F-containing cations improve the performance of perovskite solar cells
2022-02-15QinZhouChuantianZuoZilongZhangPengGaoandLimingDing
Qin Zhou, Chuantian Zuo, Zilong Zhang, Peng Gao,†, and Liming Ding,†
1Key Laboratory of Design and Assembly of Functional Nanostructures (CAS), Fujian Institute of Research on the Structure of Matter (CAS),Fuzhou 350002, China
2Center for Excellence in Nanoscience (CAS), Key Laboratory of Nanosystem and Hierarchical Fabrication (CAS), National Center for Nanoscience and Technology, Beijing 100190, China
3University of Chinese Academy of Sciences, Beijing 100049, China
4Laboratory for Advanced Functional Materials, Xiamen Institute of Rare Earth Materials, Haixi Institute (CAS), Xiamen 361021, China
Organic–inorganic halide perovskite solar cells (PSCs)have delivered power conversion efficiency (PCE) on par with that of crystalline silicon solar cells, due to the considerable effort on the optimization of perovskite materials and devices[1].The three-dimensional (3D) perovskite-based PSCs with the standard n–i–p architecture gave a certified PCE of 25.5%[2].However, the poor device stability under operating conditions remains an obstacle to commercialization.The 3D hybrid perovskite materials are susceptible to oxygen, UV light, humidity, heat, and electric fields[3].To improve device stability, two main strategies are applied: (1) improving the intrinsic stability[4]; (2) providing sufficient protection.In the first case, tremendous efforts have been devoted to optimizing the perovskite absorbers: i) compositional engineering(mixing cations or anions, adding additives); ii) dimensional engineering (Ruddlesden-Popper and Dion-Jacobson type 2D perovskites); iii) passivating 3D perovskites using low-dimensional perovskites.In the second case, three approaches are used:i) developing moisture-resistant charge-transporting materials and electrodes; ii) engineering the interface between perovskite layer, transport layer, and electrodes; iii) encapsulating devices[5].
