Biliary metal stents should be placed near the hilar duct in distal malignant biliary stricture patients
2022-06-11MitsuruSugimotoTadayukiTakagiReiSuzukiNaokiKonnoHiroyukiAsamaYukiSatoHirokiIrieYoshinoriOkuboJunNakamuraMikaTakasumiMinamiHashimotoTsunetakaKatoRyoichiroKobashiTakumiYanagitaTakutoHikichiHiromasaOhira
Mitsuru Sugimoto,TadayukiTakagi,Rei Suzuki, Naoki Konno, Hiroyuki Asama, Yuki Sato, Hiroki Irie,Yoshinori Okubo, Jun Nakamura,Mika Takasumi, Minami Hashimoto,Tsunetaka Kato,Ryoichiro Kobashi,Takumi Yanagita,Takuto Hikichi,Hiromasa Ohira
Abstract
Key Words: Endoscopic biliary drainage; Malignant biliary obstruction; Uncovered self-expandable metallic stent; Covered self-expandable metallic stent; Biliary hilar duct; Patency period
INTRODUCTION
Stricture of the common bile duct (CBD) can occur in several severe diseases (for example, bile duct cancer, pancreatic cancer, or metastasis of other cancers). Since transpapillary biliary stent insertion was first reported by Sohendra and Reynders-Frederix[1 ], it has become the first choice for biliary drainage in patients with malignant biliary obstruction. At present, uncovered self-expandable metallic stents(USEMSs) and covered SEMSs (CSEMSs) have been reported to be more effective at preventing recurrent biliary obstruction (RBO) than plastic stents (PSs) in distal malignant biliary obstruction(DMBO) patients[2 ,3 ].
Whether a USEMS or CSEMS should be used remains a topic of debate. Three reports have asserted that the patency period of CSEMSs is superior to that of USEMSs[4 -6 ]. However, others have found that the patency period is similar between CSEMSs and USEMSs[7 -9 ]. Although CSEMS insertion has some disadvantages (such as cholecystitis, pancreatitis, and migration), the stent can be removed[7 ,10 -13 ].
Based on the above findings, SEMS placement may help drain unresectable DMBOs. Determining which stent (USEMS or CSEMS) should be used has gained increasing attention. However, the optimal position of the inserted SEMS has rarely been discussed and remains unclear. Therefore, we aimed to determine the ideal position for SEMS placement.
