Cardiac stem cells: Current knowledge and future prospects
2022-02-12RadwaMehannaMarwaEssawyMonaBarkatAshrafAwaadEmanThabetHebaHamedHagarElkafrawyNehalKhalilAbeerSallamMarwaKholiefSamarIbrahimGhadaMourad
Radwa A Mehanna, Marwa M Essawy, Mona A Barkat, Ashraf K Awaad, Eman H Thabet, Heba A Hamed,Hagar Elkafrawy, Nehal A Khalil, Abeer Sallam, Marwa A Kholief, Samar S Ibrahim, Ghada M Mourad
Radwa A Mehanna, Eman H Thabet, Abeer Sallam, Medical Physiology Department/Center of Excellence for Research in Regenerative Medicine and Applications, Faculty of Medicine, Alexandria University, Alexandria 21500, Egypt
Marwa M Essawy, Oral Pathology Department, Faculty of Dentistry/Center of Excellence for Research in Regenerative Medicine and Applications, Faculty of Medicine, Alexandria University, Alexandria 21500, Egypt
Mona A Barkat, Human Anatomy and Embryology Department/Center of Excellence for Research in Regenerative Medicine and Applications, Faculty of Medicine, Alexandria University, Alexandria 21500, Egypt
Ashraf K Awaad, Samar S Ibrahim, Center of Excellence for Research in Regenerative Medicine and Applications, Faculty of Medicine, Alexandria University, Alexandria 21500, Egypt
Heba A Hamed, Ghada M Mourad, Histology and Cell Biology Department/Center of Excellence for Research in Regenerative Medicine and Applications, Faculty of Medicine, Alexandria University, Alexandria 21500, Egypt
Hagar Elkafrawy, Nehal A Khalil, Medical Biochemistry Department/Center of Excellence for Research in Regenerative Medicine and Applications,Faculty of Medicine, Alexandria University, Alexandria 21500, Egypt
Marwa A Kholief, Forensic Medicine and Clinical toxicology Department/Center of Excellence for Research in Regenerative Medicine and Applications,Faculty of Medicine, Alexandria University, Alexandria 21500, Egypt
Abstract Regenerative medicine is the field concerned with the repair and restoration of the integrity of damaged human tissues as well as whole organs.Since the inception of the field several decades ago, regenerative medicine therapies, namely stem cells, have received significant attention in preclinical studies and clinical trials.Apart from their known potential for differentiation into the various body cells, stem cells enhance the organ's intrinsic regenerative capacity by altering its environment, whether by exogenous injection or introducing their products that modulate endogenous stem cell function and fate for the sake of regeneration.Recently, research in cardiology has highlighted the evidence for the existence of cardiac stem and progenitor cells (CSCs/CPCs).The global burden of cardiovascular diseases’ morbidity and mortality has demanded an in-depth understanding of the biology of CSCs/CPCs aiming at improving the outcome for an innovative therapeutic strategy.This review will discuss the nature of each of the CSCs/CPCs, their environment, their interplay with other cells, and their metabolism.In addition, important issues are tackled concerning the potency of CSCs/CPCs in relation to their secretome for mediating the ability to influence other cells.Moreover, the review will throw the light on the clinical trials and the preclinical studies using CSCs/CPCs and combined therapy for cardiac regeneration.Finally, the novel role of nanotechnology in cardiac regeneration will be explored.
Key Words: Cardiac stem and progenitor cells; Cardiac stem cells’ secretome; Cardiac stem cells’ niche and metabolism; Nanotechnology; Clinical trials; Combined therapy
INTRODUCTION
Cardiovascular diseases are the leading cause of death globally, as stated by the latest report 2019 for the World Health Organization, with 17.9 million deaths per year, accounting for 31% of all deaths worldwide.
The heart is one of the least proliferative organs in the human body, and its minimal regenerative capacity has been dogma for decades.Such dogma has been led by the belief that the heart cannot regenerate from ischemic damage.The absence of primary tumors in the heart has further supported the notion of low proliferation.In an alleged post-mitotic organ, it has been debatable whether cardiac cells repair through activation of resident cardiac stem cells (CSCs) and cardiac progenitor cells (CPCs) or by the proliferation of pre-existing cardiomyocytes (CMs).In 2009, Bergmannet al[1] were the first to refute that notion and have reported that the heart can in fact selfrenew.Based on the results obtained from their carbon-14-labelled DNA study to track CMs, Bergmannet al[1] stated that about 50% of CMs renew over the lifespan of an adult.Hsiehet al[2] provided further evidence for the origin of newly generated CMs from progenitor cells in an alpha myosin heavy chain (MHC) transgenic model.They estimated that approximately 15% of CMs can regenerate in adult hearts following ischemic damage.With progression of research, lineage tracing of regenerated cardiac tissue confirmed that the newly regenerated CMs develop from a non-CM and possibly from stem cells (SCs)[2].
Further studies have revealed various CSC/CPC candidates that are morphologically and functionally distinct from each other yet act in a complementary fashion and contribute to the regeneration process.This complex cell aggregation is known as the CSC niche that has been a challenge to characterize and locate anatomically[3].
SC applications have been under intensive research interest since the early 20thcentury.Many types have been isolated, starting from the embryonic, amniotic, and cord blood mesenchymal stem cells (MSCs) and passing through the adult SCs till the induced pluripotent SCs (iPSCs).Adult MSCs are undifferentiated cells with the same potentials as progenitor cells regarding the ability to differentiate into all three germ layer cells[4].Exogenous MSCs from various sources, including bone marrow, adipose tissue, umbilical cord, placenta, and amniotic fluid[5], have shown promising results in the treatment of cardiovascular diseases.However, the outcome of CSC therapy has shown superior results in experimental studies but to a lesser extent in human clinical trials[6].The applications of SC therapy for cardiovascular regeneration still hold a plethora of queries to be answered as well as commandment of the molecular and signaling features for CSCs in order to standardize this therapy.Among the aspects that need optimization are the types of SCs and supporting cells to be used, the number of cells, the route of injection, the frequency, and best timing for transplantation.Standardization requires an advanced understanding of the full biological features of CSCs.
SC therapy in cardiac regeneration has dual beneficiary actions.Primarily, the transplanted exogenous SCs would directly differentiate into CMs.Concomitantly, SCs activate the endogenous progenitors through their rich secretome of extracellular vesicles, immunomodulatory and growth factors, protein, and nucleic acid families[7].These paracrine factors act to activate resident SCs and enhance vascularization to potentiate cardiac repair.
This review aims to provide insight into CSCs/CPCs regarding their embryological origin, populations, niche, metabolism, secretome, and therapeutic potentials.Also discussed is the interplay of nanotechnology with SCs in several aspects, including differentiation, tracking, imaging, and assisted therapy, showing the prospects and limitations of nanoparticle (NP)-based cardiac therapy.Finally, preclinical trials and ongoing, completed, and future clinical trials using CSCs and combined therapy are shown to delineate the potential applications in treating cardiac disease.
