基于二氧化钒的吸收带宽可调谐超材料吸收器
2022-02-22江孝伟王胜武华
江孝伟,王胜,武华
(1 衢州职业技术学院信息工程学院,浙江衢州324000)
(2 赣南师范大学物理与电子信息学院,江西赣州341000)
(3 北京工业大学光电子技术教育部重点实验室,北京100124)
0 Introduction
Adjusting the Metamaterial(MM) cell resonance structure may show many exotic electromagnetic properties, such as negative refraction[1-2], perfect absorption[3-4], electromagnetic stealth[5-6], and electromagnetic induction transparency[7].Because MM has the above-mentioned abnormal electromagnetic properties,it has gradually become a research hotspot,and has been widely used in national defense,communications,and biomedical sensing.[8-10].
The Metamaterial Perfect Absorber(MPA)has attracted widespread attention due to unprecedented characteristics compared to taper-like structure absorbers[11]or ordinary absorbers,such as high absorption efficiency,ultrathin thickness,and a scalable working wavelength[12].Since Landy et al.first proposed an MPA with perfect absorption characteristics[3],different types of MPAs have been proposed,and the absorption wavelengths over microwave[13],terahertz[14],infrared,[15]and visible bands have been identified[16].However,once the structural parameters of MPAs are fixed,their absorption characteristics are difficult to be tuned.On the other hand,demand on tunable MPA is increasing in many applications,such as modulators,optical switches,and smart reflectors.[17].
To realize the dynamic tunability of the absorption characteristics of MPA,many research groups choose to incorporate varactor diodes or graphene into the design of MPA.In the low-frequency band,diodes with changeable capacitance are generally used to change the equivalent capacitance of MPA to achieve dynamic tuning of MPA absorption wavelength and absorption efficiency[18-19].In the high-frequency band,graphene is used in MPA.By changing the chemical potential of graphene,one can achieve dynamic control of absorption wavelength,absorption efficiency,and even absorption bandwidth of MPAs[20-21].
In recent years,phase-changing material vanadium dioxide(VO2)demonstrated outstanding optical and electrical properties,and can realize the transition between metallic state and dielectric state through external excitations,such as light,electricity,and heat[22-23].Because VO2has reversible phase-changing characteristics,it is an ideal material for preparing a tunable MPA,and many research work has been done on tunable MPAs based on VO2.LEI L et al from Shenzhen University designed an absorption-bandwidth-tunable MPA based on VO2and metallic chromium materials[24].DAO Rina et al from Nanjing University of Posts and Telecommunications used VO2as a resonance unit material,and the absorption efficiency of MPA could be tuned by changing the temperature of the resonance unit[25].Our research group also demonstrated tunable absorption wavelength and absorption efficiency of MPA by using VO2and graphene in MPA[26].
To the best of our knowledge,current VO2-based MPA can realize tunable absorption efficiency,but the tuning of absorption bandwidth is rarely involved.Moreover,the tunable MPA bandwidth in visible and nearinfrared bands is even less involved.However,at present,for the application of intelligent windows,intelligent reflectors,intelligent temperature control systems,and heat emitters,MPA absorption bandwidth is required to be tunable in the visible and near-infrared bands[27-28].Therefore,in this paper,a bandwidth-tunable VO2-based MPA in visible and near-infrared bands is proposed and studied using Finite Difference Time Domain(FDTD)method.The simulation results showed that the bandwidth,Wa,of the tunable MPA proposed in this paper could reach 1.03 μm and 0.652 μm with absorption efficiency higher than 90% in the VO2dielectric and metallic state.By analyzing the electromagnetic field distribution of MPA,it was observed that as VO2was in the dielectric state,MPA achieved a wide bandwidth and high absorption owing to the Propagating Surface Plasmon(PSP),Localized Surface Plasmon(LSP),and the resonance of Fabry-Perot(FP)cavity.
1 Device structure and theory
The structure of the absorption-bandwidth-tunable MPA is shown in Fig.1.Fig.1(a)is overall view of the MPA structure,and Fig.1(b)is zoom-in view of one MPA unit cell.The MPA unit cell is composed of an Au substrate and four cylindrical resonance units with different radii.The cylindrical resonance units are of twolayered structure,with VO2as bottom layer and Au as top layer.The structural parameters are as follows:Pis the period of the MPA unit cell;r1,r2,r3,andr4are the radii of the four cylindrical resonance units respectively;hm=0.03 μm andhv=0.11 μm are the thickness of Au and VO2in the cylindrical resonance unit respectively;w1,w2,w3andw4are spacing between different cylinder resonant elements.In this work,the thickness of the Au substrate is 0.2 μm,which is thick enough to block the incident light effectively,and the transmission of the structure is nearly zero.
