血小板与淋巴细胞比值对前列腺癌预后的临床价值
2022-05-30孙照勇,张桂铭,刘勇
孙照勇,张桂铭,刘勇
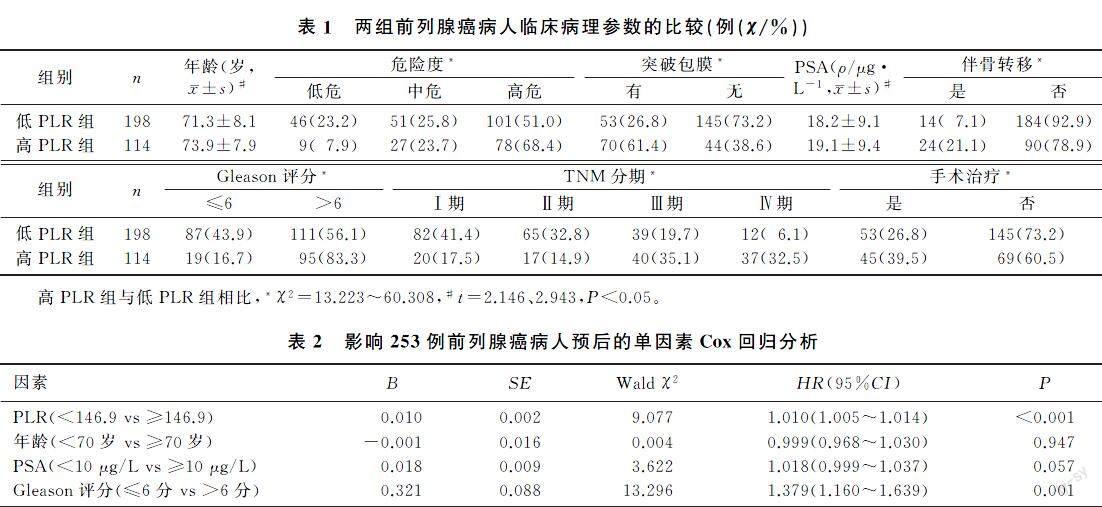
[摘要]目的 探討血小板与淋巴细胞比值(PLR)对前列腺癌预后的价值。 方法 回顾性分析2012年9月—2015年12月于青岛大学附属医院首次确诊的312例前列腺癌病人的临床资料。根据术前外周静脉血检查结果计算PLR,将病人分为高PLR组(PLR≥146.9)和低PLR组(PLR<146.9),比较两组病人的临床病理参数,使用Kaplan-Meier生存曲线分析PLR对前列腺癌病人预后的影响,采用Cox比例风险模型分析前列腺癌病人的预后影响因素。 结果 与低PLR组相比,高PLR组病人年龄大、危险度高、前列腺特异抗原水平高、肿瘤侵犯范围大、Gleason评分高、肿瘤分期晚(χ2=13.223~60.308,t=2.146、2.943,P<0.05)。Kaplan-Meier生存曲线分析显示,低PLR组病人总生存率明显高于高PLR组(χ2=23.083,P<0.05)。多因素Cox回归分析显示,PLR是前列腺癌病人的独立预后因素(HR=1.008,95%CI=1.001~1.014,P<0.05)。结论 术前外周血PLR越高,前列腺癌恶性程度越高,预后越差。
[关键词]前列腺肿瘤;血小板与淋巴细胞比值;预后
[中图分类号]R737.25
[文献标志码]A
[文章编号]2096-5532(2022)04-0559-04
doi:10.11712/jms.2096-5532.2022.58.072[HT]
[开放科学(资源服务)标识码(OSID)]
[网络出版]https://kns.cnki.net/kcms/detail/37.1517.r.20220412.1535.003.html;[JY]2022-04-1419:53:08
CLINICAL VALUE OF PLATELET/LYMPHOCYTE RATIO IN THE PROGNOSIS OF PROSTATE CANCER
SUN Zhaoyong, ZHANG Guiming, LIU Yong
(Department of Urology, Caoxian Peoples Hospital, Caoxian 274400, China)
[ABSTRACT]Objective[WTBZ] To investigate the clinical value of platelet/lymphocyte ratio (PLR) in the prognosis of prostate cancer.