EMBRYOLOGICAL ORIGIN OF CPCs
The heart is formed of a wide range of cell types originating from the mesodermal precursor cells.They include CMs and endocardial cells forming the inner layer, while epicardial-derived cells (EPDCs) and smooth muscle cells (SMCs) are found on the external layer.Differentiation of the mesodermal cells is initiated by the T-box transcriptional factors Brachyury (Bry) and Eomes.Bry+cells differentiate into insulin gene enhancer protein islet-1 (ISL1) and T-box transcription factor 5 (TBX5) expressing cells, while Eomes induce expression of mesoderm posterior 1 (MESP1).MESP1+cells are identified before the first heart field (FHF) and the second heart field (SHF) separations, so MESP1 serves as an indicator of early CPCs for both heart fields[8].Chemokine receptor type 4 (CXCR4), fetal liver kinase 1 (FLK-1), and platelet derived growth factor receptor A are other surface markers that coincide with MESP1 and are used in combination to isolate CPCs[9,10].
In addition, a novel cell surface marker known as G protein-coupled receptor lysophosphatidic acid receptor 4 is specific to CPCs and determines its functional significance.Interestingly, its transient expression peaks in cardiac progenitors after 3 to 7 d of human (h)PSCs differentiation toward cardiac lineage, then it declines.In vivo,lysophosphatidic acid receptor 4 shows high expression in the initial stages of embryonic heart development and decreases throughout development[11].
The FHF cells are the firstly differentiated myocardial cells that are derived from cells in the anterior lateral plate mesoderm; they give rise to the left ventricle, partially some of the right ventricle population, sinoatrial node, atrioventricular node, and both atria[12].Meanwhile, the SHF cells originate from the pharyngeal mesoderm to the posterior side of the heart and further divide into anterior and posterior SHF.They contribute to the right ventricle, atria, and the cardiac outflow tract (OFT) formation.Addition of the SHF-derived CMs to the ventricles depend on myocyte enhancer factor 2C (MEF2C).It has been found that MEF2C null mice die at 9.5-d post conception with severe heart defects due to failure of heart looping[13].In OFT formation, two waves of SHF progenitors and their derivatives have been identified, making a differential contribution to the aorta and pulmonary artery.The early wave of cells is favorably directed to the aorta, while the second wave of cells contributes to the pulmonary artery.Phosphoinositide-dependent kinase-1 critically regulates the second wave of cells, and its deletion results in pulmonary stenosis[14].The epicardium of the heart is formed of a transient proepicardial organ.Proepicardium is formed from homeobox protein NKx2.5 (NKx2.5) and ISL1+cells.After epicardial formation, subepicardial mesenchymal space is formed by epithelial to mesenchymal cell transformation of the epicardial cells[15] (Figure 1).
The differentiation in the posterior SHF is regulated byHoxb1gene.Stimulation ofHoxb1in embryonic stem cells (ESCs) halts cardiac differentiation, whileHoxb1-deficiency shows premature cardiac differentiation in embryos.Moreover, an atrioventricular septal defect develops as a result of ectopic differentiation in the posterior SHF of embryos deficient inHoxb1and its paralogHoxa1[16].
Multiple signaling pathways have essential roles in cardiogenesis with a sequential arrangement.The transforming growth factor-β (TGF-β) superfamily, retinoic acid, Hedgehog, Notch, Wnt, and fibroblast growth factors (FGFs) pathways comprise the chief signaling pathways involved in cardiac development.These pathways, along with transcription factors and epigenetic regulators, regulate cardiac progenitors’ specification, proliferation, and differentiation into the different cardiac cell lineages[17].
SIGNALING PATHWAYS DURING CARDIOGENESIS
TGF-β superfamily
The TGF-β superfamily members consist of over 30 structurally associated polypeptide growth factors including nodal and bone morphogenetic proteins (BMP)[18].
Nodal signaling is vital for the formation of sinoatrial node.Nodal inhibition during the cardiac mesoderm differentiation stage downregulatesPITX2c,a transcription factor recognized to inhibit the formation of the sinoatrial in the left atrium during cardiac development[19].Moreover, nodal signaling is dispensable for initiation of heart looping; however, it regulates asymmetries that result in a helical shape at the heart tube poles[20].
BMP signaling, as a member of TGF-β, has an important role in the different stages of heart development including the OFT formation, endocardium, and lastly the epicardium.The cardiac neural crest cells have a crucial role in normal cardiovascular development.They give rise to the vascular smooth muscle of the pharyngeal arch arteries, OFT septation, valvulogenesis, and development of the cardiac conduction system[21] (Figure 1).The role of BMP in OFT septation mainly depends on their gradient signaling, which arranges neural crest cell aggregation along the OFT; this Dullard-mediated tuning of BMP signaling ensures the fine timed zipper-like closure of the OFT by the neural crest cells[22].Furthermore, the BMP signaling promotes the development of endocardial cells (ECs) from hPSC-derived cardiovascular progenitors[23].It is also integrated with Notch signaling for influencing the proepicardium formation, where overexpression of Notch intracellular receptor in the endothelium enhances BMP expression and increases the number of phospho-Smad1/5+cells for enhancing the formation of the proepicardium[24].
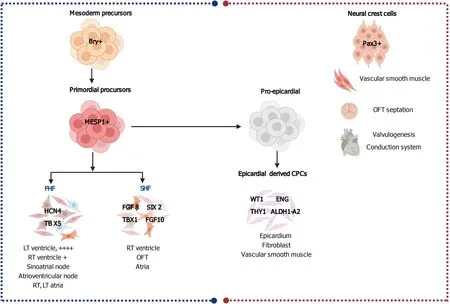
Figure 1 Embryonic cardiac progenitors, Brachyury-positive mesoderm precursors and Pax3+ neural crest cells.
Retinoic acid, hedgehog, and Notch signaling pathways
Retinoic acid signaling plays a role in heart development.It is a key factor for efficient lateral mesoderm differentiation into atrial-like cells in a confined time frame.The structural, electrophysiological, and metabolic maturation of CMs are significantly influenced by retinoic acid[25].However, it is reported that retinoic acid receptor agonists transiently enhance the proliferation of human CPCs at the expense of terminal cardiac differentiation[26].
The downregulation of the retinoic acid responsive gene, ripply transcriptional repressor 3 (RIPPLY3), within the SHF progenitors by histone deacetylase 1 is required during OFT formation[27].
Hedgehog signaling has a role in OFT morphogenesis.Lipoprotein-related protein 2 (LRP2) is a member of the LDL receptor gene family, a class of multifunctional endocytic receptors that play crucial roles in embryonic development.LRP2 is expressed in the anterior SHF cardiac progenitor niche, which leads to the elongation of the OFT during separation into aorta and pulmonary trunk.Loss of LRP2 in mutant mice results in depleting a pool of sonic hedgehog-dependent progenitor cells in the anterior SHF as they migrate into the OFT myocardium due to premature differentiation into CMs.This depletion results in aberrant shortening of the OFT[28].