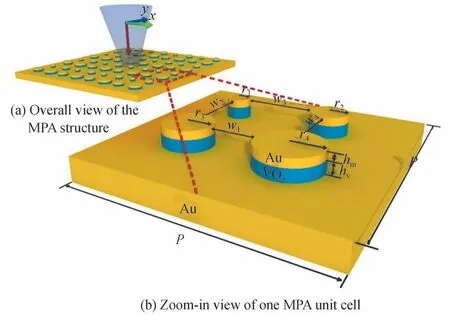
Fig.1 schematic of the structure of absorption-bandwidth-tunable MPA
With the development of material preparation technology and nanofabrication technology,the current micro-nano machining technology can fully meet the requirements of MPA processing as shown in Fig.1.Therefore,the MPA structure proposed in this paper is easier to realize in an actual experiment.The proposed structure in Fig.1 can easily be realized by current nano-fabrication technology.Firstly,a continuous VO2layer is prepared on the Au substrate by the lower-cost sol-gel method[29],then a thin Au layer was sputtered on the VO2layer by magnetron sputtering,finally the required cylindrical resonance units are patterned by electron beam lithography and ion beam etching.
The refractive indexn,and coefficientkof Au for different light wavelengths could be obtained from Ref.[30],as shown in Fig.2.According to the Bruggeman′s effective medium theory[31],The dielectric constant of VO2could be calculated by Eq.(1).In Eq.(1),εi≈9 is the dielectric constant of VO2in the dielectric state,εmis the dielectric constant of VO2in the metallic state,andfrepresents the volume ratio of VO2in the metallic state in the entire VO2.Moreover,εmcould be obtained by Eq.(2).In Eq.(2),ωpis the plasma frequency,ωis the incident light angular frequency,τ=2 μme/e is the relaxation time,meis the mass of free electrons,u≈2 cm2/V.sis carrier mobility,andeis the amount of free electron charge.Furthermore,fcould be obtained by Eq.(3),whereTrepresents the environment temperature,T0=68° C represents the VO2phase transition temperature,and ΔT=6°C is the transition width.Eqs.(1)~(3)could be combined to obtain the refractive index,nVO2,and the extinction coefficient,kVO2,of VO2for different wavelengths at different temperatures,as shown in Fig.3.
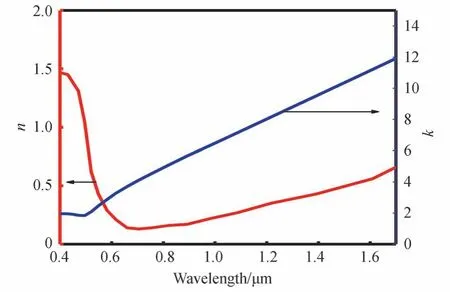
Fig.2 Refractive index and extinction coefficient of Au
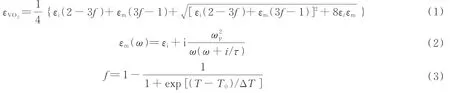
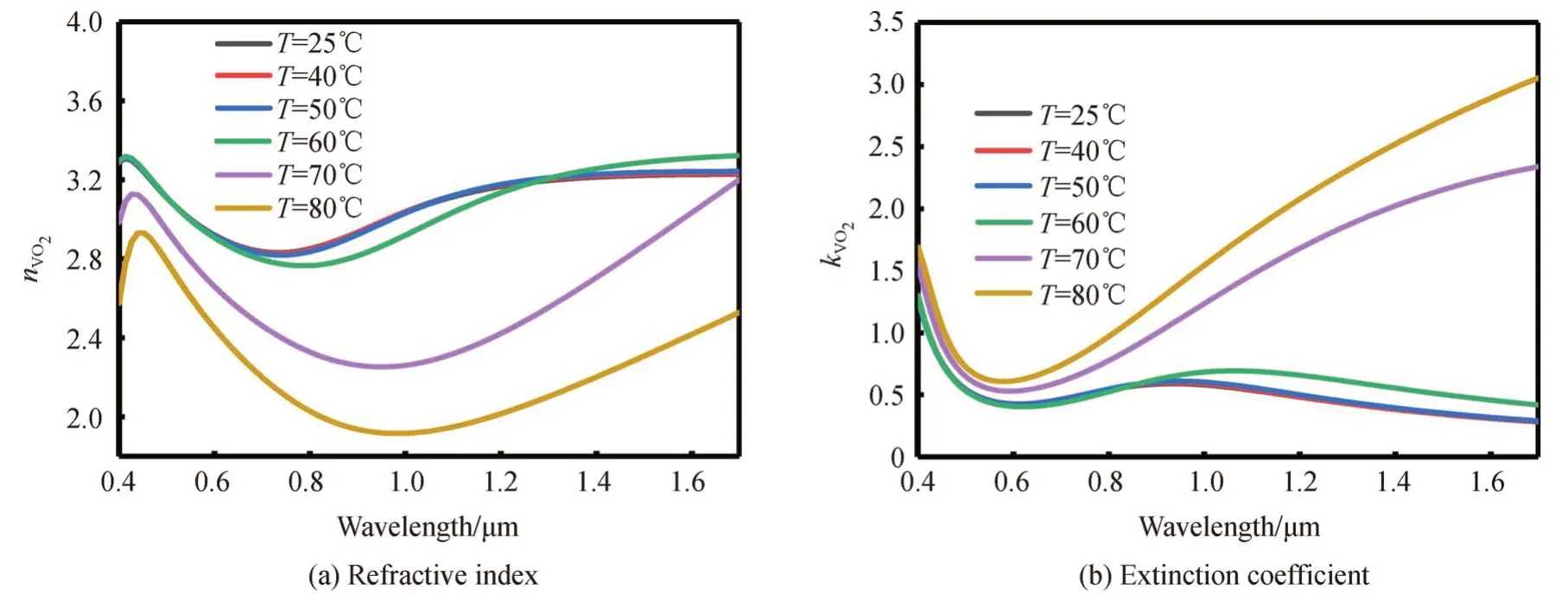
Fig.3 Refractive index and extinction coefficient of VO2 at different temperatures
2 Results and discussions