Methods A retrospective analysis was performed for the clinical data of 312 patients with prostate cancer who were first diagnosed in Affiliated Hospital of Qingdao University from September 2012 to December 2015. The PLR value was calculated according to the preoperative peripheral venous blood test results, and the patients were divided into high PLR group (PLR ≥146.9) and low PLR group (PLR<146.9). The clinicopathological parameters were compared between the two groups, the Kaplan-Meier survival curve was used to analyze the prognostic effect of PLR on prostate cancer patients, and the Cox proportional hazards model was used to analyze the prognostic factors of prostate cancer patients.
Results Compared with the low PLR group, the high PLR group had older age, higher risk, higher prostate-specific antigen level, larger tumor invasion extent, higher Gleason score, and more advanced tumor stage (χ2=13.223-60.308;t=2.146,2.943;P<0.05). The Kaplan-Meier curve analysis showed that the overall survival rate of patients in the low PLR group was significantly higher than that in the high PLR group (χ2=23.083,P<0.05). The multivariate Cox regression analysis showed that PLR was an independent prognostic factor for prostate cancer patients (HR=1.008,95%CI=1.001-1.014,P<0.05).
Conclusion A higher preoperative PLR in peripheral blood is associated with higher malignancy of prostate cancer and worse prognosis.
[KEY WORDS] prostatic neoplasms; platelet/lymphocyte ratio; prognosis
前列腺癌是世界上男性第二大癌症,也是全世界男性与癌症相关死亡的第五大原因[1]。我国前列腺癌的患病率远低于欧洲和美国,但随着人们预期寿命的增加和前列腺癌早期诊断的进步,前列腺癌的患病率和病死率显著上升[2]。早期诊断对于前列腺癌的治疗尤为重要。近年来越来越多的证据表明,炎症可能在前列腺癌的发生和发展中起主要作用[3-4]。已有研究结果表明,血清中性粒细胞计数低可以预测前列腺癌活检阳性,中性粒细胞与淋巴细胞比值(NLR)可以作为前列腺癌病人的独立预后标志物[5-6]。与NLR相类似,血小板与淋巴细胞比值(PLR)也是系统炎症的参数。大量研究表明,高PLR可独立预测肿瘤(包括胰腺癌、卵巢癌、结直肠癌、非小细胞肺癌、肝细胞癌、肾细胞癌、食管癌等)病人预后不良[7-12]。但PLR与前列腺癌之间关系的研究较少,故本研究通过回顾性分析312例前列腺癌病人的临床资料,来探讨前列腺癌病人PLR检测的临床意义。
1资料和方法
1.1研究对象
