Molecular mechanism of therapeutic approaches for human gastric cancer stem cells
2022-02-12HsiLungHsiehMingChinYuLiChingChengTaSenYehMingMingTsai
Hsi-Lung Hsieh, Ming-Chin Yu, Li-Ching Cheng, Ta-Sen Yeh, Ming-Ming Tsai
Hsi-Lung Hsieh, Li-Ching Cheng, Ming-Ming Tsai, Department of Nursing, Division of Basic Medical Sciences, Chang-Gung University of Science and Technology, Taoyuan 333, Taiwan
Hsi-Lung Hsieh, Ming-Ming Tsai, Research Center for Chinese Herbal Medicine, College of Human Ecology, Chang Gung University of Science and Technology, Taoyuan 333, Taiwan
Hsi-Lung Hsieh, Department of Neurology, Chang Gung Memorial Hospital, Taoyuan 333, Taiwan
Ming-Chin Yu, Ta-Sen Yeh, Department of General Surgery, Chang Gung Memorial Hospital at Linkou, Taoyuan 333, Taiwan
Ming-Chin Yu, Ta-Sen Yeh, College of Medicine, Chang Gung University, Taoyuan 333, Taiwan
Ming-Chin Yu, Department of General Surgery, New Taipei Municipal TuCheng Hospital, New Taipei 236, Taiwan
Ming-Ming Tsai, Department of General Surgery, Chang Gung Memorial Hospital, Chiayi 613, Taiwan
Abstract Gastric cancer (GC) is a primary cause of cancer-related mortality worldwide, and even after therapeutic gastrectomy, survival rates remain poor.The presence of gastric cancer stem cells (GCSCs) is thought to be the major reason for resistance to anticancer treatment (chemotherapy or radiotherapy), and for the development of tumor recurrence, epithelial-mesenchymal transition, and metastases.Additionally, GCSCs have the capacity for self-renewal, differentiation, and tumor initiation.They also synthesize antiapoptotic factors, demonstrate higher performance of drug efflux pumps, and display cell plasticity abilities.Moreover, the tumor microenvironment (TME; tumor niche) that surrounds GCSCs contains secreted growth factors and supports angiogenesis and is thus responsible for the maintenance of the growing tumor.However, the genesis of GCSCs is unclear and exploration of the source of GCSCs is essential.In this review, we provide up-todate information about GCSC-surface/intracellular markers and GCSC-mediated pathways and their role in tumor development.This information will support improved diagnosis, novel therapeutic approaches, and better prognosis using GCSC-targeting agents as a potentially effective treatment choice following surgical resection or in combination with chemotherapy and radiotherapy.To date, most anti-GCSC blockers when used alone have been reported as unsatisfactory anticancer agents.However, when used in combination with adjuvant therapy, treatment can improve.By providing insights into the molecular mechanisms of GCSCs associated with tumors in GC, the aim is to optimize anti-GCSCs molecular approaches for GC therapy in combination with chemotherapy, radiotherapy, or other adjuvant treatment.
Key Words: Gastric cancer stem cells; Stem cell-surface markers; Tumor niche; Tumor microenvironment
INTRODUCTION
Despite progress in the diagnosis, prognosis, and therapeutic approaches to gastric cancer (GC), it persists as one of the most commonly identified malignant tumors in the world, and with a challenging mortality rate[1-3].
The discovery of cancer stem cells (CSCs) has explained much cancer behavior.Although CSCs account for only a small percentage of the entire tumor tissues, these cells are the key components that form the entire tumor.CSCs are viewed as a tumorinitiating subpopulation of cells within tumors capable of self-renewal, which can divide and differentiate into various tumor cell types (intratumoral heterogeneity).They are highly tumorigenic, involved in metastasis, relatively resistant to chemotherapy and radiotherapy, secrete antiapoptotic factors, undergo epithelialmesenchymal transition (EMT), and display a higher performance of drug efflux pumps[4-7].Opinions regarding the existence, role, and behavior of CSCs differ, suggesting that basic research on CSCs is crucial.Understanding CSCs also gives new ideas for the treatment of cancer.On the premise that targeted killing of CSCs is the fundamental way to completely eliminate tumor tissues, methods specifically focused on CSCs have become a very important topic, the ultimate goal being to design drugs for treatment of GC[8-11].
In recent years, due to the rapid development of CSC research, methods used to identify CSCs include evaluation of tumor formation in immune-deficient mice, tumorigenicity, GCSC generationin vivo, spheroid colony formation, metastasis, EMT, chemotherapy resistance, radiotherapy resistance, and expression of specific cellsurface/intracellular markersin vitro.The GCSC topics to be discussed in this review focus on GCSC-surface/intracellular markers, intracellular markers, the regulation of GCSCs in the tumor microenvironment (TME), and the potential for GCSC-targeted treatments in the future (Figure 1).