The characteristics of proposed MPA is studied by FDTD simulation.Since the MPA is of periodic structure,by carefully setting boundary conditions,it is feasible to only simulate the unit structure as shown in Fig.1(b)in FDTD software.A periodic boundary condition was added in thex- andy-directions.A perfect match layer was added in thez-direction as the boundary condition.The polarization of the incident light was set to be TM polarization(Along thexaxis).Incident light was perpendicular to the surface of the structure,which indicated the incident angleθis 0.To ensure that the simulation results were closer to reality,the grid type was auto non-uniform during simulation,and the grid accuracy was set to the maximum value of 8.The details are shown in Fig.4.
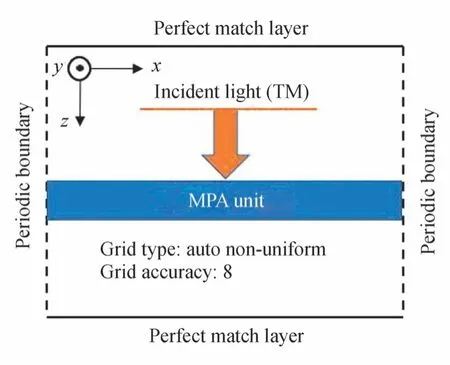
Fig.4 Detailed settings of the FDTD simulation
Fig.5 shows FDTD simulation results of absorption spectra of MPA at different temperatures.As Fig.5 shows,atT=80°C,the MPA could maintain more than 90% absorption efficiency between 0.505 μm and 1.157 μm,and the absorption bandwidthWacould reach 0.652 μm.When the temperatureTdropped from 80°C to 25°C,the MPA could maintain more than 90% absorption efficiency between 0.505 μm and 1.535 μm,and the absorption bandwidth,Wareached 1.03 μm.These findings indicate that the MPA proposed in this paper can realize the tuning of absorption bandwidth by changing the VO2temperature,and the tuning range of absorption bandwidth can reach 0.378 μm.In addition,the results show that the absorption spectrum of MPA covers both the visible and near-infrared bands.
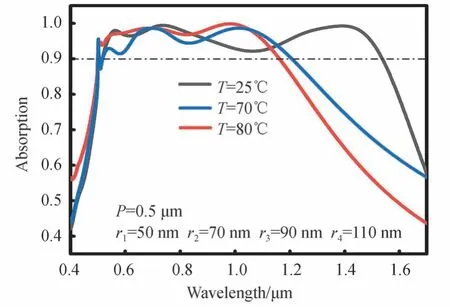
Fig.5 Absorption as a function of wavelength of MPA at different temperature
To explore the internal physical mechanism of the MPA with tunable absorption bandwidth,the electromagnetic field distribution of MPA at different resonance wavelengths is simulated and calculated at low temperature.Fig.6 shows the electric field distribution of MPA in thex-zplane(y=−0.125 μm,e.g.the center plane of cylinders with radiir3andr4)under different resonance wavelength conditions(T=25°C).As shown in Fig.6,under different resonance wavelength conditions,the electric field is basically concentrated between the gaps of the cylindrical resonance units and the corners of the Au cylinder,which indicates the incident light excites the surface plasmon polaritons(SPPs)in the MPA.Therefore,from the electric field distribution,it can be concluded that the high absorption of the MPA at each resonance wavelength is due to SPP resonance[24].