MATERIALS AND METHODS
Study design and ethics approval
This was a retrospective study. The patients were not required to provide informed consent because this study used anonymized clinical data obtained after each patient had provided written consent and agreed to undergo medical procedures. Additional details of this study are published on the home page of Fukushima Medical University (approval number 2453 ).
Patients
A total of 135 DMBO patients underwent SEMS placement between January 2011 and February 2021 (Figure 1 ). These patients did not undergo previous surgery of the upper gastrointestinal tract and were undergoing SEMS placement for the first time. Seven of these patients whose biliary obstruction was located between the junction of the cystic duct and hilar bile duct were excluded from this study. In addition, one patient who underwent double SEMS placement was excluded. Finally, 127 patients whose biliary obstruction was located between the junction of the cystic duct and Vater’s papilla were enrolled. The SEMS was placed through the upper CBD within 2 cm from the junction of the right and left hepatic ducts in 83 patients (Hilar group) (Figure 2 A and B). In the other 44 patients (Lower group),the SEMS was placed near the top of the biliary obstruction (Figure 2 C and D).
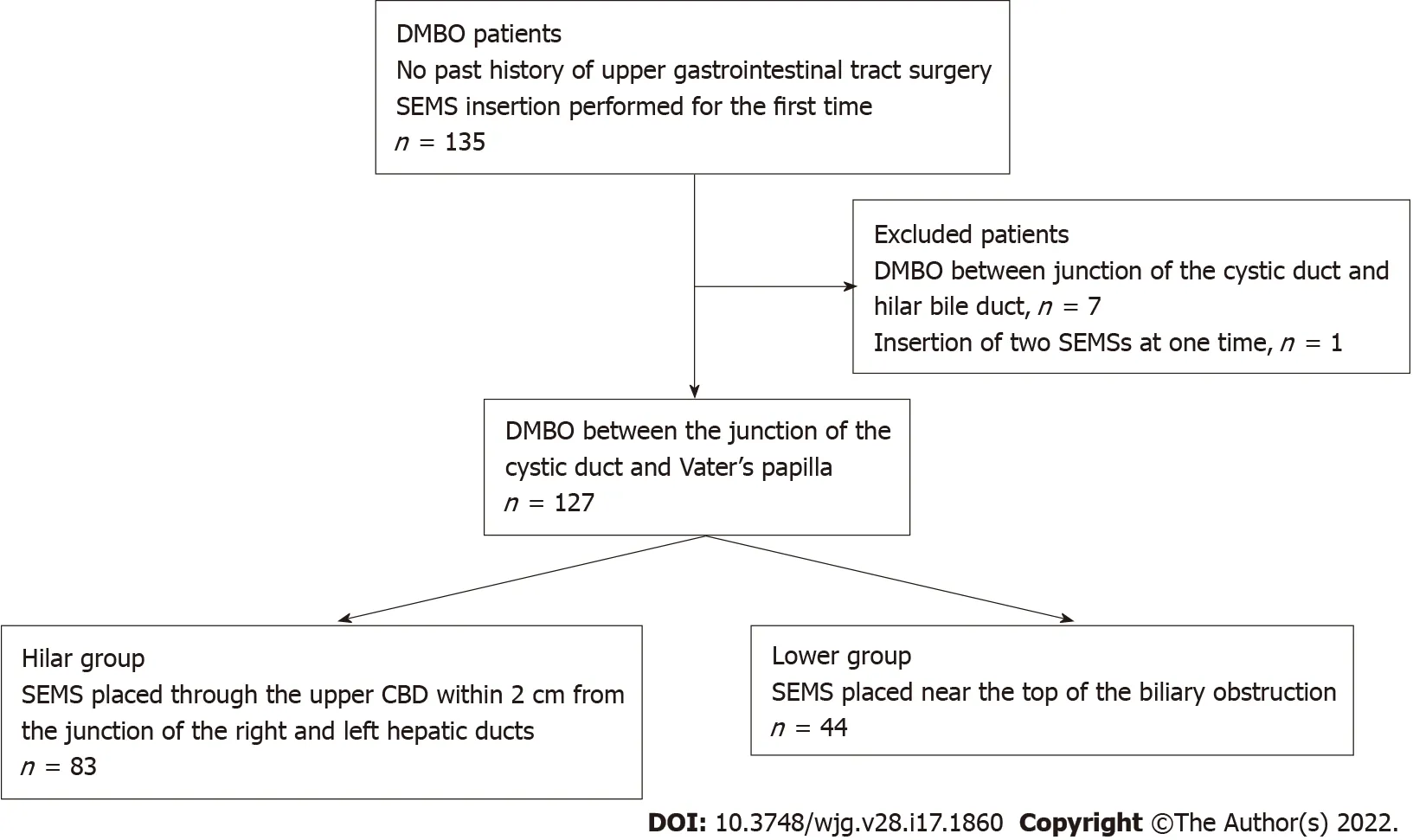
Figure 1 Patient flowchart. DMBO: Distal malignant biliary obstruction; SEMS: Self-expandable metallic stent; CBD: Common bile duct.
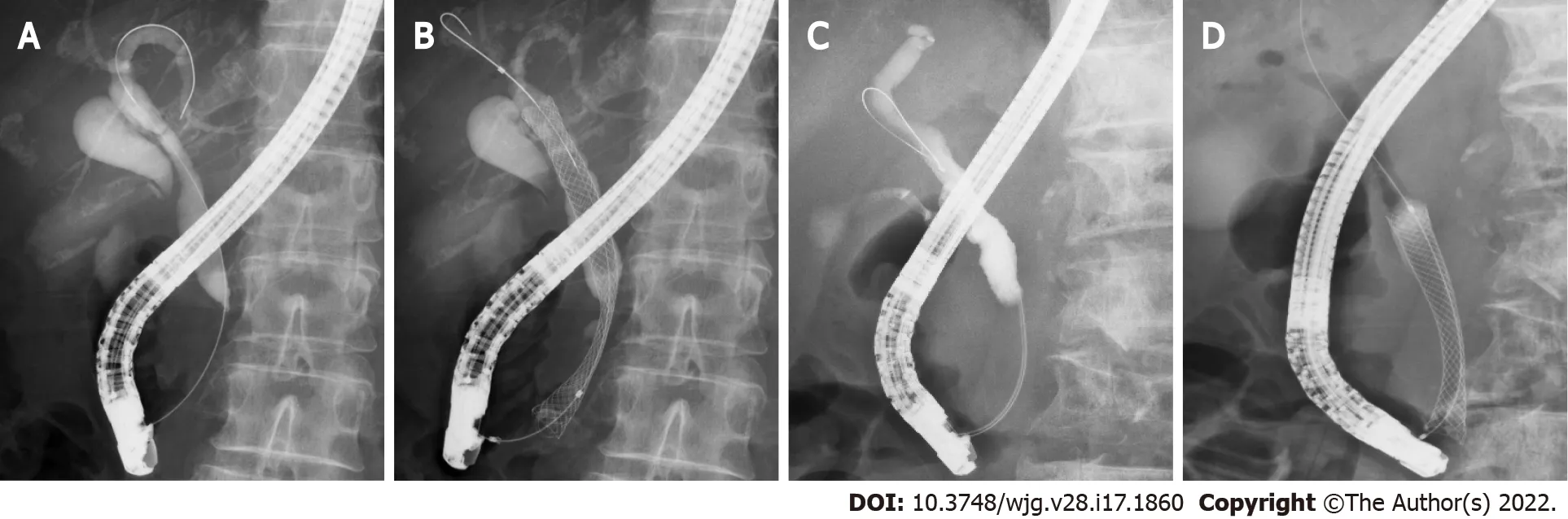
Figure 2 Representative cases from each group. A and B: A patient with distal malignant biliary obstruction (DMBO) in the Hilar group who underwent selfexpandable metallic stent (SEMS) placement near the biliary hilar duct; C and D: A patient with DMBO in the Lower group who underwent SEMS placement near the top of the biliary obstruction.