MAIN CELL-SURFACE/INTRACELLULAR MARKERS OF GCSCS
Since pathologists found that CSCs and SCs have many similarities in histological morphology, the concept that “malignant tumors arise from SCs” is an existing hypothesis[12,13].Support for this hypothesis was first published in 1994 by Dicket al[14], who discovered CSCs in acute myeloid leukemia (AML).
In 2003, Singhet al[15] showed that CSCs can also be successfully isolated from several solid tumors.CSCs are a common phenomenon in cancer, not all of a specific cancer[5,15,16].In solid tumors, the earliest successful isolation of CSCs was in breast cancer, when researchers used cell-surface markers and successfully isolated CSCs[16].Moreover, high tumor-forming ability is an extremely important biological characteristic of CSCs[15].To date, various CSCs have been shown to display different stem cell-surface markers.However, CD44 and CD133 are currently the most commonly used cell-surface markers for the identification of CSCs[5].As noted, because the proportion of each type of CSCs present in solid tumors also differs, it is likely that more cell-surface/intracellular markers will be found in the future that can more accurately determine the presence of CSCs[5].
GCSC-surface markers have also been found using flow cytometry in GC cell lines and primary GC tissues, confirming the presence of CSCs in GC[17-20], as shown in Table 1[21-49], and other potential GCSC-intracellular markers are summarized in Table 2[23,48,50-57].
CD44+, CD44H, CD44 splice variants (CD44v), CD44+/CD24+, CD44+/CD54+, EpCAM+/CD44+
CD44 is a transmembrane glycoprotein that functions as a receptor for hyaluronic acid that is involved in the Wnt/β-catenin pathway that regulates cell migration and homing[21].Takaishiet al[21] showed that CD44+ was first identified as a common GCSC-surface marker using GC cell lines, which demonstrated spheroid formation ability invitroand established tumorigenicity invivowhen injected into immunedeficient mice.Chenet al[22] reported that CD44+ cells have a sphere-forming ability that can be a cell-surface marker of GCSCs.They also provided a reasonable explanation for the chemoresistance that is frequently observed in GC patients.Takafumiet al[23] showed that CD44+ cells have higher potential for peritoneal metastasis associated with GCSC-surface markers.
CD44 has two isoforms: CD44H, which reveals a higher affinity for hyaluronic acid, and a CD44 splice variant, CD44v, which displays prometastatic ability.Ishimotoet al[25] reported that CD44v reduced glutathione (GSH) synthesis and provided protection against reactive oxygen species (ROS).Both CD44H and CD44v may act as GCSC-surface markers.
In addition, CD44+/CD24+ have been examined in GC cell lines and primary GC tissues using flow cytometric analysis[29].It was found that the presence of CD44+/CD24+ was associated with a higher tumorigenicity compared with the control cells (CD44-/CD24-) in an immune-deficient mice model.Hence, these cells have self-renewal and differentiation abilities and the combined expression of CD44+/CD24+ acts as a potential GCSC-surface marker[29].
Combined CD44+/CD54+ cells were isolated from the peripheral blood of GC patients and tumor growth similar to the original human tumor was generated when the cells were injected into immune-deficient mice.The same cells can be differentiated into gastric epithelial cells and self-renewed in vitro.These results suggest that the combined CD44+/CD54+ phenotype could be used as a potential cell-surface marker for GCSCs[30].Another group reported that GCSCs also isolated from human tumor tissues and peripheral blood carried CD44+/CD54+ GCSC-surface markers[22].
Epithelial cell adhesion molecule (EpCAM)+/CD44+ has also been identified as CSC-surface marker in various types of tumors[58-61].EpCAM+/CD44+ cells from human GC tissues grew into tumors in an immune-deficient mice model, they maintained differentiation potency, and reproduced the intratumoral heterogeneity of the original gastric tumors.EpCAM+/CD44+ cells owned anticancer agents than other subtypes of cells[31].Hanet al[31] demonstrated that EpCAM+/CD44+ cells from GC tissues are viable GCSC-surface markers by using an immune-deficient mice model.However, Roccoet al[62] reported that CD44+/CD133+ cells displayed neither SC effects nor revealed tumor-initiating effects.