Four Notch receptors (Notch1-Notch4) and five structurally similar Notch ligands [Delta-like (DLL) 1, DLL3, DLL4, Jagged1, and Jagged2] have been detected in mammals[29].Activation of Notch signaling enhances CM differentiation from human PSCs.However, the CMs derived from Notch-induced cardiac mesoderm are developmentally immature[30].In vivo, the Notch pathway plays a significant role in CPC biology.An arterial-specific Notch ligand known as DLL4 is expressed by SHF progenitors at critical time-points in SHF biology.The DLL4-mediated Notch signaling is a crucial requirement for maintaining an adequate SHF progenitor pool, in a way thatDLL4knockout results in decreased proliferation and increased apoptosis.Reduced SHF progenitor pool leads to an underdeveloped OFT and right ventricle[31].
Wnt pathway
The Wnt signaling pathway has an essential role in many developmental stages of embryogenesis.The Wnt family consists of 19 distinct Wnt proteins and other 10 types of Frizzled receptors.On the basis of their primary functions, the Wnt and Frizzled receptors are divided into two major classes, which are the canonical and noncanonical Wnt pathways[32].Accumulating evidence suggests a role for the dynamic balance between canonical and non-canonical Wnt signaling in cardiac formation and differentiation.Wnt/β-catenin signaling is required for proper mesoderm formation and proliferation of CMs but needs to be low for terminal differentiation and cardiac specification.In contrast, for cardiac specification in murine and human ESCs, noncanonical β-catenin independent Wnt signaling is essential, while the non-canonical Wnt signaling is necessary for terminal differentiation later in development[33].
The activation of non-canonical Wnt is non-catenin-independent, and the downstream proteins involve several kinases, including protein kinase C, calcium/ calmodulin-dependent kinase, and Jun N terminal kinase (JNK).Wnt11 enhances angiogenesis and improves cardiac function through non-canonical Wnt-protein kinase C-Jun N terminal kinase dependent pathways in myocardial infarction (MI)[34].In hypoxia, Wnt11 expression preserves the integrity of mitochondrial membrane and facilitates the release of insulin growth factor-1 (IGF-1) and vascular endothelial growth factor (VEGF), thus protecting CMs against hypoxia[35].Canonical dependent Wnt signaling, Wnt 3 Ligand, favors the pacemaker lineage, while its suppression promotes the chamber CM lineage[36].
TRANSCRIPTOME AND REGENERATIVE CAPACITY OF SUB-POPULATIONS
The regenerative capacity of most organs is contingent on the adult SC populations that exist in their niches and are activated by injury.Adult SC populations vary greatly in their molecular marker expression profile and hence in their possible role in regenerative medicine.The transcriptome is a representation of the gene read-outs, the cellular state, and is imperative for studying all genetic disease and biological processes.The genome-wide profiling using novel sequencing technology has made transcriptome research accessible.
c-KIT+ CPCs
Receptor tyrosine kinase (RTK) c-KIT (also referred to as SC factor receptor or CD117)-expressing CPCs are mainly located in the atria and the ventricular apex, comprising most of the ventricular and atrial myocardium[37].c-KIT+cells also express the cardiac transcription factors NKx2.5, GATA binding protein 4 (GATA4), and MEF2C but are negative for the hematopoietic markers CD45, CD3, CD34, CD19, CD16, CD20, CD14, and CD56[38,39].SC factor ligand attaches to the c-KIT receptor and activates the phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) and p38 mitogen-activated protein kinase (MAPK) signaling pathways[40].Both PI3K/AKT and MAPK pathways control various CPCs functions like self-renewal, proliferation, migration, and survival[41].During embryonic development and the early post-natal time, c-KIT+CPCs contribute to the generation of new CMs.Such capacity declines in the adult heart with only a few new CMs originating from CPCs[42].In a rat MI model, the c-KIT+CPCs have migrated through the collagen type I and type III matrices into the infarcted area.The transplanted CPCs have shown overexpressed matrix metalloproteinases (MMPs; MMP2, MMP9, and MMP14) that degrade extracellular matrix (ECM), concluding that c-KIT+CPCs hold an invasive capacity[43].Transplanted CPCs (c-KIT+CPCs and cardiospheres) also show an endogenous proliferative potentialin vivoand additionally activate endogenous CPCs[44].
SCA-1+ CPCs
Stem cell antigen 1 (SCA-1) expressing CPC population exists predominantly in the atrium, intra-atrial septum, and atrium-ventricular boundary and dispersed inside the epicardial layer of adult hearts[45].SCA-1 is a cell surface protein of the lymphocyte antigen-6(Ly6)gene family, which has roles in cell survival, proliferation, and differentiation[46].A population of SCA-1+cells from murine adult myocardium hold a telomerase activity comparable to that of a neonatal heart.This SCA-1+population is different from hematopoietic SCs as they lack CD45, CD34, c-KIT, LIM domain only 2, GATA2, VEGF receptor 1, and T-cell acute lymphoblastic leukemia 1/SC leukemia proteins.SCA-1+cells are also distinct from endothelial progenitor cells and express cardiac lineage transcriptional factors such as GATA4, MEF2C, and translation elongation factor 1 yet lack transcripts for cardiomyocytic structural genes such asBMP1r1andα-, β-MHC[47,48].Although this population exhibits the endothelial marker CD31, it is suggested to be due to the contaminating endothelial CD31+/SCA-1+cells.In vitrostudies have revealed that 5-azacytidine (5-aza), a demethylating agent, pushed SCA-1+cells to differentiate into CMs[48,49].Further studies have isolated SCA-1+cells that lack CD31 and CD45 markers, referring to them as lineage negative (Lin-).The SCA-1+/Lin-cells display a mesenchymal cell-surface profile (CD34-, CD29+, CD90+, CD105+, and CD44+) and are able to differentiate, to a certain extent, into CMs and endothelial and smooth muscle-like cells[50,51].
Human SCA-1+-like cells also express early cardiac transcription factors (GATA4, MEF2C, insulin gene enhancer protein ISL-1, and Nkx-2.5) and can differentiate into contractile CMs[52].Although a human ortholog of the SCA-1 protein has not been yet identified, an anti-mouse SCA-1 antibody is used to isolate SCA-1+-like cells from the adult human heart.
MESP1+ CPCs
MESP1 expressing cells mainly contribute to the mesoderm and to the myocardium of the heart tube during development[53].Transient expression of MESP1 seems to accelerate and enhance the appearance of cardiac progenitor.However, homologous disruption of theMESP1gene has resulted in aberrant cardiac morphogenesis.MESP1interacts with the promoter area of main cardiac transcription factors, including heart and neural crest derivatives expressed 2, Nkx2-5, myocardin, and GATA4[54].These factors induce fibroblasts to express a full battery of cardiac genes, form sarcomeres, develop CM-like electrical activity, and in a few cases elicit beating activity[55].Several studies have shown that the addition of MESP1 could enhance the efficacy of direct reprogramming of fibroblasts into CMs[56,57].The transdifferentiation of fibroblasts to CMsvia MESP1suggests thatMESP1chiefly modulates the gene regulatory network for cardiogenesis[52].