Endoscopic biliary drainage
With the patient in a prone position, a duodenoscope was inserted after the patient was sufficiently sedated with midazolam. When the duodenoscope reached Vater’s papilla, biliary cannulation was initiated. After the range of the DMBO was confirmed by cholangiography, an SEMS was inserted from the upper part of the obstruction to Vater’s papilla. Endoscopic sphincterotomy (EST) was performed for first-time endoscopic biliary drainage with a PS or before SEMS insertion. The position and type of SEMS (USEMS or CSEMS) were randomly determined by each endoscopist. All the procedures were performed by pancreaticobiliary specialists or trainees under the guidance of specialists.
The USEMSs used in this study were as follows: BileRush, 8 mm × 6 cm, 10 mm × 6 or 8 cm (Piolax,Kanagawa, Japan); Bonastent, 10 mm × 8 cm (Standard Sci Tech, Seoul, Korea); HANARO, 10 mm × 7 cm (Boston Scientific, Tokyo, Japan); Niti-S Large cell, 10 mm × 5 , 6 , 8 , or 10 cm (Taewoong Medical,Gyeoenggi-do, Korea); WallFlex, 10 mm × 6 , 8 , or 10 cm (Boston Scientific); X Suit NIR, 10 mm × 8 cm(Olympus Medical, Tokyo, Japan); and Zilver, 10 mm × 6 cm, and Zilver 635 , 10 mm × 6 , 8 , or 10 cm(Cook Medical Japan, Tokyo, Japan). The CSEMSs used in this study were as follows: Bonastent, 10 mm× 7 cm (Standard Sci Tech); HANARO, 10 mm × 5 , 6 , or 8 cm (Boston Scientific); Niti-S Comvi, partially covered, 10 mm × 6 , 7 , or 8 cm (Taewoong Medical); WallFlex, fully covered, 10 mm × 6 cm, and partially covered, 10 mm × 6 or 8 cm (Boston Scientific); and X Suit NIR, 10 mm × 4 , 6 , or 8 cm (Olympus Medical).
Outcomes of interest
The primary outcome was the stent patency period. The secondary outcomes were the technical success rate, functional success rate, adverse events (pancreatitis, post-EST bleeding), severity of adverse events,and stent dysfunction rate. These outcomes were defined according to partially revised versions of the reported criteria[14 ]. The stent patency period was determined as the time from first SEMS insertion to SEMS dysfunction. SEMS dysfunction was defined as the recurrence of hepatic dysfunction, jaundice, or dilated bile tract on ultrasonography or computed tomography (CT), which required secondary SEMS placement. Technical success was defined as successful placement of an SEMS that reached from the upper part of the obstruction to Vater’s papilla. Functional success was defined as the return of alanine transaminase (ALT) or total bilirubin (TB) levels to normal values (ALT < 27 U/L, TB < 1 .2 mg/dL) or less than half of the pretreatment values. Adverse events and the severity of adverse events were defined according to Cotton’s criteria[15 ]. Posttreatment pancreatitis was also confirmed by contrastenhanced CT.
In addition, the patient characteristics (age, sex, serum ALT level, serum TB level, cause of stricture,chemotherapy, duodenal stricture, CBD diameter above the stricture, CBD stricture diameter, CBD stricture length), year of procedure (2011 -2015 , or 2016 -2021 ), stent diameter, type of SEMS used(USEMS or CSEMS), SEMS shortening, and observational period were compared between the Hilar group and the Lower group. The maximum serum ALT and TB values recorded in the previous week up to endoscopic SEMS insertion were used. The cause of stricture was divided into pancreaticobiliary and metastatic. Duodenal stricture was defined as a stricture that was difficult for the upper gastrointestinal scope to pass through. The diameter and length of the CBD stricture were measured by endoscopic retrograde cholangiography. The year of the procedure was compared between the groups because the techniques and devices have advanced over the approximately ten-year study duration.SEMS shortening was determined as more than 1 cm of shortening evident on X-ray or CT imaging after SEMS placement.
Statistical analyses
Student’s t test or Welch’s t test was used to compare continuous variables. Fisher’s exact test was used to compare nominal variables. To analyze the SEMS patency period, the log-rank test was used. To analyze the factors that influenced SEMS dysfunction, a Cox proportional hazard model was used.P<0 .05 was considered statistically significant. All statistical analyses were performed using EZR (Saitama Medical Centre, Jichi Medical University, Saitama, Japan).
RESULTS
The patient characteristics are shown in Table 1 . There was no significant difference in age, sex, serum ALT level, serum TB level, stricture cause, chemotherapy, duodenal stricture, CBD diameter above the stricture, CBD stricture diameter, or CBD stricture length between the Hilar group and the Lower group.