Ionic defects cause hysteresis and poor stability[6].In conventional 3D PSCs (Fig.1(a)), the perovskites suffer from two types of defects.One type is the intrinsic point defect in the bulk.These defects can interact with carriers through electrostatic interaction, harming charge transport.The built-in field is not enough to extract the carriers, and some of them can be trapped by these defects.The other type of defect originates from the dangling bonds, which act as the recombination centers.Two methods were used to passivate these defects: (1) adding additives into precursor solution[7−11]or antisolvents[12]to control crystal growth and passivating defects at grain boundaries (GBs); (2) coating functional molecules onto perovskite layer[13].
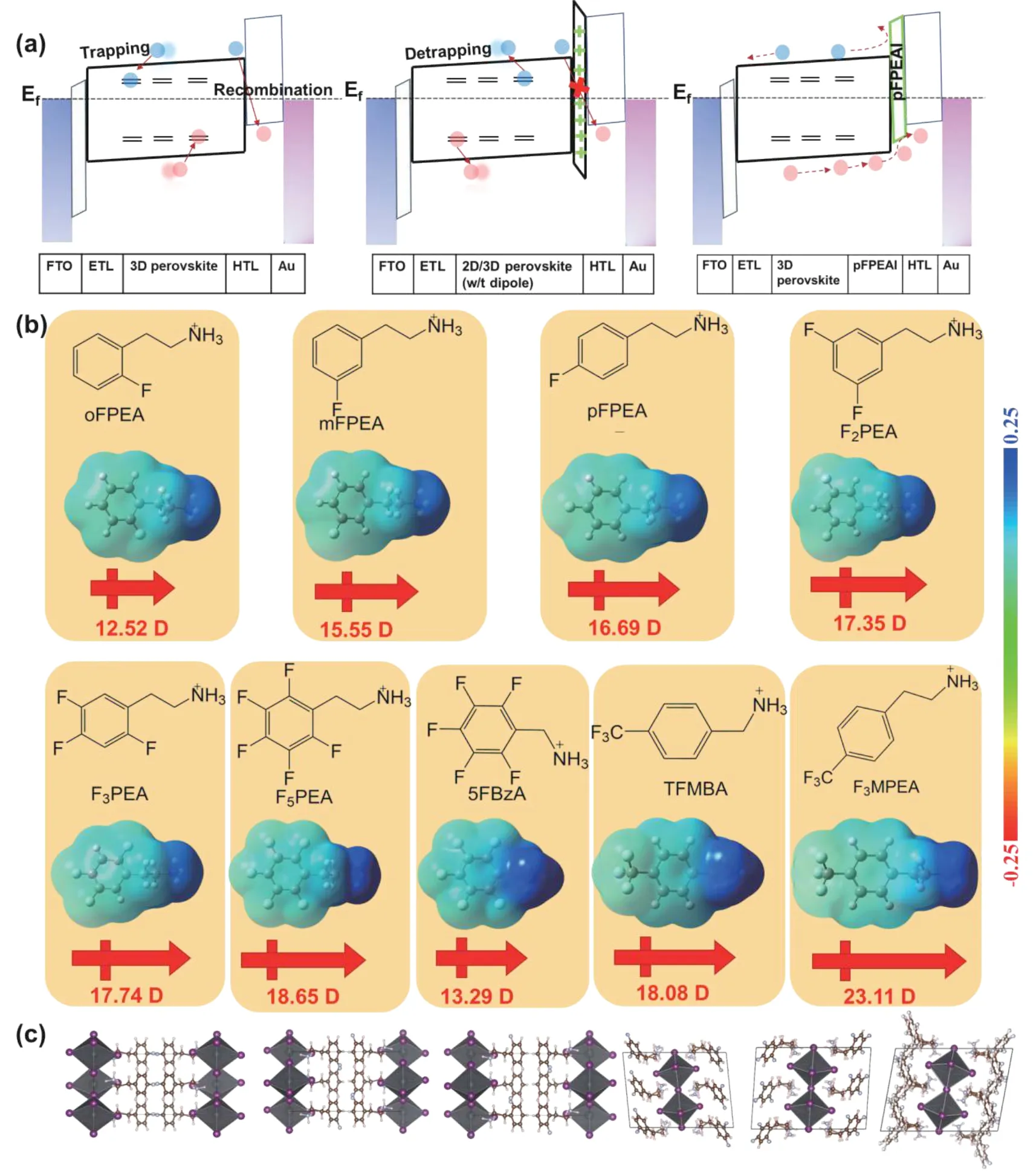
Fig.1.(Color online) (a) Energy level diagrams for the devices with pristine 3D perovskite, 2D/3D bilayer perovskite, and pFPEAI passivated perovskite.(b) Calculated electrostatic potential surface (EPS) and electric dipole moment (EDM) for the ammonium salts.(c) Crystal structures of 2D perovskites.From left to right, (pFPEA)2PbI4, (mFPEA)2PbI4, (oFPEA)2PbI4, (F2PEA)2PbI4, (F3PEA)2PbI4, (F5PEA)2PbI4.
The gain in long-term stability was often accompanied by the sacrifice of PCEs[14].2D/3D heterostructures can improve device stability without sacrificing the efficiency.A common approach to form a 2D/3D structure is to use bulky ammonium salts to do post-treatment.Huet al.first developed a cation infiltration process to prepare layered perovskite (LP)structures on MAPbI3film[15].The bottom MAPbI3layer ensures efficient light absorption and charge generation, while the top layer serves as the charge extraction layer and moisture barrier.Among the bulky cations for LP structure, nbutylammonium (BA+)[16,17]and 2-phenylethylammonium(PEA+) are the most popular[18,19].
To further enhance the hydrophobicity of the bulky cations, fluorination is an effective strategy (Fig.1(b))[20].Recently, Biet al.used an aliphatic fluorinated amphiphilic ammonium salt, 1,1,1-trifluoro-ethyl ammonium iodide (FEAI), to improve the stability and performance of PSCs[21].In 2017, Karunadasaet al.reported that the aromatic amino (e.g., PEA)-based 2D perovskites have lower exciton binding energies than the aliphatic amino (e.g., BA)-based 2D perovskites due to the higher dielectric constant of the polarizable aromatic groups[22].They also found that F atoms could improve the moisture resistance.