KDR+ CPCs
Kinase insert domain receptor (KDR), also known as Flk-1, is one of the earliest discovered cardiogenic progenitor cell markers acting during the early stages of cardiac development in human[58].Nelsonet al[59] have reported that Flk-1 has a distinctive transcriptome that has been evident at day 6, immediately after gastrulation but prior to the expression of the cardiac transcription factors.KDR+population lack the pluripotent octamer-binding transcription factor 4, sex determining region Y-Box transcription factor (SOX) 2, and endoderm SOX17 markers.On the other hand, KDR+CPCs have shown a noteworthy upregulation in SOX7, a vasculogenic transcription factor, overlapping with the emergence of primordial cardiac transcription factors GATA4, myocardin, and NKx2.5.Moreover, KDR subpopulations that overexpress SOX7 are associated with a vascular phenotype rather than a cardiogenic phenotype.These outcomes offer insights for refining the therapeutic regenerative interventions.
CPCs from the first and second heart fields
The FHF cells express hyperpolarization activated cyclic nucleotide gated potassium channel 4 and TBX5, while SHF progenitors express TBX1, FGF 8, FGF10, and sine oculis homeobox2 (Figure 1).Cells from the SHF exhibit high proliferative and migratory capacities and are mostly responsible for the elongation and winding of the heart tube.Moreover, SHF cells differentiate to CMs, SMCs, fibroblasts, and endothelial cells (ECs) along their journey in the heart tube to form the right ventricle, right ventricular OFT, and most of the atria[60,61].However, FHF cells hold less proliferative and migratory potentials and differentiate predominantly to CMs that form the left ventricle and small parts of the atria[62].The cells of the cardiac crescent, theoretically the progeny of FHF CPCs, are terminally differentiated cells expressing the markers of CMs, such as actin alpha cardiac muscle 1 and myosin light chain 7[63,64], hence they are unlikely to be multipotent progenitors.Therefore, it is difficult to identify FHF before Nkx2.5 and TBX5 expressions.Conversely, multipotent SHF CPCs were validated with a clonal tracing experiment and identified by ISL1 expression[65].However, ISL1 expression is not specific for SHF and has been proposed to represent only the developmental stages[66].Tampakakiset al[67] generated ESCs by using hyperpolarization activated cyclic nucleotide gated potassium channel 4-green fluorescent protein and TBX1-Cre; Rosa-red fluorescent protein reporters of the FHF and the SHF respectively, and also by using live immunostaining of the cell membrane CXCR4, a SHF marker and the reporters.The ESC-derived progenitor cells have shown functional properties and transcriptome similar to theirin vivoequivalents.Thus, chamber-specific cardiac cells have been generated for modelling of heart diseasesin vitro.
Epicardium-derived CPCs
The EPDCs are important as a signaling source for heart development, cardiac regeneration, and post-MI heart repair.Throughout the development of the heart in mice, EPDCs aid in the formation of various cardiac cell types and secrete paracrine factors for myocardial maturation[68].In the adult heart, EPDCs are normally dormant and become stimulated following myocardial injury.Transcriptional analysis of the EPDCs derived from human (h)iPSCs cells have revealed several markers of EPDCs including Wilm’s tumor protein 1, endoglin, thymus cell antigen 1, and aldehyde dehydrogenase 1 family member A2[69] (Figure 1).Following MI in mice, EPDCs undergo an epithelial-to-mesenchymal transition, with overexpression of Wilms tumor protein 1, and differentiate mainly into SMCs/fibroblasts[70,71].EPDC-secreted paracrine factors include VEGF-A, FGF2, and PDGF-C, which support the growth of blood vessels, protect the myocardium, and recover cardiac functions in an acute MImouse model[70].
Side population-derived CPCs
Side population (SP) cells have been detected in the heart and other various tissues and hold enhanced stem and progenitor cell activity[72].SP cells, when stainedin vitro,hold the ability to flush out the DNA Hoechst dye from their nuclei[73].Gene expression profiling of SP cells after MI has revealed a downregulation of Wnt-related signals coupled with increased SP cell proliferation.This has been validatedin vitroby treatment of isolated SP cells with canonical Wnt agonists or recombinant Wnt, where the proliferation of SP cells has been repressed with partial arresting the G1 cell cycle phase[74].Consistent with this observation, delivery of secreted Frizzled-related proteins (SFRP; the Wnt antagonizer) improves post-MI remodeling[75,76].
SP cells can be identified by surface marker adenosine triphosphate (ATP) binding cassette subfamily G member 2 (ABCG2), also referred to as the breast cancer resistance protein1[77].ABCG2+cells have been also observed in the adult heart and can differentiatein vitrointo CMs[78].When SP cells have been injected into the injured hearts of rats, they have been recruited to the injured regions, where they differentiate into CMs, ECs, and SMCs, suggesting that they may be endogenous SP cells[79].However, ABCG2-CreER based genetic lineage tracing has demonstrated that ABCG2+cells could only differentiate into the multiple cardiac cell lineages during the embryonic stages but not in adulthood[80,81].The combination of ABCG2+cells with pre-existing CMs is more likely to stimulate CM proliferation rather than differentiation into CMs directly[82].Therefore, genetic fate mapping investigations have disproved the SP cells property of the adult endogenous ABCG2+SP and theirin vivorenewing myogenic ability[83].
Cardiosphere-derived CPCs
Cardiospheres contain a combinatio n of stromal, mesenchymal, and progenitor cells that are isolated from cultures of human heart biopsy[39,84].They represent a nichelike environment, with cardiac-committed cells in the center and supporting cells in the periphery of the spherical cluster[85].The cardiosphere-derived cells (CDCs) were originally isolated from mouse heart explants and human ventricular biopsies based on their ability to form three-dimensional (3D) spheroids in suspension cultures[86].CDCs have grabbed much attention due to their proliferation and differentiation abilities by inherent stimulation of cardio-specific differentiation factors [GATA4, MEF2C, Nkx2.5, heart and neural crest derivatives expressed 2, and cardiac troponin T (TNNT2)] using a clustered regularly interspaced short palindromic repeat/dead Cas9 (CRISPR/dCas9) assisted transcriptional enhancement system[87,88].Sanoet al[89] have postulated that the CRISPR/dCas9 system may provide a proficient method of modifyingTNNT2gene activation in SCs.Consequently, CRISPR/dCas9 can improve the therapeutic outcomes of patients with ischemic heart disease by enhancing the transplanted CDCs differentiation capacity within the ischemic myocardium.Heart tissue is usually obtained by endomyocardial biopsy or during open cardiac surgery and grown in explants to form CDCs.CDCs have shown a superior myogenic differentiation potential, angiogenesis, and paracrine factor secretion as compared to other cell types.In heart failure animal models, the injected CDCs potentially differentiated into CMs and vascular cells.Additionally, CDCs have diminished unfavorable remodeling and infarct size, and hence improve cardiac function[90].Accordingly, cardiospheres and CDCs may be some of the most promising sources of CPCs for cardiac repair.
CSC niche
The niche in the heart integrates several heterogeneous cell types, including CSCs, progenitors, fibroblasts, SMCs, CMs, capillaries, and supporting telocytes (TCs)[91], together with the junctions and cementing ECM that hold the niche together.Such architectural arrangement is essential for protection against external damaging stimuli and for preserving the stemness of the CSCs (Figure 2).Without the niche microenvironment, CSCs lose their stemness and initiate differentiation eventually, leading to the exhaustion of the CSC pool.Similarly,in vitrostudies require feeder layers and cytokines supplements in the culture media to ensure that SCs remain in their undifferentiated state[37].