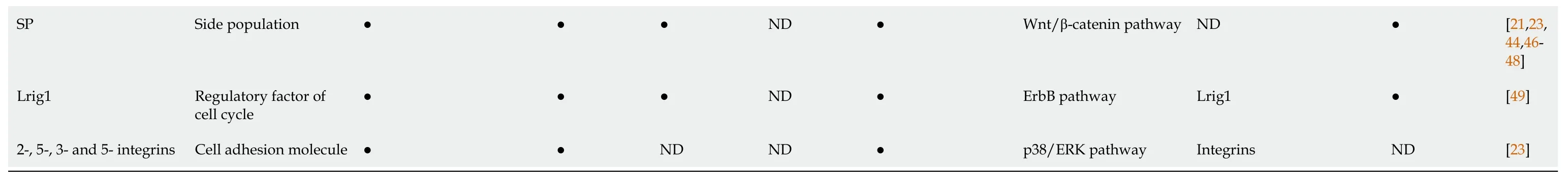
●: Determined; ND: Not determined.CD44: Cluster of differentiation 44; CD44v: CD44 splice variant; Lgr5: Leucine-rich repeat-containing G-protein coupled receptor 5; ALDH1: Aldehyde dehydrogenase 1; CXCR4: C-X-C chemokine receptor type 4; GCSCs: Gastric cancer stem cells; GC: Gastric cancer; EpCAM: Epithelial cell adhesion molecule; Lgr5: Leucine-rich repeat-containing G-protein coupled receptor 5; ABCB1: ATP-binding cassette subfamily B member 1; ABCG2: ATP-binding cassette subfamily G member 2; MDR1: Multidrug resistance protein 1; SP: side population; Lrig1: Leucine rich repeats and immunoglobulin like domains protein 1; SDF-1: Stromal cell-derived factor-1; SSZ: Sulfasalazine; TGF-β: Transforming growth factor-β; HH: Hedgehog; PTCH1: Patched homolog 1; MAPK: Mitogen-activated protein kinase; ERK: Extracellular-signal-regulated kinase; JAK :Janus kinase; STAT3: Signal transducer and activator of transcription 3; mTOR: Mechanistic target of rapamycin; EMT: Epithelial-mesenchymal transition; EGFR: Epidermal growth factor receptor; miRNA: MicroRNA.
CD71-
CD71 belongs to the transferrin receptor that mediates the uptake of transferrin/iron complexes on the surface of red cells.Ohkumaet al[32] reported that the CD71-cell surface marker was increased in the G1/G0 phase causing cell-cycle arrest, and CD71- cell the invasive heads of cancer motivations, showing CD71- cells have high tumorigenicity, multipotency, and invasiveness abilities.Thus, they suggested that CD71- is a suitable GCSC-surface marker for discovering GC in patients.
CD90
CD90 belongs to the immunoglobulin superfamily and the Thy-1 cell-surface antigen (adhesion molecule) family, which is involved in several signal pathways (such as the Notch pathway).Jianget al[33] tested CD90+, a well-known CSC-surface marker in gastric primary tumors.They found that CD90+ GC cells can format spheroid populations, undergo self-renewal, and form a tumor hierarchy from human gastric primary tumorsin vitroand tumorigenicity of gastric primary tumor modelsin vivo.Thus, they suggested that CD90+ is a potential GCSC-surface marker.
CD133
CD133 belongs to the pentaspan transmembrane glycoprotein, which is considered to be a hematopoietic SC-surface marker.Zhaoet al[63] verified that CD133 is significantly related to CSC-surface markers in various tumors; however, whether CD133 is also a significant GCSC-surface marker remains unclear[62,63].
Tandaleah, the Fire Goddess of the Volcano, had finally arrived! The next day I quit my jobs and invested my last paycheck in art supplies and began doing what I loved. I hadn t painted a picture in fifteen years because we d barely scratched out a living on the farm in Missouri and there hadn t been money for the tubes of paint and canvas and frames. I wondered if I could still paint or if I d forgotten how. My hands trembled the first time I picked up a brush, but before an hour had passed I was lost in the colors spreading across the canvas in front of me. I painted pictures of old sailing ships, and as soon as I started believing in myself, other people started believing in me, too. My first painting sold for fifteen hundred dollars before I even had time to frame it.
Leucine-rich repeat-containing G protein-coupled receptor 5
Leucine-rich repeat-containing G protein-coupled receptor 5 (Lgr5) was identified as a novel normal SC-surface marker of the gastrointestinal tract[34,35,64].CD44+, ALDH1+, and CD133+ cell-surface marker cells co-combined with Lgr5+ cells in the SCs of adjacent normal gastric mucosa in GC[34].One group has shown that Lgr5+ cells continuously produced SCs in the gastric glands of transgenic mice under normal homeostatic conditions[35].Another group suggested increased LGR5+ cell-surface marker GCSCs during gastric tumorigenesis may play a role in the development and progression of GC[36].