The outcomes of SEMS placement are shown in Table 2 . There was no difference in the procedure year, technical success rate, functional success rate, adverse events, or SEMS shortening between the two groups. The rate of CSEMS use and the SEMS diameter were also not significantly different between the two groups. Regarding the type of SEMS used, the covered WallFlex (Boston Scientific) stent was used significantly more frequently in the Hilar group than in the Lower group (28 /83 (33 .7 %) vs 7 /44 (15 .9 %),Pvalue = 0 .038 ), and the X Suit NIR stent was used significantly more frequently in the Lower group than in the Hilar group (6 /44 (13 .6 %) vs 0 /83 (0 %), P value < 0 .01 ). SEMS dysfunction was observed significantly more often in the Lower group than in the Hilar group [18 /44 (41 %) vs 2 /83 (2 .4 %),Pvalue< 0 .01 ]. The causes of SEMS dysfunction were as follows: Ingrowth (1 ) and overgrowth (1 ) in the Hilar group, and ingrowth (3 ), overgrowth (2 ), ingrowth and overgrowth (8 ), top edge closed by the CBD wall(4 ), and dislocation (1 ) in the Lower group. In the cases in which the top edge was closed by the CBD wall, the SEMSs used were the Zilver 635 (Cook Medical), WallFlex (Boston Scientific), Niti-S large cell(Taewoong Medical), and HANARO (Boston Scientific) stents. A representative case in which the top edge of the SEMS was closed by the CBD wall is shown in Figure 3 . The observational period was longer in the Lower group than in the Hilar group (9 .12 ± 12 .07 mo vs 4 .16 ± 5 .76 mo, P value = 0 .012 ).
The results of the stent patency comparison are shown in Figure 4 and Supplementary Figure 1 . The stent patency period was significantly longer in the Hilar group than in the Lower group (Figure 4 A,Pvalue < 0 .01 ). The stent patency period was not significantly different between the groups when the patients were divided according to the use of a covered WallFlex stent, use of a covered X Suit NIR stent, observational period (Figure 4 B-D), age, sex, serum ALT level, serum TB level, metastatic or pancreaticobiliary status, presence or absence of chemotherapy, presence or absence of duodenal stricture, CBD diameter above the stricture, CBD stricture diameter, CBD stricture length, year of procedure, USEMS or CSEMS, presence or absence of SEMS shortening (Supplementary Figure 1 ).
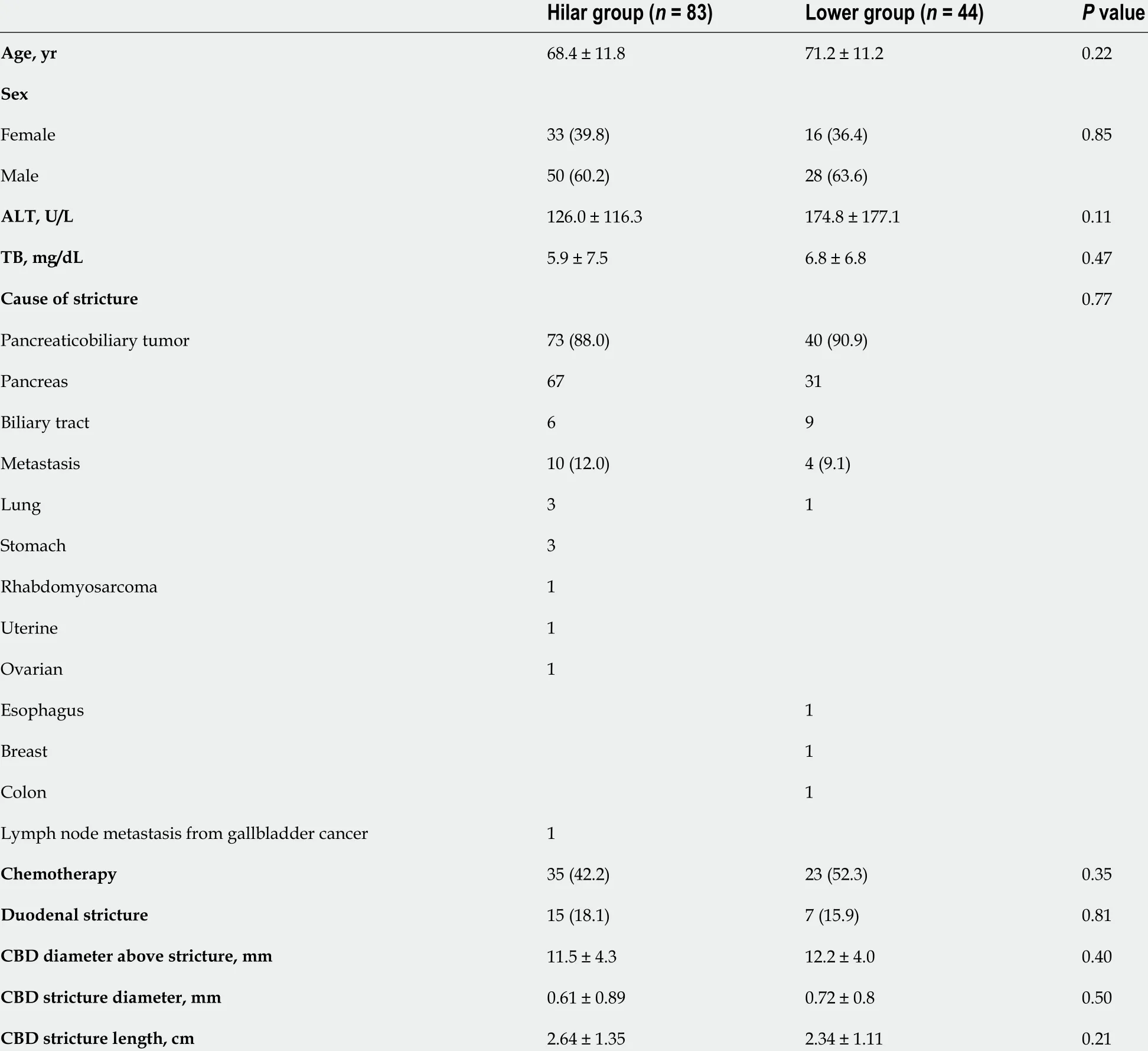
Table 1 Patient characteristics
The risk factors for SEMS dysfunction are shown in Table 3 . Serum ALT level and lower placement were statistically significant factors in the univariate analysis [ALT: hazard ratio (HR): 1 .003 , 95 %confidence interval (CI): 1 .001 -1 .01 , P value < 0 .01 ; Lower group: HR: 11 .42 , 95 %CI: 2 .61 -49 .83 ,Pvalue< 0 .01 ]. However, the only statistically significant risk factor in the multivariate analysis was lower placement (HR: 9 .94 , 95 %CI: 2 .25 -44 .0 , P < 0 .01 ).