In vitrostudies have recapitulated the niche theory using cardiospheres, which are 20-150 μm spheres (Figure 2) of cells generated from the explant outgrowth of heart tissues[92,93].Cardiospheres consist of CSCs in the core and cells committed to the cardiac lineage such as myofibroblasts, while vascular SMCs and ECs form the outer layer of the spheres.The 3D structure of cardiospheres protects the interiorly located CSCs from oxidative stress as well as maintain their stemness and function[84].
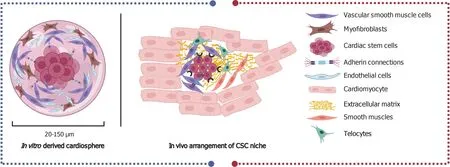
Figure 2 In vivo arrangement of the central cardiac stem cells and the surrounding cells that comprise the niche (right side) and the in vitro derived cardio spheres (left side).
Accurate anatomical identification of CSCsin vivoremains a challenge due to the lack of basal-apical anatomical orientation as seen in epithelial organs such as the intestines[94].Moreover, the heart does not comprise a specific compartment, where cells form a well-defined lining as seen in the bone marrow osteoblasts[95].The adult heart epicardial lining anatomically contains several classes of niches, which are not limited to the sub epicardium[96] but dispersed throughout the myocardium, more in the atria and apex away from hemodynamic stress[97].Some niches have been described in the atrio-ventricular junction of adult mouse and rat hearts[98] and interestingly in the human hearts[99].The young mouse heart has been studied morphometrically to identify the location of CSCs niche and has been defined as a randomly positioned ellipsoid structure consisting of cellular and extracellular components.Within the niches, undifferentiated CSCs are usually assembled together with early committed cells that express c-KIT on surface, Nkx2.5 in the nucleus, and the contractile protein α-sarcomeric actin in the cytoplasmic[97].
CSCs niche consists of clusters of c-kit+, MDR1+, and Sca-1+cells[98] but lack the expression of the transcription factors and cytoplasmic or membrane proteins of cardiac cells[99,100].Cardiac c-kit+/CD45-cells comprise about 1% of the CSC niche[97], are self-renewing clonogenic, and possess a cardiac multilineage differentiation potential comprise[101].
Within the niche, gap junctions (connexins) and (cadherins) connect SCs to their supporting cells, myocytes/fibroblasts.Conversely, ECs and SMCs do not act as supporting cells.Hence, the communication between CSCs with CMs and fibroblasts has been investigated by usingin vitroassays[102].The transmission of dyesviagap junctions between CSCs and CMs or fibroblasts was demonstrated previously and verified the functional coupling of these three cell populations[97].In addition, micro ribonucleic acid (miRNA-499) translocates from CMs to CSCs comprising to the initiation of lineage specification and formation of myocytes[103].
Identification of SC niches is contingent upon the fulfillment of explicit criteria, including the recognition and determination of the affixing of SCs to their supporting cells as well as assuring the existence of an ancestor-progeny association[104].Chemical and physical signals modulate the behavior of SCs within the niche.Amongst these signals are cytokines, cell surface adhesion molecules, shear forces, oxygen tension, innervation, and ions that serve as major determinants of SCs function[97].Cell-to-cell signaling mediates the fate of SCs within the niches to promote selfrenewal and favors their migration and differentiation.The fine-tuned crosstalk between SCs and their supporting cells regulates the state of the niche regarding quiescence or activity[105].
CSC niches, similar to the bone marrow, characteristically live in low oxygen tension, which favors a quiescent primitive state for SCs[106].The longstanding perpetuation of the CSC niche requires a hypoxic environment, while physiological normoxia could be required for active cardiomyogenesis[107].Hypoxic c-KIT+CSCs within niches have been found throughout the myocardium, especially at the atria and apex.Throughout all ages, bundles of CSCs with low oxygen content coexist with normoxic CSCs niches.Hypoxic CSCs, especially in the atria, are quiescent cells undergoing cell cycle arrest and cannot divide.Normoxic CSCs are pushed into intense proliferation and differentiation with continuous telomere erosion, resulting finally in dysfunctional aged CMs[108].Additionally, Nkx2.5 and GATA4 expressions are only restricted to the normoxic CSC niche.A balance between the hypoxic and normoxic niche is essential for the preservation of the CSC compartment and for the maintenance of myocardial homeostasis during the organ lifespan.Some factors such as aging cause an imbalance by expanding the hypoxic quiescent CSCs so that less pools of cycling CSCs maintain cell turnover[100].Hypoxic cardiac niches are abundant in the epicardium and subepicardium in an adult mouse heart, which also fosters a metabolically distinctive population of glycolytic progenitor cells[109].
The pool of CSCs seems to be heterogeneous, incorporating quiescent and actively proliferating cells, migratory and adherent cells, uncommitted and early committed cells, with young and senescent cells.Additional surface epitopes remain to be disclosed to classify pools of CSCs holding specific properties.Surface Notch1 expression distinguishes multipotent CSCs that are poised for lineage commitment, while c-Met and ephrin type-A receptor 2 receptors reveal cells with particular migratory potential out of the niche area.A specific compartment of CSCs, expressing IGF-1 receptor, can be stimulated to regenerate damaged myocardium, while those expressing IGF-2 receptor hold higher probability for senescence and apoptosis.Although this arrangement of cells seems to equip properly the CSC with homeostasis regulation, it does not effectively protect against aging or ischemic injury of the heart[100].
CSCs RELATIONSHIP WITH OTHER CELLS
Circulatory angiogenic cells
Circulatory angiogenic cells (CACs) are endothelial progenitor cells involved in vasculogenesis, angiogenesis, and stimulating myocardial repair, mainly through paracrine action.Lathamet al[110] demonstrated that conditioned medium from CAC-CSC co-cultures exhibited greatly mobilized CACs, with induction of tubule formation in human umbilical vein endothelial cells, mainly through the upregulation of the angiogenic factors angiogenin, stromal cell-derived factor 1 (SDF-1α), and VEGF.Moreover, administration of CACs and CSCs in infarcted hearts of non-obese/severe combined immunodeficient mice restored substantially the left ventricular ejection fraction (LVEF), with reduction of scar formation as revealed by echocardiography.Successful yet modest SMCs, ECs, and CM differentiation has been also reported.
Saphenous vein-derived pericytes
Pericytes (also called Rouget cells, mural cells, or perivascular mesenchymal precursor cells) are mesodermal cells that border the endothelial lining.They are highly proliferative cells and express neural/glial antigen 2, SOX-2, PDGFR-β, CD34, and several mesenchymal markers such as CD105, CD90, and CD44.It was previously reported that the transplantation of saphenous vein-derived pericytes (SVPs) into an ischemic limb of an immunodeficient mice restored the local circulatory networkviaangiogenesis[111].Moreover, treatment with SVP reduced fibrotic scar, CM death, and vascular permeability in a mouse model of MIviamiRNA-132 facilitated angiogenesis[112].Avolioet al[113] were the first to describe the relationship between SVP and the endogenous CSCs.Combined CSC and SVP transplantation in the infarcted myocardium of severe combined immunodeficient/Beige-immunodeficient mice showed similar results to treatment with CSCs or SVP cells per se, regarding scar size and ventricular function, indicating that SVPs alone are as potent as CSCs.