选取2012年9月—2015年12月于青岛大学附属医院泌尿外科首次确诊的前列腺癌病人312例,对其临床资料进行回顾性分析。纳入标准:①均行B超引导下前列腺穿刺活检术;②病理检查证实为前列腺癌。排除标准:①合并免疫系统疾病、服用免疫抑制剂病人;②合并其他肿瘤;③合并急性或慢性感染;④患有脾病或凝血功能障碍。收集病人年龄、术前血清前列腺特异抗原(PSA)、术前血常规、术前MR检查、骨扫描结果、手术病理或穿刺病理结果等临床资料。98例病人接受了前列腺癌根治术,其余214例病人接受了内分泌治疗或放化疗。治疗后定期电话随访,最后一次随访时间在2018年12月。本研究经青岛大学附属医院伦理委员会批准,研究对象均签署知情同意书。
1.2研究方法
收集病人的血常规指标,获取血小板绝对值和淋巴细胞绝对值,计算PLR。建立受试者工作特征(ROC)曲线,计算PLR预测前列腺癌高危风险的最佳临界点。根据截断值将病人分为高PLR组和低PLR组。比较两组间临床病理参数的差异,使用Kaplan-Meier生存曲线分析PLR对前列腺癌病人预后的影响,采用Cox比例风险模型分析前列腺癌病人的预后影响因素。前列腺癌病人的危险度被分为低危、中危和高危,其中低危病人PSA<10 μg/L、Gleason评分≤6分、临床分期≤T2a,中危病人PSA 10~20 μg/L、Gleason评分=7分、临床分期为T2b,高危病人PSA>20 μg/L、Gleason评分≥8分、临床分期≥T2c。总生存期(OS)指从诊断日期开始到因任何原因死亡或最后一次随访的时间。
1.3统计学分析
采用SPSS 22.0软件对数据进行统计分析。服从正态分布的计量资料以[AKx-D]±s表示,组间比较采用t检验;计数资料组间比较采用卡方检验。P<0.05认为差异有统计学意义。
2结果
2.1两组病人临床病理参数比较
ROC曲線分析结果显示,PLR最佳临界点为146.9,曲线下面积为0.664。以146.9为PLR截断值,将病人分为高PLR组(PLR≥146.9,114例)和低PLR组(PLR<146.9,198例)。与低PLR组比较,高PLR组病人年龄大、危险度高、Gleason评分高、肿瘤分期晚、肿瘤侵犯范围大、PSA水平高,二者差异均有统计学意义(χ2=13.223~60.308,t=2.146、2.943,P<0.05)。见表1。
2.2PLR对前列腺癌病人预后的影响
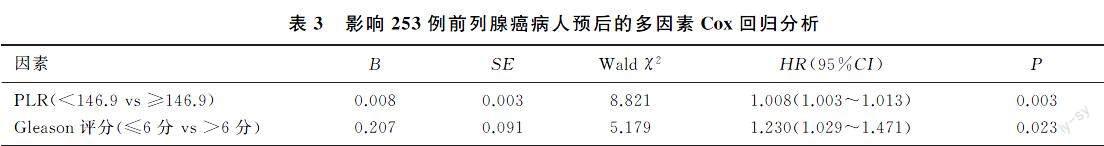
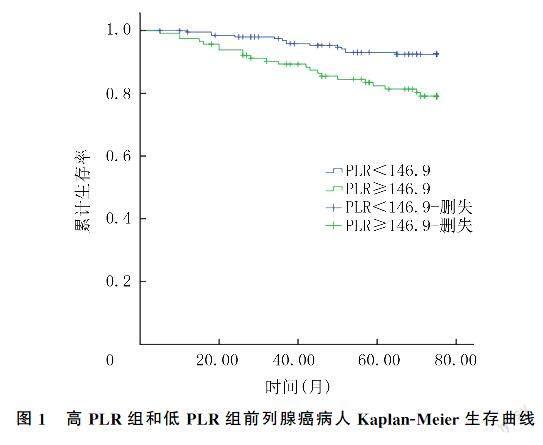
本文312例病人中位随访时间为50个月(5~75个月),失访59例,死亡36例。Kaplan-Meier生存曲线分析结果显示,低PLR组病人总生存率明显高于高PLR组(χ2=23.083,P<0.05)。见图1。为了探讨前列腺癌病人OS的预后影响因素,采用Cox回归分析PLR、年龄、PSA、Gleason评分对前列腺癌病人预后的影响。单因素及多因素Cox回归分析显示,PLR和Gleason评分是前列腺癌病人的独立预后因素。见表2、3。
3讨论
近年来,随着对肿瘤炎症微环境认识的不断深入,炎症与肿瘤的关系已成为一个前沿研究热点。许多研究表明,恶性肿瘤细胞的侵袭能力不仅取决于肿瘤细胞的生物学行为,还取决于肿瘤微环境,尤其是各种炎症因子的相互作用[13]。通过刺激或抑制肿瘤细胞,炎症反应在肿瘤的发生、发展和预后中起着重要作用[14]。据评估,潜在的感染和炎症反应与全世界15%~20%的癌症相关死亡有关[15]。慢性炎症会增加患癌的风险,并且会促进肿瘤的进展和转移[16]。外周血计数的变化可以反映癌症病人的炎症反应。高PLR既反映了血小板依赖的促肿瘤反应升高,也反映了淋巴细胞介导的抗肿瘤免疫反应降低,而这两者都可能导致癌症进展和不良预后。众所周知,血小板在止血和血栓形成中有着重要地位。最近的研究结果表明,血小板可以介导癌细胞的生长、扩散和血管生成[17]。BOUCHARA-
BA等[18]研究表明,血小板衍生的溶血磷脂酸对乳癌的骨转移形成至关重要。DASHEVSKY等[19]的研究结果表明,血小板衍生的微粒通过上调基质金属蛋白酶2(MMP2)表达增强前列腺癌细胞的侵袭性。而肿瘤细胞可以介导血小板聚集[20]。血小板聚集在肿瘤细胞周围可以保护肿瘤细胞免受自然杀伤(NK)细胞的杀伤[21]。此外,肿瘤浸润的分化簇CD8+和CD4+淋巴细胞被证明对抗肿瘤活性有重要的作用[22]。有大量的研究结果表明,淋巴细胞是癌症免疫监视的细胞基础,可抑制肿瘤细胞的增殖和转移[23]。作为血小板计数和淋巴细胞计数相结合的参数,PLR反映了机体内血小板和淋巴细胞的增减变化,也是促肿瘤炎性反应状态与抗肿瘤免疫状态的平衡指标,可以为癌症病人的预后提供相对准确的信息。