DISCUSSION
In this study, we investigated the ideal position for SEMS insertion in DMBO patients. The results demonstrated that the SEMS patency period was longer when the stent was placed near the hilar duct.
This finding suggests that this position overcomes several causes of SEMS dysfunction. As shown in Table 2 , the main causes of SEMS dysfunction were tumor ingrowth and/or overgrowth and a top edge closed by the CBD wall; notably, overgrowth and a top edge closed by the CBD wall were prevented by using a longer SEMS. Longer SEMSs can delay stent dysfunction due to tumor overgrowth. In the four patients in the Lower group, the top edge of the SEMS was closed by the CBD wall, which may be caused by linearization of the SEMS. The axial force on the stent is thought to be related to the linearization and closing of the top edge by the CBD wall. However, in the four patients with SEMS dysfunction caused by closure of the top edge by the CBD wall, the SEMSs were not necessarily affectedby high axial force, except for the WallFlex (Boston Scientific) stent. When a short SEMS is placed near the top edge of the DMBO, the axial force might be enhanced by the biliary stricture. Using longer SEMSs overcomes this problem because the axial force decreases with increasing distance between the top edge of the SEMS and CBD stricture[16 ]. In fact, a biliary obstruction was relieved by placing a second SEMS near the biliary hilar duct (Figure 3 C).