In 2019, we first introduced 2-(4-fluorophenyl)ethylamine, 4-FC6H4C2H4NH2(pFPEA), to grow a 2D perovskite layer atop 3D perovskite to realize both high efficiency and high stability[23].The laser scanning confocal microscope was used to observe the distribution ofin-situformed 2D perovskite over 3D perovskite.We used density functional theory (DFT)to calculate the surface energy of the slab model ((100)plane).(pFPEA)2PbI4has the lowest surface energy, consisting with the observed stability trend, i.e., (pFPEA)2(MA)2Pb3I10> (PEA)2(MA)2Pb3I10> MAPbI3[22](Fig.2(a)).The calculation also indicated that the defect formation energy of(pFPEA)2PbI4is much higher than that of (PEA)2PbI4, which benefits the stability of 3D perovskite.(pFPEA)2PbI4capping layer can protect 3D perovskite from moisture, reduce charge recombination, and facilitate hole transport.(Fig.1(a))

Fig.2.(Color online) (a) DFT calculation for slab surface energy.From left to right, 3D perovskites, 2D (PEA)2PbI4 perovskites, 2D (pFPEA)2PbI4 perovskites.Reproduced with permission[23], Copyright 2019, Wiley-VCH.(b) The formation energy for PEA2PbI4, oFPEA2PbI4, mFPEA2PbI4, and pFPEA2PbI4.Reproduced with permission[25], Copyright 2019, Nature Publishing Group.(c) XRR profiles for 2D, 3D, and 2D/3D perovskite films.(d) GIXD data for 2D, 3D, and 2D/3D perovskite films.Reproduced with permission[28], Copyright 2019, American Association for the Advancement of Science.(e) F 1s XPS depth profiles of the perovskite film with TFMBAI.Reproduced with permission[32], Copyright 2021, ACS Publications.(f) Electrostatic potential at the surface of 2D perovskites containing FPEAI and 5FBzAI cations.(g) 2D-GIWAXS plots and azimuthally integrated intensity of pristine 3D and 2D/3D films.Reproduced with permission[29], Copyright 2020, Wiley-VCH.
Later, Zhanget al.used time-resolved microwave conductivity (TRMC) measurements to compare in-plane and out-ofplane transport in (PEA)2PbI4and (pFPEA)2PbI4films[24].The out-of-plane microwave mobility of (pFPEA)2PbI4is ~7 times larger than that of (PEA)2PbI4.Fluorine substitution favors intermolecular packing and charge transport.As a result, the quasi-2D perovskite (pFPEA)2MA4Pb5I16gave a PCE above 13%, which is higher than that of PEA-based perovskite.In addition, the thermal stability of pFPEA perovskites is significantly enhanced.Huet al.demonstrated that mono-fluorination of PEA at different positions could influence the structure of 2D perovskites (empirical (xFPEA)2(MA)3Pb4I13) and device performance[25].They concluded that phase distribution, surface morphology, and crystal orientation determined the properties of 2D perovskite films and device performance.By performing photoluminescence (PL) and transient absorption (TA) measurements, more random phase distribution was found in oFPEA 2D perovskite film than mFPEA and pFPEA 2D perovskite films.This trend is consistent with DFT-calculated formation energies (oFPEA2PbI4>> PEA2PbI4>mFPEA2PbI4≈ pFPEA2PbI4) (Fig.2(b)).
The influence of F position on the surface dipole(Fig.1(b)), photophysical properties, electrochemical properties, and photovoltaic performances of corresponding 2D/3D perovskites was elucidated by Zhouet al.[26].The combined theoretical and experimental studies revealed that oFPEAI provides the best passivation, possibly due to the highest formation energy.By using (oFPEA)2PbI4as the passivation layer on 3D perovskite, the planar PSCs demonstrated a PCE of 23.80% with aVocdeficit of 0.39 V, which is the highest PCE for the 2D/3D architectures[27].
As for interface passivation, the bulky cations only anchor on perovskite surface.Jianget al.proposed an effective passivation by using pFPEAI[13].They confirmed that monofluorinated cations could passivate the defects at interfaces and GBs, increase moisture resistance, and improve device performance.