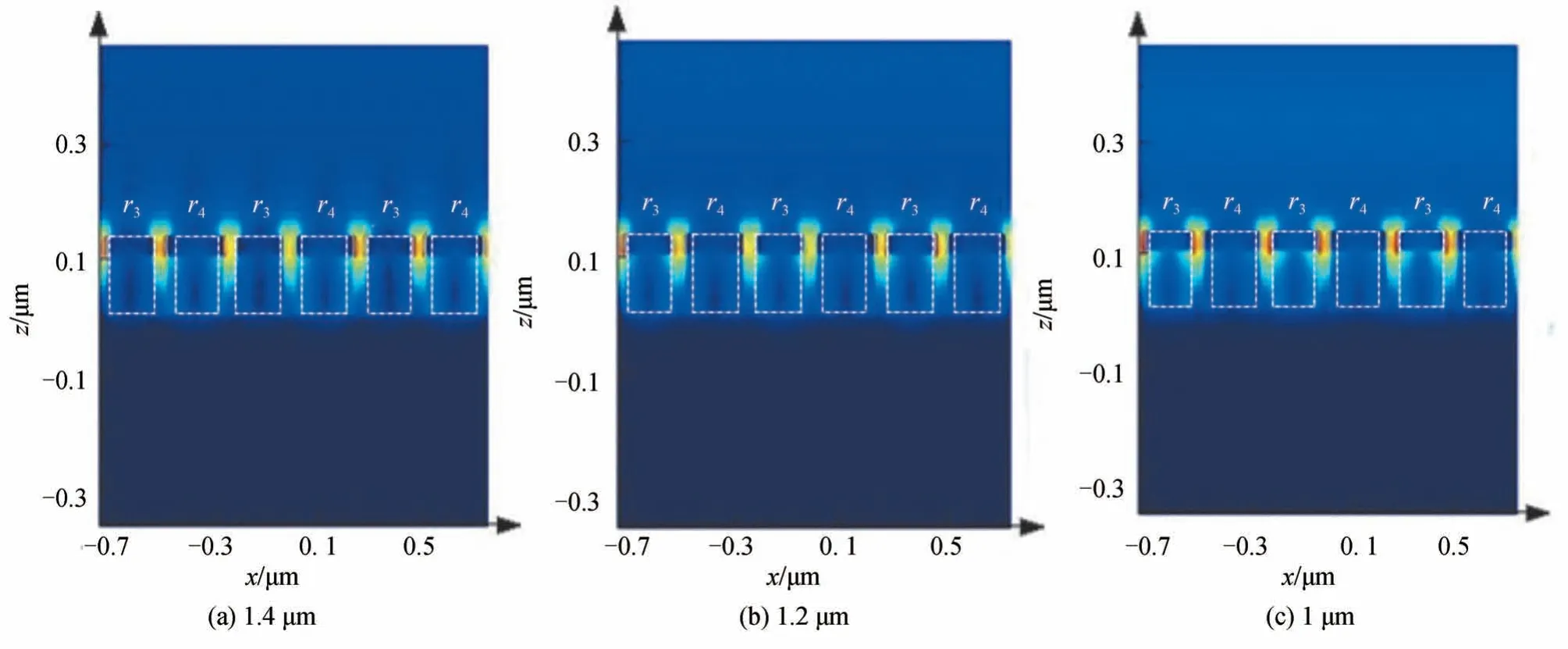

Fig.6 Electric field distribution of MPA at the different resonance wavelength(T=25°C)
Unlike the electric field distribution,the magnetic field distribution(T=25°C)of the MPA under different resonance wavelength conditions is very different.As shown in Fig.7(a)and 7(b),LSP resonance is the mainreason for the high absorption efficiency of MPA at long wavelengths(1.4 μm and 1.2 μm).Moreover,most of the magnetic field is confined to the VO2layer,which is between the Au cylinder and the Au substrate[23].Fig.7(e)shows that the high absorption efficiency of MPA at a short wavelength(0.505 μm)is mainly due to PSP resonance,because only a small part of the magnetic field is restricted to the VO2layer,and most of magnetic field is located between adjacent cylindrical resonance units,which is a significant PSP resonance feature[32-33].The specific proof can also be seen in Fig.9(a).As for the resonance absorption of MPA at wavelengths of 0.8 μm and 1 μm,Fig.7(c)and 7(d)show that it is not only caused by LSP resonance and PSP resonance,but is also mixed with FP cavity resonance,that is,the light wave oscillated back and forth between the Au cylindrical layer and the Au substrate.In this case,the Au cylindrical layer and Au substrate act as mirrors to form a FP resonator.