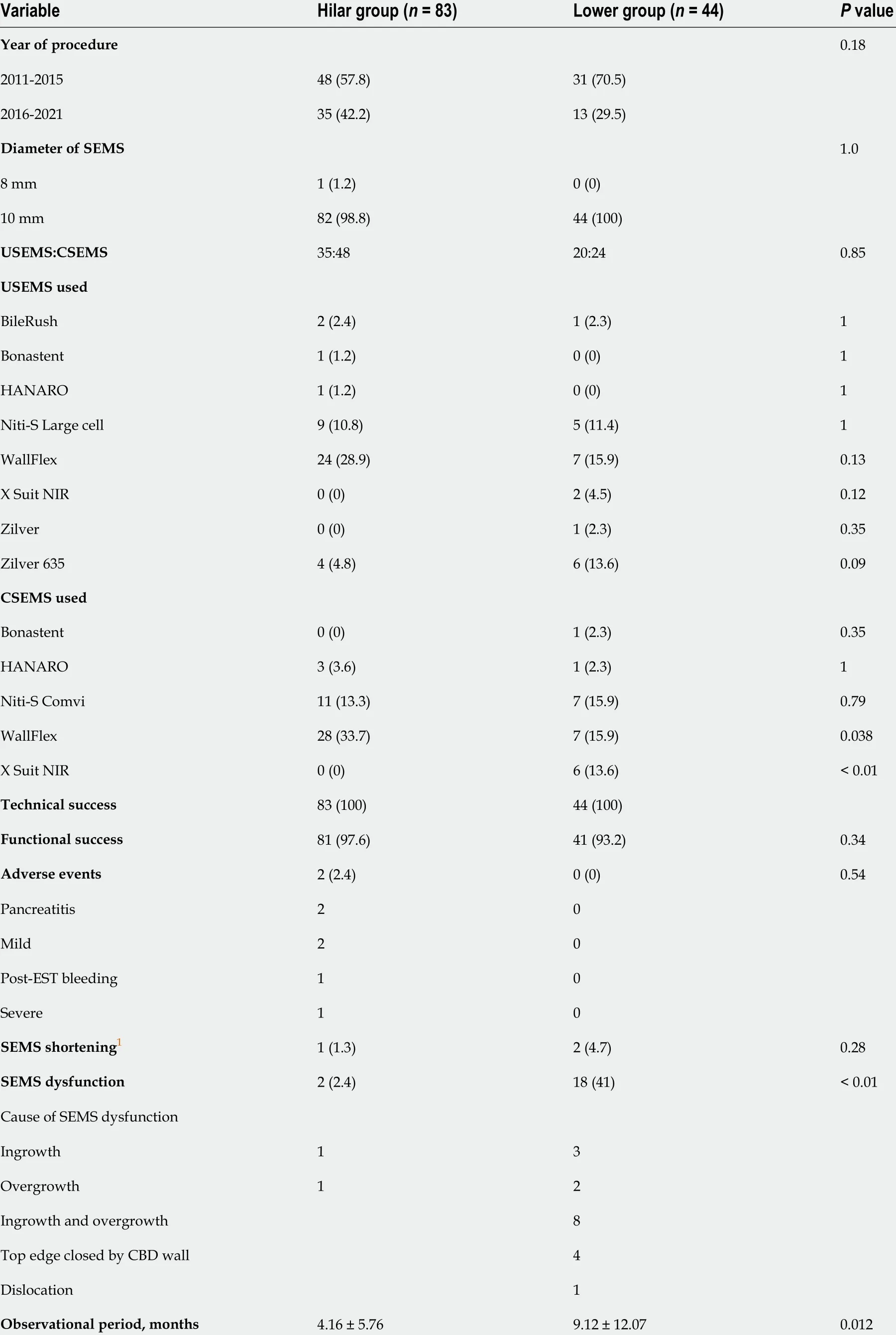
Table 2 Outcome of biliary self-expandable metallic stent placement
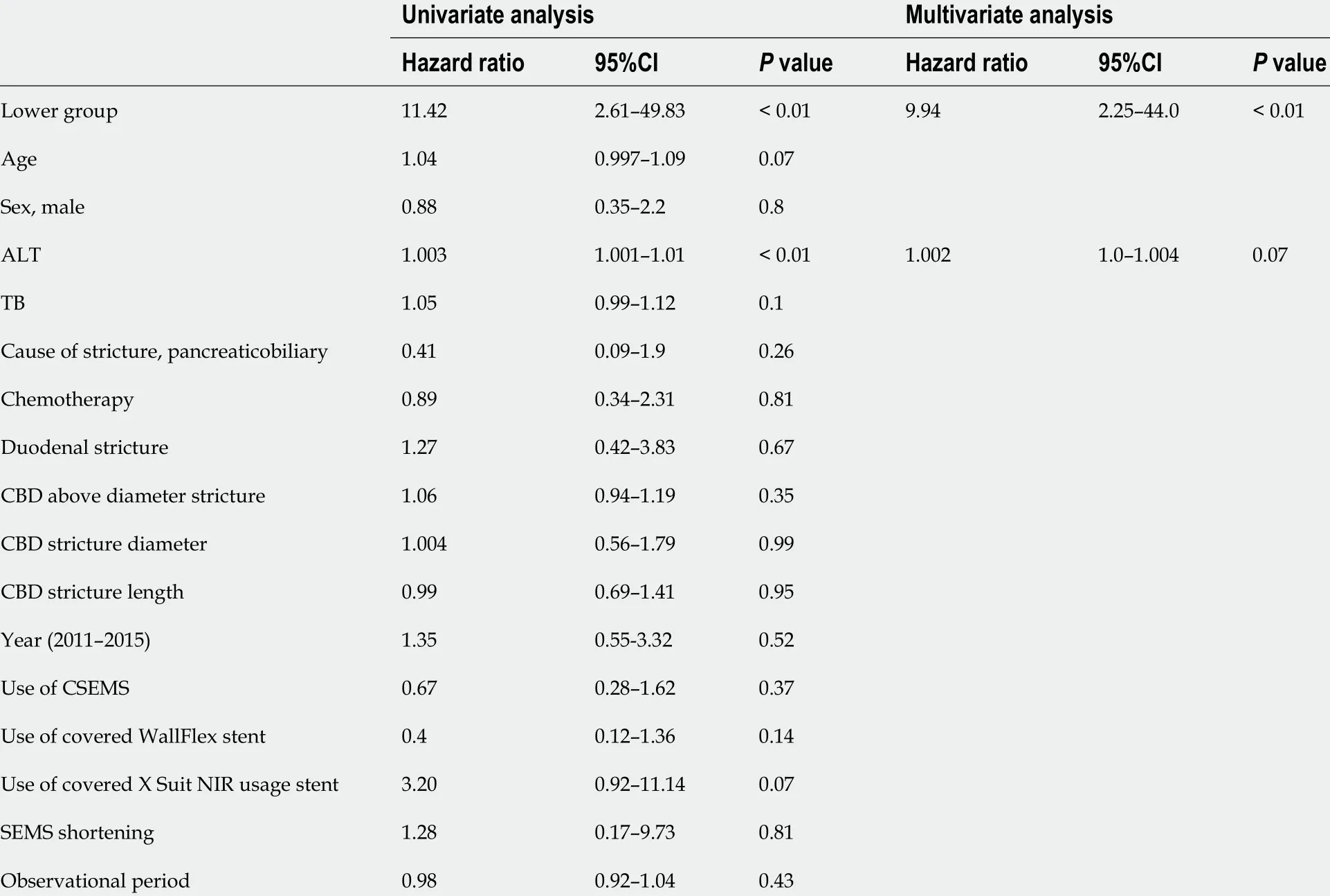
Table 3 Risk factors for self-expandable metallic stent dysfunction
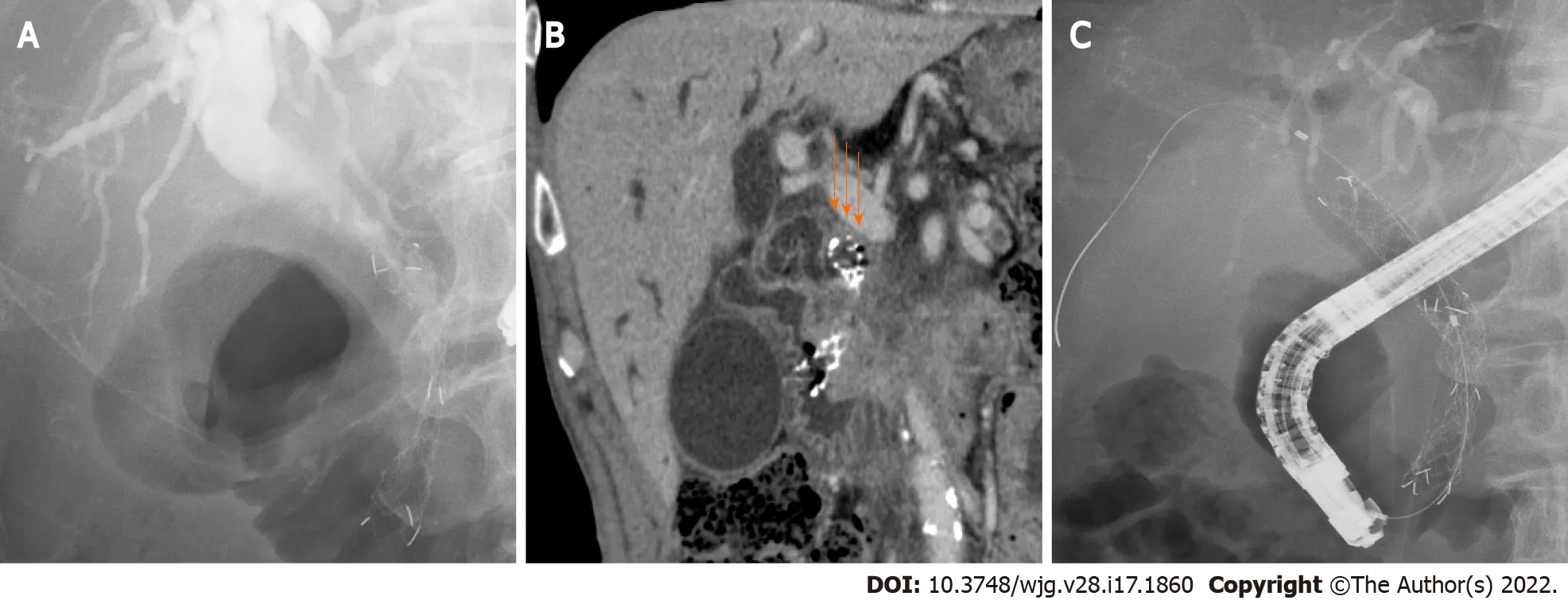
Figure 3 A patient with closure of the top edge of the self-expandable metallic stent by the common bile duct wall. A: A patient with distal malignant biliary obstruction who underwent uncovered self-expandable metallic stents (USEMS) placement near the top of the biliary obstruction; B: The top edge of the SEMS was closed by the common bile duct wall (arrows). Upper bile tract dilation was observed; C: An additional USEMS was placed near the biliary hilar duct.
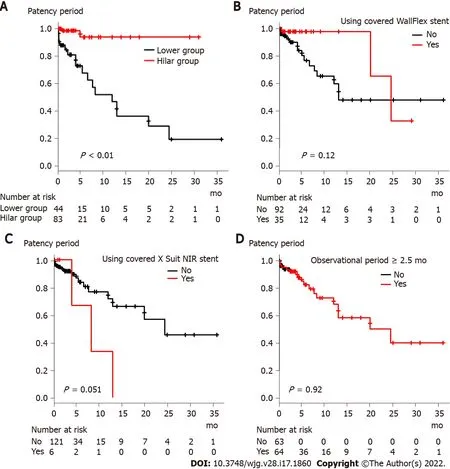
Figure 4 Comparison of stent patency period based on factors that were significantly different between the Hilar group and Lower group.A: Stent placement position (Hilar group vs Lower group); B: Use of the covered WallFlex stent; C: Use of the covered X Suit NIR stent; D: Observational period (<2 .5 mo vs ≥ 2 .5 mo).
In past reports, time to adequate expansion, degree of CBD stricture[17 ], duodenal invasion[18 ],duodenal SEMS[19 ], and anticancer treatment[18 ,20 ] were reported as risk factors for RBO. The factors related to SEMS expansion and CBD stricture were not an issue in this study because functional success was achieved in almost all the patients. Anticancer treatment has been reported to cause RBO as follows.Although anticancer treatment reduces the tumor burden, it can dislocate the CSEMS or induce neutropenia and bacterial overgrowth and, ultimately, cholangitis or sludge formation in the bile duct[20 ]. However, anticancer treatment was not proposed as a risk factor for RBO in a study that involved patients with a USEMS[21 ]. This study involved both USEMSs and CSEMSs. Therefore, anticancer treatment may not be a risk factor for SEMS dysfunction. Duodenal invasion from tumors reduces peristalsis and causes food impaction in the biliary duct, and a duodenal SEMS prevents the outflow of bile juice[19 ]. In this study, any RBO requiring additional SEMS placement was defined as SEMS dysfunction so that SEMS occlusion caused by tumors could be properly evaluated and cases of SEMS occlusion by food impaction could be excluded. Therefore, SEMS placement near the biliary hilar duct was revealed as a new factor related to longer SEMS patency.