Aldehyde dehydrogenase 1
Aldehyde dehydrogenase 1 (ALDH1) belongs to the aldehyde dehydrogenase family of enzymes that catalyzes the oxidation of aromatic aldehydes to carboxyl acids.Katsunoet al[39] found ALDH1 to be another novel GCSC-surface/intrcellular marker.The ALDH1+ cells showed higher tumorigenic potential, self-renewal, and produced heterogeneous cell populations compared with ALDH1- cells for a human GC cell linein vitroandin vivo.In addition, they used transforming growth factor- (TGF-) therapy to destroy ALDH1+ cells and their tumorigenicity through downregulation of ALDH1 and regenerating islet-derived protein 4 (REG4)[39].
C-X-C chemokine receptor type 4
By using microarray analysis, studies have found that C-X-C chemokine receptor type 4+ (CXCR4+) cells can be a novel GCSC-surface marker.CXCR4+ cells can form spheroid colonies, and they have high metastatic ability and chemotherapy resistancein vitro.Moreover, CXCR4+ cells have tumorigenicity and GCSC generation capacity in immune-deficient micein vivo.CXCR4+ cells were also found in clinical tissues[42].
Side population, Nanog homeobox, octamer-binding transcription factor 3/4, a2, a5, b3, and b5 integrins
Side population (SP) is well known as a CSC-rich population in many tumors[23,44,46,47].GC cell lines have also isolated SP cells[23,46,65].Furthermore, studies have confirmed that SP cells showed higher engrafted tumor formation and peritoneal metastasis with overexpression levels of adhesion molecules (such as 2, 5, 3, and 5 integrins) and CD44 compared with those of the non-SP cells in GC cell lines.Moreover, the mRNA overexpression of GCSC markers ALDH1, CD44, Nanog homeobox (NANOG), and octamer-binding transcription factor 3/4 (OCT3/4) was significant in SP cells, which are similar to CSCs[23].Fukudaet al[46] described similar results and found that SP cells are more tumorigenic and chemoresistant compared with non-SP cells in GC cell lines and human GC tissues, which remain in an undifferentiated state and display a different hierarchy in malignancy.
Ehataet al[66] found that SP cells display greater tumorigenicity, self-renewal activity, and multipotency of SC phenotypesin vivocompared with non-SP cells in human diffuse-type GC cells but not intestinal-type GC cells.Another group reported that SP cells were smaller and expressed CD133 and MSI-1, which yielded SP and non-SP cells in recultivation experiments[44].Elsewhere it was reported that SP cells also have GCSC properties in the MKN-45 GC cell line.However, SP cells did not have GCSC properties in BGC823 and other GC cell lines[21,48,67].Nevertheless, using the SP cell assay to isolate GCSCs remains debatable.
ATP-binding cassette subfamily B member 1/multidrug resistance protein 1 and ATP-binding cassette subfamily G member 2
Jianget al[33] demonstrated the overexpression of the GCSC markers ATP-binding cassette subfamily B member 1/multidrug resistance protein 1 (ABCB1/MDR1) and ATP-binding cassette subfamily G member 2 (ABCG2) of SP cells in human GC tissues and several GC cell lines.Previous studies found that ABCB1/MDR1 and ABCG2 belong to ABC transporters, which can remove toxic multidrugs extracellularly causing chemoresistance of GCSCs.The expression of these transporters is related to the response to therapy and survival for GC patients[23,43-45].
Dedicator of cytokinesis 6
Chiet al[50] revealed that overexpression of dedicator of cytokinesis 6 (DOCK6) promoted chemoresistance, radioresistance, GC progression, and independent biomarkers of GC prognosis through the Rac family small GTPase 1 (Rac1) activation in the WNT/β-catenin signaling pathway.DOCK6 acts as a guanine nucleotide exchange factor (GEF) for Rac1 and CDC42.These results suggest that DOCK6 is a novel GCSC marker in GC cell lines, animal models, and clinical GC tissues[50].
Muscle, intestine, and stomach expression 1
Leucine-rich repeats and immunoglobulin-like domains protein 1
Leucine-rich repeats and immunoglobulin-like domains protein 1 (Lrig1)+ (a pan-ErbB inhibitor) GCSCs are involved in cell-cycle repression and response to oxidative damage.Loss of APC in Lrig1+ cells leads to intestinal-type GC, or genetic loss of Lrig1 resulting in higher expression of ErbB1-3[56].