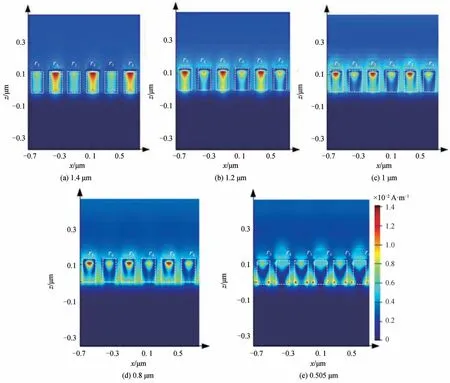
Fig.7 The magnetic field distribution of MPA at the different resonance wavelength(T=25°C)
To understand the intrinsic physical mechanism of the high absorptivity of MPA in the VO2metallic state,the electromagnetic field distribution of MPA at the wavelength of 0.505 μm and 1 μm atT=80°C is calculated,as shown in Fig.8.Fig.8(a)shows the magnetic field distribution of the MPA at a wavelength of 0.505 μm.Compared with Fig.7(e),the magnetic field distribution of VO2at the short wavelength is basically the same regardless of whether VO2is in the dielectric state or metallic state.That is,the physical mechanism leading to the high absorption efficiency of MPA at the short wavelength is the same,which is because of the PSP resonance.
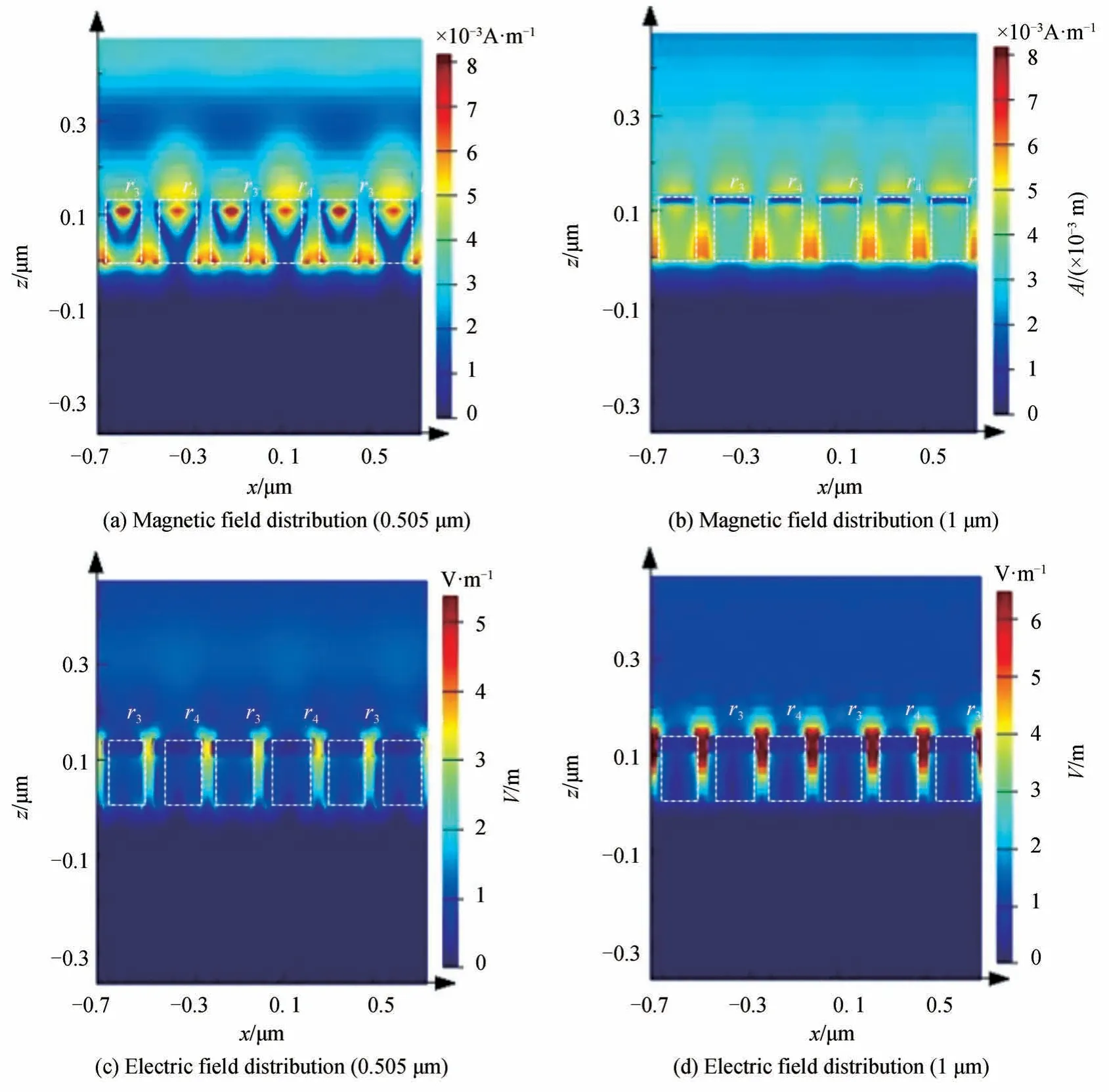
Fig.8 The electromagnetic field distribution of MPA at resonance wavelength(T=80°C)
Comparing Fig.8(b)with Fig.7(c),it can be seen that when VO2is in the metallic state,the high absorption efficiency of MPA at long wavelengths(1 μm)is not only caused by LSP or PSP resonance,but also mainly because of FP cavity resonance.The FP cavity resonance here is mainly composed of an adjacent cylinder resonance units and air gap[24].The FP cavity length is the thickness of cylinder resonance unit(hm+hv).The width of FP cavity isw1.Owing to the formation of the FP cavity,more magnetic fields are concentrated between the air gaps of the adjacent cylinder resonance units.Figs.8(c)and 8(d)show the electric field distribution of MPA at a wavelength of 0.505 μm and 1 μm when VO2is in a metallic state,respectively.It can be seen the high absorption efficiency of MPA at high temperature is also due to the SPP resonance.