TCs
TCs represent a recently described cell population in the stromal spaces located in many organs, including the heart.They are broadly dispersed throughout the heart and comprise a network in the three cardiac layers, heart valves, and in CSC niches.TCs have been documented also in primary culture from heart tissues[114,115].The ratio of cardiac TCs (0.5%-1%) exceeds that of CSCs.Although they still represent a minute portion of human cardiac interstitial cells, their extremely long and extensive telopodes allow them to occupy more surface area, forming a 3D platform probably that extends to support other cells[116].The telopodes act as tracks for the sliding of precursor cells towards mature CMs and their integration into heart architecture[91].TCs form a tandem with CSCs/CPCs in niches, where they communicate through direct physical contact by atypical junctions or indirect paracrine signaling[115].
TC-CSC co-culturing have suggested that TCs and CSCs act synergistically to control the level of secreted proteins, as shown by the increased levels of monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein1 α and 2 (MIP-1 α and MIP-2), and interleukin (IL)-13.Whereas, the level of IL-2 decreased compared to the monoculture of CSCs or TCs.IL-6 found in TC culture is behind the upregulation of these chemokines.Chemokines elucidated the role of TCs in directing the formation of CMs.Within the context, MIP-1α and MCP-1 play roles in the formation of SMCs in the airway.Additionally, MCP-1 is also involved in mouse skeletal muscle regeneration by recruiting macrophages.The enhancement of MCP-1 secretion serves as an activator of another cell population, primarily macrophages, which are generally involved in such processes[117].
IL-6 also activates downstream signaling pathways and contributes to cardioprotection and vessel formation in the heart through activation of gp130/signal transducer and activator of transcription 3.The Gp130/signal transducer and activator of transcription 3 is essential for the commitment of cardiac SCA-1+cells into endothelial lineage[118].
Furthermore, IL-6 targetsVEGFand hepatocyte growth factor (HGF) genes.VEGF has a mitogenic effect on CMs[119].It is known to mobilize bone marrow-derived mesenchymal stem cells (BM-MSCs) into the peripheral blood in MI patients[120].HGF and its receptor (c-Met) are also involved in cardiogenesis, as it is expressed early during cardiac development[121].The level of HGF mRNA is normally low in the heart, but it is upregulated for at least 14 d after ischemic insult in rats, enhancing CMs survival under ischemic conditions[122,123].Moreover, it has the potential to generate an adhesive micro-environment for SCs, as demonstrated in a study of transplantation of HGF transfected BM-MSCs in the infarcted myocardium[124].HGF is also a powerful angiogenic agent, conducting its mitogenic and morphogenic effects through the expression of its specific receptor in various types of cells, including myocytes.Moreover, HGF exerts antifibrotic and antiapoptotic effects on the myocardium[125,126].
Transcriptomic analysis also has disclosed that TCs express pro-angiogenic miRNAs including let-7e, miRNA-21, miRNA-27b, miRNA-126, miRNA-130, miRNA-143, miRNA-503, and miRNA-100[127].The TCs and CSCs interactin vitroforming atypical junctions, such as puncta adherentia and stromal synapses.The puncta adherentia consists of cadherin-catenin clusters.It controls the symmetry of division by facilitating the proper positioning of centrosomes.Therefore, an increased number of CSCs has been reported to be encountered in the presence of cardiac TCs[128,129].
CSCs SECRETOME
The paracrine potential of CSCs/CPCs has been recently under focus.CSC-derived cytokines and growth factors include epidermal growth factor (EGF), HGF, IGF-1, IGF-2, IL-6, IL-1α, and TGF-β1[130,131].Exosomes appear to harbor relevant reparative signals, which mechanistically underlie the beneficial effects of CSCs transplantation[132].
Structurally, exosomes are lipid bilayer nano-sized organelles, 20-150 nm in diameter, secreted from all cell types, and function as intercellular communicators.Exosomes are highly heterogenic in content, and this stems from the unique packaging process that occurs inside progenitor and SCs.Exosomes carry lipids, proteins, and nucleic acids, with an abundance of miRNAs that hold profound post-transcriptional gene regulatory effects[133].
Protein content of exosomes
Amongst the distinctive protein content of cardiac exosomes are the chaperone proteins heat shock protein (HSP) 70 and HSP60.The HSP70 and HSP60, which under normal conditions assist in protein folding processes and deter misfolding and protein aggregation under pathological states induced by stress, also play major roles in apoptosis[134].Circulating exosomes from healthy individuals have been found to activate cardioprotective pathways in CMsviaHSP70 through extracellular signalregulated kinase ½ and HSP27 phosphorylation[135].
The exosome protein cargo of CPCs is distinct from BM-MSCs, fibroblasts, and other sources as it contains ample amounts of the pregnancy-associated plasma protein-A (PAPP-A).PAPP-A is present on the surface of human exosomes and interacts with IGF binding proteins (IGFBPs) to release IGF-1[136].The cardioprotective role of CPCs-exosomes has been proven experimentally inin vitroischemia/reperfusion and MI models and on CMs apoptosis to surpass that of BM-MSC-exosomes owing to their rich content of PAPP-A[137].
Exosomes’ surface and intra-vesicular markers
Like all exosomes, mouse CPCs-derived exosomes are positive for the surface markers CD63, CD81, and CD9, TSG-101, and Alix, however, they express a high-level of GATA4-responsive-miRNA-451.MiRNA-451 has been shown to inhibit CM apoptosis in an acute mouse myocardial ischemia-reperfusion model through inhibition of the caspases 3/7.The expression of miRNA-21 in the mouse CPCs-exosomes additionally justifies their CM protection against oxidative stress and antiapoptotic effectsviainhibition of programmed cell death protein 4 (PDCD4)[138].Human CPCs-exosomes are enriched with miRNA-210, miRNA-132, and miRNA-146a-3p, which account for the diminished CM apoptosis, enhanced angiogenesis, and improved LVEF[139].MiRNA-146a-5p is the most highly upregulated miRNA in human CPCs-exosomes and targets genes involved in inflammatory and cell death pathways[137].
The CDCs contain CD34+stromal cells of cardiac origin and are multipotent and clonogenic but not self-renewing[140].CDCs secrete exosomes that induce cardiomyogenesis and angiogenesis, regulate the immune response, downgrade fibrosis, and improve the overall cardiac function[141,142].Moreover, CDCs homogeneously express CD105 but not CD45 or other hematopoietic markers.They also exhibit a high expression of miRNA-126[143].Circulating miRNA-126 may participate in cardiac repair during acute MI and has been demonstrated to be downregulated in heart damage[144].