Polyfluorinated cations were also used, which produced an ultra-hydrophobic layer, protecting perovskite from ambient moisture while mitigating ion diffusion in the device.Liuet al.treated 3D perovskite with pentafluoro-phenylethylammonium iodide (F5PEAI) to form an ultra-hydrophobic 2D perovskite (F5PEA)2PbI4[28].They estimated the thickness of the 2D perovskite layer to be ~8 nm by X-ray reflectivity (XRR,out-of-plane direction) and grazing incidence X-ray diffraction (GIXRD, in-plane direction) measurements (Figs.2(c)and 2(d)).A PCE of 22.2% was achieved.The unsealed 2D/3D PSCs retained 90% of their initial efficiency after 1000 h operation in humid air under simulated sunlight.Paeket al.used a highly hydrophobic cation, perfluorobenzylammonium (5FBzA+), to form 2D perovskite with reinforced intermolecular interactions[29].Owing to the strong halogen-halogen interaction, (5FBzA)2PbI4layer aligned in the in-plane orientation (Figs.2(f) and 2(g)) and induced an increase inVoc(60 mV), which is much higher than its monofluorinated analog pFPEAI.A high PCE of 21.65% and enhanced operational stability were obtained.
Moreover, Qiuet al.studied dipole moments of fluorinated cations pFPEA+and 4-(trifluoromethyl)phenethylammonium (F3MPEA+) to understand the effect of fluorine numbers on the properties of 2D/3D perovskite and device performance[30].They found that the enlarged dipole moment can upshift Fermi level and increase the built-in field across 2D/3D perovskite heterojunction, thus facilitating charge transport.The 2D/3D PSCs delivered a PCE of 22.4%.Recently,2D/3D perovskites with a series ofin-situgrown (FxPEA)2PbI4(x= 1, 2, 3, 5) were tested[31].They systematically studied the performance and stability of the PSCs with different numbers of F.Among them, F3PEA-based 2D/3D perovskite gave the best PCE (23.04%).F5PEA-based device retained 95.0% of its initial PCE under ambient atmosphere (RH 60%) without encapsulation for 300 h.
F-containing bulky cations were also used for interface modification.Zhuet al.employed 4-(trifluoromethyl)benzy-lammonium iodide (TFMBAI) as an amphiphilic modifier for interfacial defect mitigation.4-(trifluoromethyl)pyridine (TFP)dopant was used to further enhance HTL’s hydrophobicity[32].By using X-ray photoelectron spectroscopy (XPS) depth profiling, the thickness of TFMBAI layer is estimated to be ~5 nm(Fig.2(e)).The nonradiative recombination is suppressed, and the PCE was enhanced from 20.9% to 23.9% with suppressed hysteresis.Besides the post-treatment, Wanget al.incorporated 0.3 mol% F5PEAI into MAPbI3solution and obtained a maximum PCE of 21.1% for the inverted cell[33].
Fluorinated aryl ammonium salts have recently been used in other fields.Shiet al.used fluorinated cations to make 2D perovskite ferroelectrics[34].They found that the position of fluorine is particularly essential.(oFBA)2PbCl4(oFBA: 2-fluorobenzylammonium) is the first ferroelectric 2D perovskite, possessing a high phase transition temperature of 448 K.However, (3-fluorobenzylammonium)2PbCl4and (4-fluorobenzylammonium)2PbCl4showed no ferroelectric properties.Jianget al.used polar pFPEA molecule to make quasi-2D perovskites for light-emitting diodes (LEDs)[35].The LEDs presented a peak external quantum efficiency (EQE) of 20.36%.
In short, fluorinated cations can improve the performance and stability of perovskite solar cells.To develop new F-containing cations is an interesting project in PSC field.
Acknowledgements
P.Gao acknowledges the financial support from the National Natural Science Foundation of China (21975260) and the NSFC-CNR Exchange Program (22011530391).L.Ding thanks the National Key Research and Development Program of China (2017YFA0206600) and the National Natural Science Foundation of China (51773045, 21772030, 51922032, and 21961160720) for financial support.
杂志排行
Journal of Semiconductors的其它文章
- I nvestigation on the passivation, band alignment, gate charge,and mobility degradation of the Ge MOSFET with a GeOx /Al2O3 gate stack by ozone oxidation
- I nvestigation into the InAs/GaAs quantum dot material epitaxially grown on silicon for O band lasers
- High-operating-temperature MWIR photodetector based on a InAs/GaSb superlattice grown by MOCVD
- A 58-dBΩ 20-Gb/s inverter-based cascode transimpedance amplifier for optical communications
- Band gap tuning and p to n-type transition in Mn-doped CuO nanostructured thin films
- Vertical nanowire/nanosheet FETs with a horizontal channel for threshold voltage modulation