To explore the influence of MPA structural parameters on its absorption characteristics at low temperature(VO2is in dielectric state,T=25℃),the MPA absorption spectra(T=25°C)under the conditions of different periodsPand cylindrical resonance unit radius were simulated and calculated.Fig.9(a)shows the influence ofPon the absorption spectrum of MPA.AsPincreased,the absorption bandwidth of MPA gradually narrowed,and the absorption wavelength of MPA at the short-wavelength gradually showed redshift.WhenPincreased from 0.5 μm to 0.6 μm,the initial wavelength of the MPA absorption spectrum shifted from 0.505 μm to 0.599 μm.It can be found that the absorption wavelength of MPA at short wavelengths is very close toP.This phenomenon is due to the fact that high absorption efficiency of MPA at short wavelengths is caused by PSP resonance.The PSP resonance wavelength,λPSP,is related to the MPA period,P,which can be seen in Eq.(4)[32].In Eq.(4),mis an integer,λ0is the free-space wavelength.Because the incident light is perpendicular to the surface of the MPA,θis 0,thereforeλPSPequals toP.
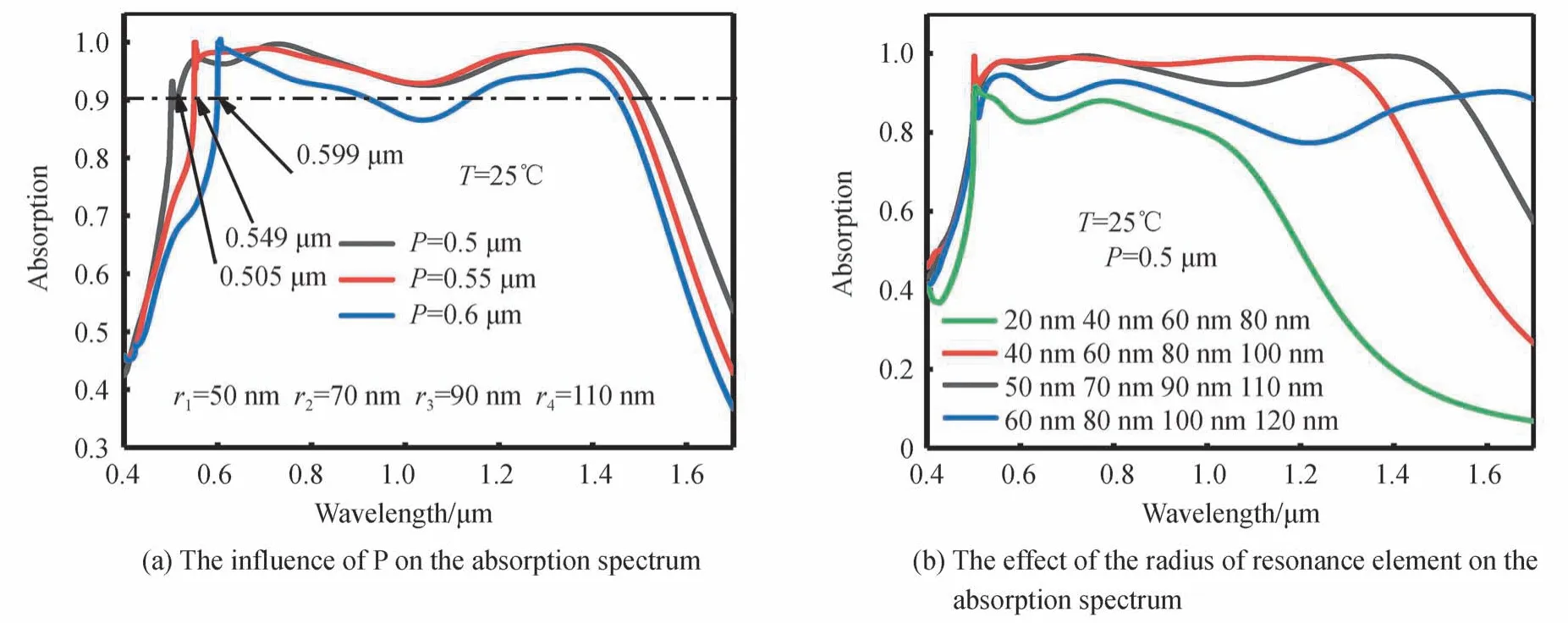
Fig.9 The influence of MPA structural parameters on the absorption spectrum of MPA(T=25°C)
As Fig.9(a)shows,although thePof MPA gradually increased,there is no obvious redshift or blueshift at the MPA wavelength of 1.4 μm.This is because,as can be seen from Fig.7(a),the high absorption efficiency of MPA at 1.4 μm is caused by LSP resonance,and periodPhas almost no effect on the LSP resonance wavelength.

Fig.9(b)shows the MPA absorption spectra versus the radii of different cylindrical resonance units.As can be seen from Fig.9(b),with an increase in the radius of the resonance unit,the MPA absorption bandwidth,Wa,increased firstly and then decreased.In the short wavelength range between 0.5 μm and 1 μm,in all the 4 simulated conditions,the MPA could maintain a relatively high absorption efficiency(>75%).The reason for this is that the high absorption efficiency of MPA at short wavelength is caused by the PSP resonance,as shown in Fig.7(e).From Eq.