Sex-determining region Y-box 2
Microarray studies have found that aberrant expression of sex-determining region Ybox 2 (SOX2) has been observed in GCs.SOX2 transcriptional activity can promote cell proliferation and migration, antiapoptosis, and chemoresistance, and suppress changes in cell cycle and tumorigenic potential in vitro and in vivo for GC development and progression[54].These putative markers may be suitable to isolate GCSCs and offer new insights into novel approaches for GC therapy by targeting GCSCs in clinical trials.It is important to note that most published markers are not specific to GC.Moreover, different GCSCs may coexist within the same tumor mass.Thus, these putative marker GCSCs may also contain SCs and progenitor cells.Therefore, in terms of targeted therapy, identification of a single cell-intracellular marker on GCSCs may not be enough to kill all GCSCs, and thus, a combination of these GCSC markers is essential.
GCSCS REGULATE SIGNALING PATHWAYS IN THE TUMOR NICHE
In recent years, it has been discovered that it is impossible to treat a differentiated tumor without knowing exactly the true group of CSCs.However, normal cancer cells (non-CSCs) can be affected by the TME, epigenetic regulation, and other factors leading to dedifferentiation and a manifestation of CSCs.Therefore, CSCs are a dynamic balance.CSCs can be regulated by stromal cells or could differentiate into tumors with several populations.While internally differentiated tumor populations can occur, dedifferentiation under the influence of the microenvironment can cause cells to revert to CSCs.This idea has greatly affected our biological knowledge of malignant tumors.Awareness of cancer treatment, especially evaluation of treatment that is effective for CSCs, with novel methods of clinical trials should be rethought[10,68].
In addition, CSCs express specific cell-surface/intracellular antigens, and the signaling pathways also differ from non-CSCs, reflecting the characteristics of the cells.Figure 2 shows that non-CSCs can be regulated in the external environment, in signal transmission, and in epigenetic modification[69].The ability to dedifferentiate under genetic control displays a specific characteristic of CSCs[6,70-72].
There are several signaling pathways involved in the drive and maintenance of CSCs in both normal and cancer cells (non-CSCs) (Figure 3).
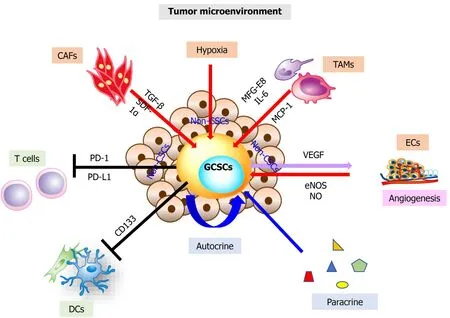
Figure 2 The roles of gastric cancer stem cells in the tumor microenvironment and activated in gastric cancer stem cells.
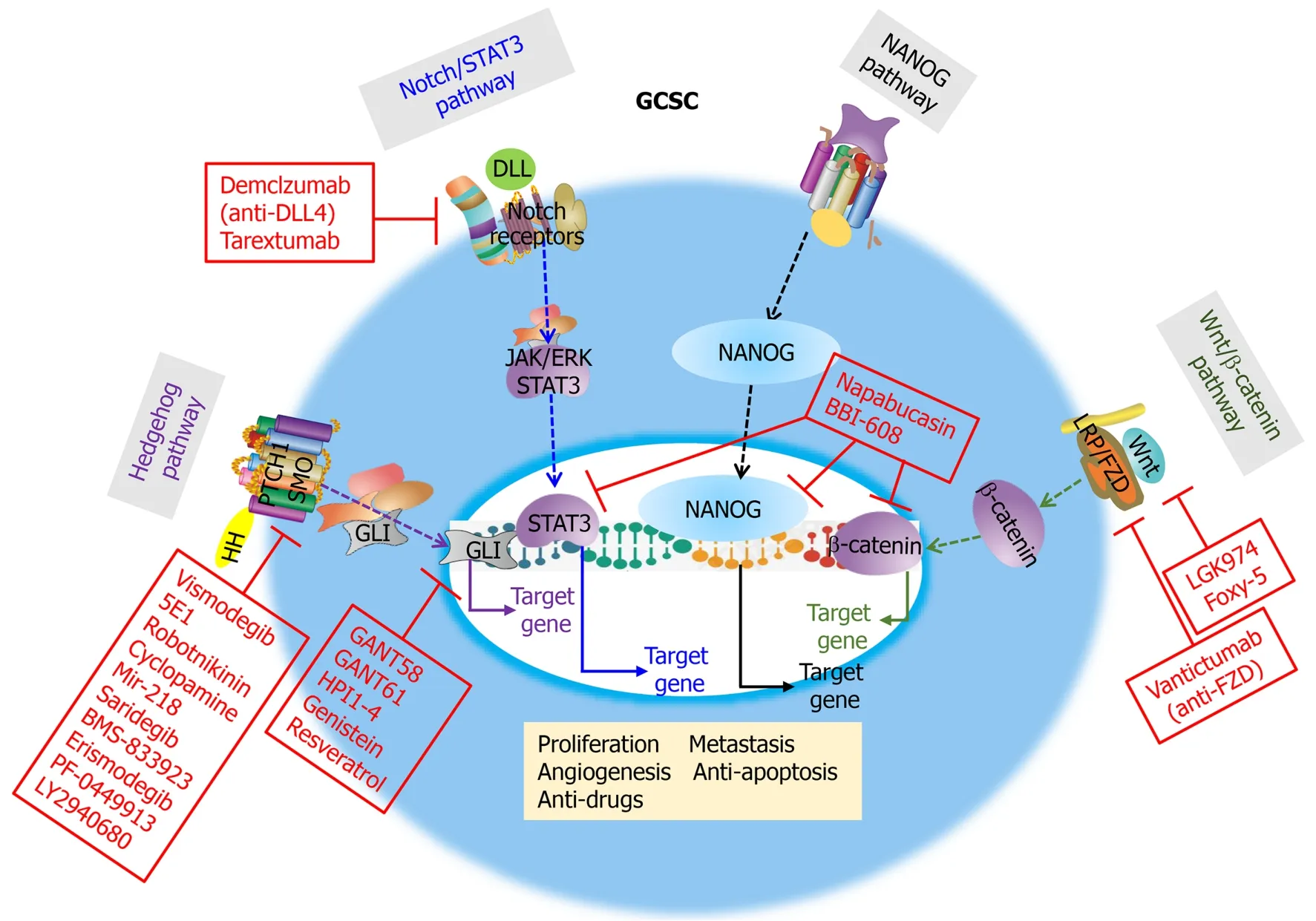
Figure 3 Four signal pathways contribute to stemness properties of cancer stem cells: Hedgehog, Notch/STAT3, NANOG, and Wnt/βcatenin pathways.
Hedgehog pathway