Exosome secretion and function
While exosomes are constitutively secreted, changes in the surrounding microenvironment, such as hypoxia, can induce modifications in CPCs- and CM- derived extracellular vesicles.Hypoxic CMs secrete large extracellular vesicles containing long noncoding RNA neat 1 (LNCRNA NEAT1), which is transcriptionally regulated under basal conditions by p53, while during hypoxia it is regulated by the hypoxia inducible factor 2A.An uptake of the hypoxic CM-derived extracellular vesicles by fibroblasts can prompt the expression of profibrotic genes[145].Oxidative stress may also induce the release of cardiac CPCs exosomes, which in turn inhibit apoptosis when taken up by H9C2 (rat cardiomyoblast cell line)[132].Furthermore, oxidative stress stimulates secretion of miRNA-21 rich exosomes, which could inhibit H9C2 apoptosis by targeting PDCD4 and hence can be accounted as a new method to treat ischemiareperfusion[138].
Intercellular communicationviaexosomes occurs as part of various biological processes, including immune modulation, vasculogenesis, transport of genetic materials, and pathological conditions such as inflammation, apoptosis, and fibrosis, which can lead to cardiovascular disease when altered[146].Hence, isolation and analysis of cardiac exosomes contents, mainly miRNA and proteins, could offer diagnostic information for several cardiovascular diseases[147] (Figure 3).
Functionally, exosomes mediate several intra-cardiac inter-cellular communications such as:
CPC-CM crosstalk through factors, such as miRNA-146a and PAPP-A, which activate extracellular signal-regulated kinases 1/2 pathway and inhibit apoptosis[139].
CPC-macrophage (M1) crosstalkviamiRNA-181b and Y-RNA fragment transforms M1 to M2 macrophages with attenuated proinflammatory cytokines and increased IL-10[148,149] (Figure 4).
CPC-fibroblast interactionviaexosomes primes the fibroblasts and increases expression of VEGF and SDF-1.Experimental injection of fibroblasts primed with CPCs-exosomes into the myocardium of a MI model proved to reduce infarct size and improve cardiac function.In addition, cardiosphere-isolated exosomes have been used to prime inert fibroblasts, leading to an intensification of their angiogenic, cardiomyogenic, antifibrotic, and collective regenerative effects[150] (Figure 4).
CPC-self regulatory mechanisms: Exosomes derived from CPCs may play critical roles in maintaining the self-renewal state of CPCs themselves and balance their differentiation,i.e.preserve their stemness[151] (Figure 4).The CPC-derived exosomes activate the endogenous CPCs by transferring signal molecules directly within their niche[152].
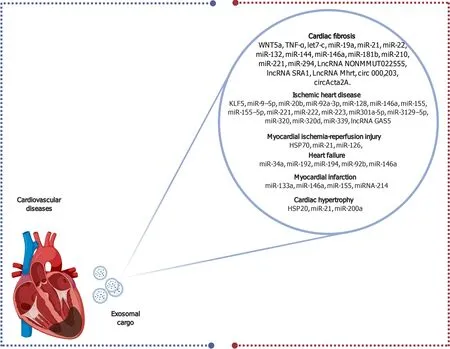
Figure 3 Schematic diagram elucidating the diverse exosomal contents that serve as biomarkers for several cardiovascular diseases.
CPC-derived exosomes release various RNA species in the extracellular space, modulating endogenous SC plasticity and tissue regeneration through their cytoprotective, immunomodulatory, pro-angiogenic, and anti-apoptotic actions[153].
Fibroblasts and pericytes interact after transdifferentiating to myofibroblasts and deposit ECM causing cardiac fibrosis.These fibrotic changes are usually induced by cardiac damage and lead to scar formation.Exosomes serve as messengers for cell-tocell communication during cardiac fibrosis[154].Molecular mechanisms of cardiac fibrosis are primarily related to TGF-β pathways, IL-11 signaling pathway, nuclear factor-κβ pathway, and Wnt pathways[155].Accordingly, the bioactive substances targeted at these pathways could hypothetically be applied in the treatment of cardiac fibrosis.Wnt3a, being highly expressed in exosomes, could activate the Wnt/β-catenin pathway in cardiac fibroblasts by restricting GSK3β activation[156].Moreover, tumor necrosis factor α contained in exosomes can be transferred between cardiac myocytes.In general activation/inhibition of the exosomes conveying remodeling substance secretion or uptake can control the myocardial remodeling and repair following MI[154,157].
The highlighted complex cell-to-cell communication from endogenous or exogenous CSCs provides an optimal microenvironment for resident CPC proliferation and differentiation (Figure 4), rendering the environment receptive to transplanted CPCs.This adaptation is promoted through activation of pro-survival kinases, leading to the induction of a glycolytic switch in recipient CPCs[158].
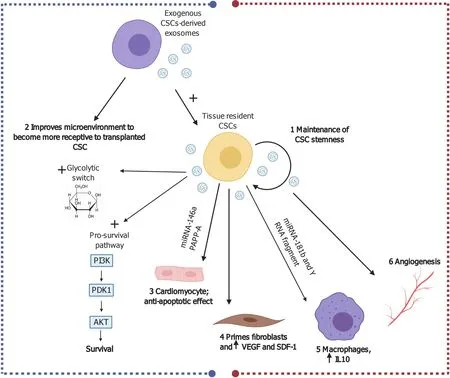
Figure 4 Possible cardiac reparative effects of cardiac stem cell/cardiosphere-derived cell-derived exosomes in myocardial ischemia and ischemia/reperfusion injury.
Therapeutic efficiency of CPCs/CDCs exosomes
Data from experimental models suggest that the exosomal component of the CPC secretome can fully recapitulate the effects of cellular therapy on ischemic and nonischemic heart models[140].In an ischemia-reperfusion injury rat model, Ciullo and partners[159] have shown that the systemic injection of exosomes (genetically manipulated to overexpress CXCR4-ExoCXCR4) improve cardiac function.Additionally, expression of hypoxia-inducible factor 1 (HIF-1) in the infarcted myocardium is upregulated through the stimulation of SDF-1α.The latter is one of the CXC chemokine family overexpressed in heart post-MI that readily attaches to the CXCR4 receptor and acts as a potent chemoattractant for CXCR4 expressing circulating progenitor cells.The ExoCXCR4 are more bioactive in the infarcted zone than naturally occurring exosomes injectedviatail-vein, confirming their superior homing and cardioprotective properties in the damaged heart.
Galletet al[160] postulated the safety and efficiency of CDC-derived exosomes in acute and chronic myocardial injury animal models.Within the context of experimental research to validate the paracrine hypothesis for CDCs-derived exosomes, it has been proven that human CDC-exosomes can recapitulate CDC therapy and boost cardiac function post-MI in pig models.Intramyocardial injection of human CDCexosomes has resulted in higher exosome retention and efficacy as compared to intracoronary injection, with great reduction of scar size and increased ejection fraction.This indicates that the route of administration is imperative for full functional capacity of the exosomes.Subsequently, the researchers have devised a randomized preclinical study by means of a NOGA-guided intramyocardial exosome injection.Decreased collagen content in the infarct and border zone and increased neovascularization and Ki67+CMs are indicative of the reparative functions of CDC-exosomes.Notably, human CDC-exosomes have shown a lack of an immune reaction, as seen by the lack of inflammatory reactions or CM necrosis in pig models.These observations strongly support the view that CDC-exosomes are ready to be tested in clinical trials.