There were some limitations to this study. First, this was a retrospective observational study performed at a single institution. In the future, it is hoped that a prospective multicenter study will confirm our findings. Second, the type of SEMS was not unified. The axial force or shortening length varied among the SEMSs. Measurement of the axial force was difficult in this study; instead, different kinds of SEMSs were compared. As a result, the type of SEMS did not influence SEMS dysfunction. The WallFlex stent (Boston Scientific), which has a high axial force and a high shortening rate[22 ], was used significantly more often in the Hilar group. However, remarkable shortening was rarely observed (the presence or absence of shortening was confirmed in 23 patients 24 h after SEMS placement and in 99 patients more than 48 h after SEMS placement). This was likely due to the placement of an SEMS with a longer than established length because the SEMS could not fully expand in the area of the stricture. As described above, the axial force decreases with increasing SEMS length. Because of these factors, the difference in the type of SEMS did not influence the outcomes. Third, SEMS obstruction of sludge or food debris was not considered SEMS dysfunction. In past reports, sludge formation has been proposed to be a cause of SEMS dysfunction[4 ,5 ,21 ]. This factor is surely important for comparisons of patency periods between USEMS and CSEMS. If SEMS obstruction of sludge or food debris was considered stent dysfunction, the patency period was also significantly longer in the Hilar group than in the Lower group (Supplementary Figure 2 ). Therefore, the obstruction of sludge or food debris did not influence the results of this study. As described above, the SEMS obstruction of sludge or food debris was excluded from SEMS dysfunction to properly evaluate the relationship between the positions of the SEMS and tumor in this study.
CONCLUSION
The results of our study revealed that placement of an SEMS near the biliary hilar duct could delay tumor overgrowth and prevent closure of the top edge of the SEMS by the CBD wall. Thus, in DMBO patients, the SEMS should be placed near the biliary hilar duct to achieve a longer patency period.
ARTICLE HIGHLIGHTS

ACKNOWLEDGEMENTS
We thank the entire staff at the Department of Gastroenterology of Fukushima Medical University, the Department of Endoscopy of Fukushima Medical University Hospital, and the gastroenterology ward of Fukushima Medical University Hospital.
FOOTNOTES
Author contributions:Sugimoto M wrote the paper and designed and performed the study; Takagi T and Ohira H designed and oversaw the study; Suzuki R, Konno N, HA, Hikichi T, Nakamura J, Takasumi M, Sato Y, Irie H,Okubo Y, Hashimoto M, Kato T, Kobashi R, and Yanagita T provided clinical advice; and all the authors read and approved the final version of the manuscript.
Institutional review board statement:This study was approved by the Institutional Review Board of Fukushima Medical University, No. 2453 .
Informed consent statement:The patients were not required to give informed consent because this study used anonymous clinical data obtained after each patient had agreed to medical activities by written consent. For full disclosure, the details of this study are published on the home page of Fukushima Medical University.
Conflict-of-interest statement:The authors declare that they have no competing interests.
Data sharing statement:The datasets analyzed during the current study are available from the corresponding author upon reasonable request.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4 .0 ) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4 .0 /
Country/Territory of origin:Japan
ORCID number:Mitsuru Sugimoto 0000 -0002 -4223 -613 X; Tadayuki Takagi 0000 -0003 -0696 -5973 ; Rei Suzuki 0000 -0002 -4049 -0484 ; Naoki Konno 0000 -0001 -9830 -4317 ; Hiroyuki Asama 0000 -0002 -0102 -0404 ; Yuki Sato 0000 -0001 -8000 -0972 ;Hiroki Irie 0000 -0002 -4805 -6244 ; Yoshinori Okubo 0000 -0003 -0567 -3805 ; Jun Nakamura 0000 -0001 -6006 -1778 ; Mika Takasumi 0000 -0002 -6025 -8084 ; Minami Hashimoto 0000 -0002 -5750 -7182 ; Tsunetaka Kato 0000 -0002 -2529 -2463 ; Ryoichiro Kobashi 0000 -0003 -0991 -6042 ; Takumi Yanagita 0000 -0002 -1236 -857 X; Takuto Hikichi 0000 -0002 -9815 -1557 ; Hiromasa Ohira 0000 -0003 -4331 -0634 .
S-Editor:Fan JR
L-Editor:A
P-Editor:Fan JR
杂志排行
World Journal of Gastroenterology的其它文章
- Current status and future of targeted peptide receptor radionuclide positron emission tomography imaging and therapy of gastroenteropancreatic-neuroendocrine tumors
- Clinical outcomes of endoscopic papillectomy of ampullary adenoma: A multi-center study
- New insights in diagnosis and treatment of gastroenteropancreatic neuroendocrine neoplasms
- Gut homeostasis, injury, and healing: New therapeutic targets
- Sirtuin1 attenuates acute liver failure by reducing reactive oxygen species via hypoxia inducible factor 1 α
- Peroxisome proliferator-activated receptor-alpha activation and dipeptidyl peptidase-4 inhibition target dysbiosis to treat fatty liver in obese mice