本文结果显示,PLR升高的前列腺癌病人具有更多与进展高风险相关的临床病理特征,包括高龄、Gleason评分高、肿瘤分期晚、肿瘤侵犯范围大和高PSA水平。以上结果表明,PLR可以作为前列腺癌恶性进展的指标。LI等[2]研究表明,与低PLR组相比,高PLR组的年龄更大、肿瘤分期更晚、Gleason评分和器官受累程度更高,与本研究结果一致。然而,WANG等[24]研究发现,低PLR组与高PLR组前列腺癌病人在年龄、血清PSA水平、Gleason评分、风险分层、转移发生率等方面差异无显著性。
本文Kaplan-Meier生存曲线分析显示,与低PLR组相比,高PLR组病人的6年总生存率明显降低。Cox回归分析结果显示,高PLR和Gleason评分是前列腺癌病人预后的独立危险因素。本研究结果进一步证实了PLR在肿瘤发生发展中的作用,为炎症和肿瘤的研究提供了更多的证据。LI等[2]研究表明,高PLR组病人3年生存率显著降低,PLR可能是与病死率相关的危险因素,是随访中全因病死率的独立预测因子。WANG等[24]研究结果表明,高PLR前列腺癌病人的肿瘤特异性生存率、无进展生存率和总生存率明显低于低PLR病人。然而,高PLR導致前列腺癌预后不良的机制目前尚不清楚,推测预后不良可能是由炎症和营养不良状态引起的[25]。
本研究存在一定的局限性:①为回顾性研究,可能会出现错误的数据收集;②研究对象相对较少,随访时间不够长。因此,本研究结果需在更大受试者规模和更长随访时间的进一步研究中进行验证。
综上所述,PLR与前列腺癌病人的临床病理参数密切相关,是前列腺癌病人随访期间死亡的独立影响因素,对前列腺癌的预后具有重要的临床意义。总体而言,PLR是一种简单、廉价且方便的指标,可以帮助预测前列腺癌的进展和预后。
[参考文献]
[1]TORRE L A, BRAY F, SIEGEL R L, et al. Global cancer statistics, 2012[J]. CA: A Cancer Journal for Clinicians, 2015,65(2):87-108.
[2]LI F, HU H B, GU S, et al. Platelet to lymphocyte ratio plays an important role in prostate cancer's diagnosis and prognosis[J]. International Journal of Clinical and Experimental Medicine, 2015,8(7):11746-11751.
[3]DE NUNZIO C, KRAMER G, MARBERGER M, et al. The controversial relationship between benign prostatic hyperplasia and prostate cancer: the role of inflammation[J]. European Urology, 2011,60(1):106-117.
[4]TAVERNA G, PEDRETTI E, DI CARO G, et al. Inflammation and prostate cancer: friends or foe[J]? Inflammation Research: Official Journal of the European Histamine Research Society, 2015,64(5):275-286.
[5]FUJITA K, IMAMURA R, TANIGAWA G, et al. Low se-rum neutrophil count predicts a positive prostate biopsy[J]. Prostate Cancer and Prostatic Diseases, 2012,15(4):386-390.
[6]LANGSENLEHNER T, THURNER E M, KRENN-PILKO S, et al. Validation of the neutrophil-to-lymphocyte ratio as a prognostic factor in a cohort of European prostate cancer patients[J]. World Journal of Urology, 2015,33(11):1661-1667.