(4),we know the resonance wavelength of PSP is mainly determined by the periodP,so as long asPdid not change,the MPA could maintain the PSP resonance at the short wavelength.However,the absorption efficiency varied greatly at long wavelengths(>1 μm).The reason for this is that the high absorption efficiency of MPA at long wavelength is caused by LSP resonance,as shown in Fig.7(a)and 7(b).According to Ref.[32],LSP resonance is mainly affected by the shape and size of the MPA resonance unit.Therefore,when the radius of the resonance unit is small,the absorption efficiency of MPA is extremely low because it cannot meet the conditions to excite LSP resonance;when the radius of the resonance element increases,it gradually meets the excitation conditions of LSP resonance,and increases the absorption efficiency of MPA at the long-wavelength range.
In order to understand the influence of structural parameters on the absorption characteristics of MPA at high temperature(VO2is in metallic state,T=80℃),we changed the thickness of top Au layerhmand the radius of the resonance units.Fig.10(a)shows the influence ofhmon the absorption characteristics of MPA as VO2is in metallic state(T=80℃).As can be seen from Fig.10(a),whenhmincreases,the absorption wavelength of MPA at short wavelength almost unchanged,but the absorption efficiency gradually declines.Refer to Fig.8(a),when VO2is in metallic state,the high absorption efficiency of MPA at the short wavelength is caused by the PSP resonance.On the contrary,the absorption wavelength of MPA in the long wavelength is red-shifted.This is because when VO2is the metallic state(T=80℃),the high absorption efficiency of MPA at the long wavelength is due to the FP cavity resonance rather than LSP resonance.The relationship between the FP cavity resonance wavelengthλFPandhmis shown in Eq.(5)[34],whereneffis the FP cavity effective refractive index.According to Eq.(5),with the increase ofhm,the resonant wavelength of FP cavityλFPwill increase,resulting in the red shift of MPA at the long wavelength.