The Hedgehog pathway has a primary role in cell differentiation and normal vertebrate embryonic development[27].The Hedgehog pathway, which regulates adult SCs in tissue maintenance and repair, is inactive in most adult tissues.However, activation of the Hedgehog pathway induces tumor progression and radiation resistance in several cancers to cause mutations in the patched homolog 1 (PTCH1) and smoothened (SMO; frizzled family receptor) genes in patients[31].
Notch/STAT3 pathway
In several cancers, the activati on of the STAT family (such as STAT1, STAT3, STAT4, STAT5a, STAT5b, and STAT6)viaphosphorylation of a specific tyrosine kinase residue promotes tumor growth and metastasis.Among them, STAT3 activation plays a key role in tumor progression[73].Studies show that the action of many protein tyrosine kinases, oncogenes, IL-17, and viruses is mediatedviaactivation of the downstream phosphorylated STAT3 transcription factor pathway.These actions include cellular proliferation, invasion, migration, antiapoptosis, and angiogenesis in GCSCs[74].Additionally, STAT3 upregulated cyclin D1 and c-Myc expression, contributing to enhanced cell-cycle progression in cancer cells.Interestingly, targeting STAT3 activation suppresses tumor growth and metastasis, without affecting normal cellsin vitroandin vivo, suggesting that STAT3 could be effective for GCSC-targeting therapy[75].
NANOG pathway
In embryonic SCs, signal transducer and activator of transcription 3 (STAT3) forms a complex with NANOG for maintaining SC pluripotency.Various studies propose that NANOG may act as an oncogene to be activated in several cancers in which overexpression correlates with poor survival promoting oncogenesis in patients[76].
Wnt/β-catenin pathway
Wnt ligands are produced from cells in the SC microenvironment, and the Wnt/βcatenin pathway has been identified for its role in CSC self-renewal and radiationresistant pathways[30,77].Wnt signaling is associated with EMT with a poor clinical outcome[78].
Due to CSCs have chemotherapy resistant treatments associated with GCSC-related signaling pathways and develops CSCs targeted therapy[79,80].The research on CSC signaling pathways is still preliminary.Their functional regulatory mechanisms are worthy of advanced discussion regarding the similarities and differences of signaling pathways between differentiated cancer cells (non-CSCs) and CSCs.A solution to these problems can help understand the resistance phenomenon caused by clinical treatment of tumors, and design approaches that could provide an important reference for anti-CSCs targeting drugs[81].Therefore, these CSC-related pathways are commonly new targets for the development of anticancer drugs or inhibitors in various clinical trials[69,82] (Table 3)[27,28,73,75-78,83-86].
