaccharides must be evaluated in additional studies.
conflicts of interest
The authors declare that there is no conflict of interest.
Acknowledgements
This work was supported by the National Natural Science Foundation of China Program [31871773 and 31820103010]; the Key Scientific and Technological Research Projects in the Key Areas of the Xinjiang Production and Construction Corps [2018AB010];National First-Class Discipline Program of Food Science and Technology [JUFSTR20180102]; the BBSRC Newton Fund Joint Centre Award; and Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province.
杂志排行
食品科学与人类健康(英文)的其它文章
- Hypoglycemic natural products with in vivo activities and their mechanisms: a review
- Bacteroides utilization for dietary polysaccharides and their beneficial effects on gut health
- Natural compounds may contribute in preventing SARS-CoV-2 infection: a narrative review
- A comprehensive review on the effects of green tea and its components on the immune function
- A review on current and future advancements for commercialized microalgae species
- Effects of silkworm pupa protein on apoptosis and energy metabolism in human colon cancer DLD-1 cells