[7]STOTZ M, GERGER A, EISNER F, et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer[J]. British Journal of Cancer, 2013,109(2):416-421.
[8]ASHER V, LEE J, INNAMAA A, et al. Preoperative platelet lymphocyte ratio as an independent prognostic marker in ova-rian cancer[J]. Clinical & Translational Oncology: Official Publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico, 2011,13(7):499-503.
[9]KWON H C, KIM S H, OH S Y, et al. Clinical significance of preoperative neutrophil-lymphocyte versus platelet-lymphocyte ratio in patients with operable colorectal cancer[J]. Biomar-kers: Biochemical Indicators of Exposure, Response, and Susceptibility to Chemicals, 2012,17(3):216-222.
[10]LIU H B, WU Y, WANG Z F, et al. Pretreatment platelet-to-lymphocyte ratio (PLR) as a predictor of response to first-line platinum-based chemotherapy and prognosis for patients with non-small cell lung cancer[J]. Journal of Thoracic Di-sease, 2013,5(6):783-789.
[11]PINATO D J, STEBBING J, ISHIZUKA M, et al. A novel and validated prognostic index in hepatocellular carcinoma: the inflammation based index (IBI)[J]. Journal of Hepatology, 2012,57(5):1013-1020.
[12]FOX P, HUDSON M, BROWN C, et al. Markers of systemic inflammation predict survival in patients with advanced renal cell cancer[J]. British Journal of Cancer, 2013,109(1):147-153.
[13]AZAB B, BHATT V R, PHOOKAN J, et al. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients[J]. Annals of Surgical Oncology, 2012,19(1):217-224.
[14]HUSSEIN M R, HASSAN H I. Analysis of the mononuclear inflammatory cell infiltrate in the normal breast, benign proli-
ferative breast disease, in situ and infiltrating ductal breast carcinomas: preliminary observations[J]. Journal of Clinical Pathology, 2006,59(9):972-977.
[15]WANG J F, ZHOU X F, HE Y H, et al. Prognostic role of platelet to lymphocyte ratio in prostate cancer: a meta-analysis[J]. Medicine, 2018,97(40):e12504.
[16]MANTOVANI A. Cancer: inflaming metastasis[J]. Nature, 2009,457(7225):36-37.
[17]SHARMA D, BRUMMEL-ZIEDINS K E, BOUCHARD B A, et al. Platelets in tumor progression: a host factor that offers multiple potential targets in the treatment of cancer[J]. Journal of Cellular Physiology, 2014,229(8):1005-1015.
[18]BOUCHARABA A, SERRE C M, GRS S, et al. Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer[J]. The Journal of Clinical Investigation, 2004,114(12):1714-1725.
[19]DASHEVSKY O, VARON D, BRILL A. Platelet-derived microparticles promote invasiveness of prostate cancer cells via upregulation of MMP-2 production[J]. International Journal of Cancer, 2009,124(8):1773-1777.
[20]GOUBRAN H A, STAKIW J, RADOSEVIC M, et al. Platelets effects on tumor growth[J]. Seminars in Oncology, 2014,41(3):359-369.
[21]ZHENG S, SHEN J, JIAO Y, et al. Platelets and fibrinogen facilitate each other in protecting tumor cells from natural killer cytotoxicity[J]. Cancer Science, 2009,100(5):859-865.
[22]ADAMS S, GRAY R J, DEMARIA S, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase Ⅲ randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199[J]. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 2014,32(27):2959-2966.
[23]DUNN G P, OLD L J, SCHREIBER R D. The immunobiology of cancer immunosurveillance and immunoediting[J]. Immunity, 2004,21(2):137-148.
[24]WANG Y Q, XU F, PAN J H, et al. Platelet to lymphocyte ratio as an independent prognostic indicator for prostate cancer patients receiving androgen deprivation therapy[J]. BMC Cancer, 2016,16:329.
[25]SUN Z H, JU Y, HAN F Y, et al. Clinical implications of pretreatment inflammatory biomarkers as independent prognostic indicators in prostate cancer[J]. Journal of Clinical Laboratory Analysis, 2018,32(3):e22277.
(本文編辑马伟平)