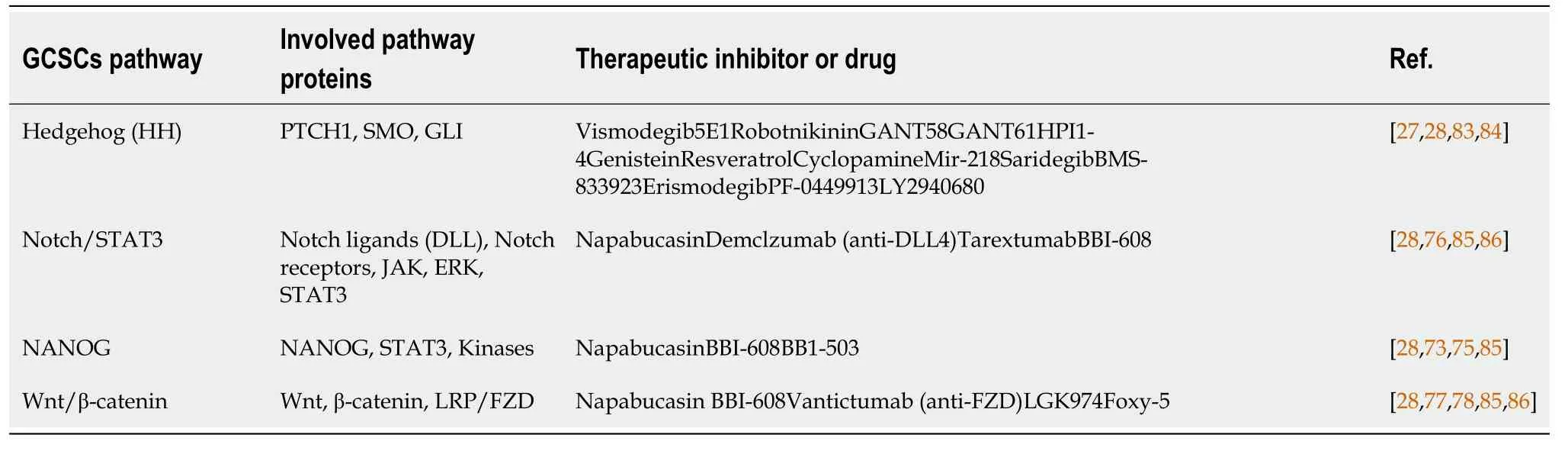
Table 3 Regulators of gastric cancer stem cells-related pathway stimuli in gastric cancer stem cells stemless properties and gastric cancer stem cells-targeted therapy
CURRENT TREATMENT OF THE POTENTIAL FOR TARGETING GCSCS
In general, most of the initial tumor appears reduced in size after treatment.However, the appearance of drug resistance after treatment is common because a subpopulation of differentiated cancer cells (non-CSCs) develops drug resistance.CSCs are more resistant to conventional chemotherapy, EMT, more antiapoptotic factors, and higher performance of drug efflux pumps than differentiated cancer cells (non-CSCs)[6,8,71,72].
The reason CSC theory has received considerable attention in recent years is because CSCs can escape the poison of chemotherapy drugs, which can explain the failure of chemotherapy.
Usually CSCs are parked in the cell cycle G0phase, that is, they will not enter the cell cycle.Currently, most chemotherapeutic drugs focus on inhibiting the cell cycle in a growing cell.The growth of differentiated cancer cells (non-CSCs) in the cell cycle can be prevented, but the drugs cannot kill CSCs.Once chemotherapy is over, the surviving CSCs will regenerate and proliferate, leading to the recurrence of cancer.Because some studies show that chemotherapeutic drugs may promote CSCs after treatment, it is recommended to use two-stage therapy to kill CSCs.Therefore, the first stage is to promote CSCs into the cell cycle, and the second stage uses chemotherapy drugs or targeted CSC therapy to kill CSCs[87].Several studies have indicated that CSCs promote recurrence after chemotherapy[6].
It has also been reported that CSCs will increase tumor cell invasion, EMT, and metastatic ability[4-7].Another group pointed out that CSCs will increase the protein level of the chemokine receptor CXCR4; thus, CSCs will follow the of its stromal cellderived factor-1 (SDF-1; CXCR4 ligand) concentration degree of metastasis, from the original position (low levels of SDF-1) in CSCs microenvironment (tumor niche) transfer to any tissue that exhibits high levels of SDF-1.CXCR4 neutralizing antibody can effectively inhibit CSC metastasis in nude mice.These results confirm that CSC cells can indeed promote cancer EMT, metastasis, and invasion abilities[47].