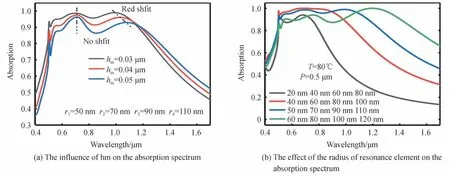
Fig.10 The influence of MPA structural parameters on the absorption spectrum of MPA(T=80°C)

Fig.10(b)shows the influence of the MPA resonance unit radius on the absorption characteristics of the MPA.Different from VO2in dielectric state,when VO2is in metallic state,the absorption efficiency of MPA at long wavelength gradually increases with the increase of the resonance unit radius.As comparison,when VO2is in dielectric state,as shown in Fig.9(b),with the increase of the resonance unit radius,the absorption efficiency of MPA at long wavelength first increases and then decreases.This is mainly because when VO2is metallic state,the high absorption efficiency of MPA at long wavelength is due to the FP cavity resonance.With the increase of resonance unit radius,the FP equivalent refractive index gradually meets the resonance condition of long wavelength(see Fig.11),so that the absorption efficiency of MPA at long wavelength is gradually improved.
The relationship between the width of FP cavitywand the equivalent refractive index of FP cavityneffcan be obtained by Eqs.(6)~(8).εmandεd=1 are the dielectric constants of Au and air respectively.k0=2π/λ,whereλis the incident light wavelength.Fig.11 shows the relationship betweenwandneffwhen the wavelength of incident light is 1 μm(εm=−47.84+3.11i).It can be seen from Fig.11 that aswdecreases,that is,as the radius of the cylinder resonance unit increases,neffwill gradually increase.Because the length of the FP cavity unchanged,the resonance wavelengthλFPof the FP cavity will increase when theneffincreases.Therefore,as shown in Fig.10(b),when the radius of the cylindrical resonance unit increases,the absorption efficiency of MPA at the long wavelength increases.
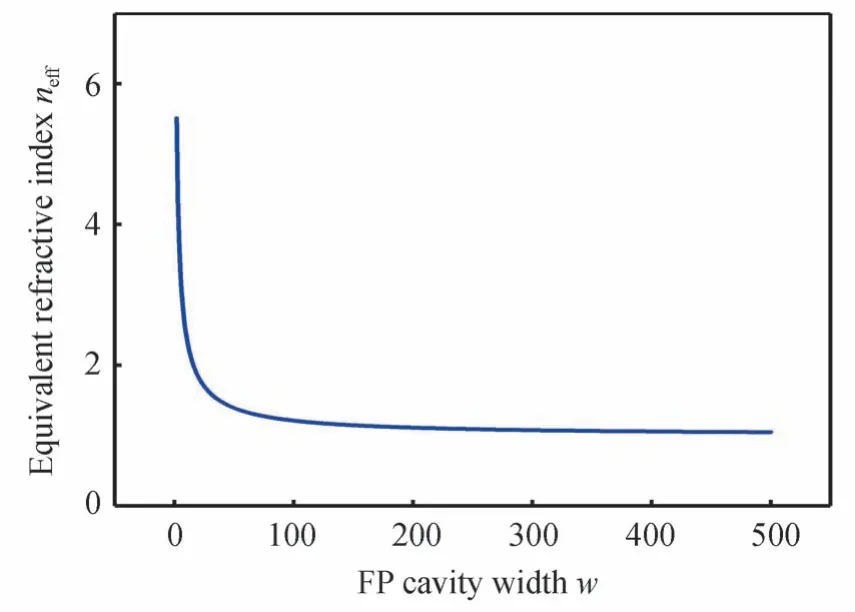
Fig.11 The influence of FP cavity wdith w on the FP cavity equivalent refractive index neff

3 Conclusions
We designed a MPA with high absorption efficiency and tunable absorption bandwidth in visible and nearinfrared light bands.The simulation results indicate that by changing temperature,the absorption bandwidthWaof the MPA can be tuned,and the tuning range can reach 0.375 μm.We also studied the effects of structural parameters on absorption bandwidth.By analyzing the electromagnetic field of the MPA at absorption wavelength,it can be found that when VO2is at a low temperature(T=25°C),the high absorption efficiency of the MPA in the near-infrared band is due to LSP resonance,and the high absorption efficiency of the MPA in the visible band is due to the PSP resonance.However,when VO2is at high temperature,the high absorption efficiency of the MPA in the near-infrared band is caused by FP resonance.The research in this paper can provide a theoretical basis for design and fabrication of a high-performance,dynamic,adjustable MPA in the future.