Wanget al[88] and Ricci-Vitianiet al[89] found that glioma cells are derived from neuron-like glioma stem cells.They also found that the tumor tissue and the tumor inside vascular ECs (vascular endothelial cells) both have the same aberrant genes.Researchers have found that these tumor-derived vascular ECs come from CSCs, that is, CSCs can differentiate into vascular ECs to form new blood vessels.This result challenges the existing belief that the internal vascular ECs of a tumor are normal cells.It also explains why combretastatin A-4 (a vascular disrupting agent) can selectively abolish the existing tumor vascular ECs but is less harmful to normal blood vessels.
FURTHER CHALLENGES OF ANTIGCSCS THERAPY
Currently, scholars have different opinions on the origin of GCSCs.Additionally, various tumors do not have the same origin of CSCs and our understanding needs to be deepened.Importantly, GCSCs play an important role in the occurrence and development of tumors, EMT, metastasis, recurrence, and prognosis.
If only GCSCs are removed, is such treatment sufficient? Obviously, differentiated cancer cells (non-CSCs) also need targeted therapy.Moreover, both GCSCs and their differentiated cancer cells (non-CSCs) require effective targeted therapy.In the future, newly developed drugs will provide opportunities for successful cancer treatment to improve the patient’s prognosis.Efficacy of these drugs must first be confirmed by clinical trials.
Given that research in GCSCs is an emerging field, many details remain unclear, and future GCSC research will need to focus on several key issues: (1) The determination of GCSC-specific molecules of cell-surface/intracellular markers; (2) The environment of the GCSC niche, the interaction and molecular mechanism between the GCSC tumor niche and GCs; (3) The molecular mechanism of malignant transformation/EMT of normal SCs; (4) The need to verify and clarify the mechanism of GCSC chemo-/radioresistance therapy; (5) Exploration and development of therapeutic approaches for GCSCs; and (6) Overall, monitoring of the role of GCSCs to evaluate the indicators (monitoring circulating tumor cells), it means that may appear for new metastatic lesions which can be early detected.Therefore, with the evaluation of clinical trials involving GCSCs, the results of GCSC therapy will help improve anticancer treatment.GCSC-targeted drug therapy alone may not be sufficient to fight cancer, but combined treatment can improve treatment results.Finally, GCSC theory provides a new way to evaluate anticancer drugs in clinical trials and provide a framework for effective drug development.We believe that cancer can be cured, or at least, become a chronic and controllable disease; this goal will surely be achieved in the future.
Following improvements in research technology, it is hoped that a better understanding of GCSC-surface/intracellular markers and related signal pathways will help antitumor growth, early diagnosis, GCSC-targeted drug therapy, antimetastasis, anti-EMT, as well as recurrence prevention and prognosis judgment, all of which have significant therapeutic application.
CONCLUSION
In recent years, an increasing number of studies have highlighted that CSCs are indeed tumor-initiating cells in cancer tissues.These cells may not be a single homogeneous cell: different tissues or different patients have different tumor initiation cells.The more we understand these GCSCs or tumor-initiating cells, the better we can design drugs to kill them, such as using second-stage therapy to kill GCSCs[87] or designing a monoclonal Ab (antibody) or small interference RNA (siRNA) that blocks GCSCsurface/intracellular markers.For example, CD44 can promote the differentiation of GCSCs to complete the treatment.With the joint efforts of basic researchers and clinicians, the hope is that cancer can be effectively controlled in the future.
ACKNOWLEDGEMENTS
The corresponding author would like to thank theWorld Journal of Stem Cellsfor the opportunity and acknowledge the hard work of all supporting authors.
杂志排行
World Journal of Stem Cells的其它文章
- Cardiac stem cells: Current knowledge and future prospects
- Multiple roles of mothers against decapentaplegic homolog 4 in tumorigenesis, stem cells, drug resistance, and cancer therapy
- Modified mesenchymal stem cells in cancer therapy: A smart weapon requiring upgrades for wider clinical applications
- Epigenetic regulation by long noncoding RNAs in osteo-/adipogenic differentiation of mesenchymal stromal cells and degenerative bone diseases
- Therapeutic effects of menstrual blood-derived endometrial stem cells on mouse models of streptozotocin-induced type 1 diabetes
- Stem cell therapy applied for digestive anastomosis: Current state and future perspectives