Similar promising outcomes were observed in a Duchenne muscular dystrophy model (mdx), in which intramyocardial injection of CDC-exosomes efficiently recapitulated the effects of CDC injection on cardiac function, leading to recovery of movement.Administration of CPC-derived exosomes has resulted in transient restoration of partial expression of full-length dystrophin in mdx mice[161].Further studies assessed the therapeutic potential of CPC-exosomes in a doxorubicin cardiotoxicity model and non-ischemic heart disease[162].In addition, two concluded phase I clinical trials in patients with heart failure and revealed the capacity of CDCs to enhance cardiac function by reducing ventricular remodeling and scar formation.Despite receiving a single injection at the beginning of the study, the improvement in cardiac function was noted after the 1-year follow-up.This finding consequently leads to the proposition that transplanted CDCs mainly have imposed their actions at the site of injury by secreting paracrine factors including exosomes.In other words, CDCexosomes achieved a biphasic beneficiary regenerative effect involving acute cardio protection coupled with long-term stimulation of endogenous cardiac repair[163].
METABOLISM OF CSCs
While the fetal heart obtains most of its ATP supplyviaglycolysis[164], the adult heart relies mainly on fatty acid oxidation to fulfill the contracting myocardium high energy demand[164,165].The loss of the regenerative phenotype is related to the oxidative metabolism of glucose and fatty acids[166,167] and is mediated by various physiological changes including increased workload and the demand for growth, which cannot be solely met by glycolysis[168,169], as well as postnatal increase in both circulating levels of free fatty acids and blood oxygen levels[164,165].Studies have shown the involvement of the HIF-1 signaling pathway[170], peroxisome proliferatoractivated receptor α (PPARα)[171], and peroxisome proliferator-activated receptor γ coactivator-1 (PGC-1) in the switch toward oxidative metabolism[172], which is accompanied by dramatic increase in the number of mitochondria in CMs[173].
Notably, similar metabolic reprogramming occurs during differentiation from cardiac SCs to CMs[167].Studies reported that after differentiation into CMs, there is an increase in the mitochondrial number and activity[174], increased oxidative metabolism[175], and increased respiratory capacity resulting in an increased adenosine diphosphate:ATP ratio[173] after differentiation into CMs.
The fact of the various metabolic changes that accompany the transition from glycolysis to fatty acids oxidation affect cardiac cell maturation[164,167] has mandated the consideration of substrate composition in cardiac differentiation protocols[167].
A study by Malandraki-Milleret al[176] investigated the effect of fatty acid supplementation, which mimics the metabolic switch from glucose to fatty acid oxidation, on adult cardiac progenitors.The study used radiolabeled substrate consumption for metabolic flux to investigate the role of the PPARα/PGC-1 axis during metabolic maturation.Oleic acid stimulated the PPARα pathway, enhanced the maturation of the cardiac progenitor, and increased the expression of MHC and connexin after differentiation.Moreover, total glycolytic metabolism, mitochondrial membrane potential, the expression of glucose, and fatty acid transporter increased.The recorded results contributed greatly in highlighting the role of fatty acids and PPARα in CPC differentiation.
Another study by Correiaet al[177] has linked substrate utilization and functional maturation of CMsviastudying the effect of the metabolic shift from glucose to galactose and fatty acid-containing medium in the maturation of hPSCs-derived CMs (hPSCs-CMs).The shift accelerated hPSC-CM maturation into adult-like CMs with higher oxidative metabolism, mature transcriptional signatures, higher myofibril density, improved calcium influx, and enhanced contractility.Galactose improved total oxidative capacity with reduction of fatty acid oxidation, thereby protecting the cells from lipotoxicity.
In CDCs, oxidative metabolism and cell differentiation reciprocally affect each other.In vitrocultures for CDCs revealed a PPARα agonist that triggers fatty acid oxidation.Metabolic changes have been characterized as the CDC differentiated towards a cardiac phenotype.Addition of a PPARα agonist at the onset of differentiation has induced a switch towards oxidative metabolism, as shown by changes in gene expression with decreasing glycolytic flux and increasing oxidation of glucose and palmitate.Undifferentiated CDCs have generated high levels of ATP from glycolysis and from oxidation of acetoacetate.Upon differentiation, oxidative metabolism of glucose and fatty acids is upregulated with decreased oxidation of acetoacetate, a metabolic phenotype similar to that of the adult heart[178].
Taken together, the metabolic hallmarks of differentiated CMs vary from their undifferentiated SCs.Energy substrate metabolism during cardiac development and differentiation shows gradual decrease in the contribution of glycolysis to ATP synthesis with simultaneous increase in fatty acid-dependent mitochondrial respiration[179].
Common methods for the investigation of substrate metabolism include the measurement of metabolic fluxes using radio-labeled substrates, such as D-U-14Cglucose[180,181] as well as measurement of mitochondrial oxygen consumption rate and extracellular acidification rate using the XF Extracellular Flux Analyzer (Seahorse Bioscience, North Billerica, MA, United States)[182,183].
Recently, a detailed protocol for metabolic characterization of hiPSCs-CMs has been developed.The hiPSCs are obtained from adult somatic cellsvianovel cell reprogramming approaches, followed by differentiation to CMs.The novelin vitrocardiac cellular model provided new insights into studying cardiac disease mechanisms and therapeutic potentials.The characterization protocol measures small metabolites and combines gas- and liquid-chromatography-mass spectrometry metabolic profiling, lactate/pyruvate, and glucose uptake assays as important tools[184].Integration between the implemented assays has provided complementary metabolic characteristics besides the already established electrophysiological and imaging techniques, such as monitoring ion channel activities[185], measurement of action potentials, changes in Ca+2fluxes[186], and mitochondria viability and apoptosis[187].
An alternative pathway for glucose metabolism in CMs involves the entry of glucose-6-phosphate (G6P) in the pentose phosphate pathway, with resultant generation of reduced nicotinamide adenine dinucleotide phosphate (NADPH)[188].Reduced NADPH helps to regenerate reduced glutathione and thus acts protectively against reactive oxygen species induced cell injury.
The cardioprotective role of the pentose/G6P/NADPH/glutathione pathway has been emphasized by Jainet al[189] who demonstrated that G6P dehydrogenase (G6PD) lacking mice have more severe heart damage induced by the myocardial ischemia reperfusion injury in Langendorff-perfused hearts as compared with wild-type mice.
Moreover, Katareet al[190] studied this pathway in CPCs isolated from hearts of diabetic mice.They reported that both G6PD and transketolase activities were markedly reduced in diabetes mellitus, which resulted in apoptosis of CMs.Interestingly, they have also reported that apoptosis was induced under high glucose conditionsviainhibition of the pentose phosphate pathway, which mediates prosurvival signaling pathways.
Cellular metabolic transcriptome profile is an important determinant of many critical cell functions such as survival, growth, differentiation, and reprogramming.With the fast-track advancements in CSCs research, in-depth and thorough metabolic transcriptome analyses on CSCs are needed.It has been also suggested that metabolic genes can be targeted to manipulate the differentiation of ESCs into specific CM phenotypes or to modulate the maturation grade of CMs derived from ESCs[179].
As mentioned earlier in the review, the energy demand of the contracting myocardium of an adult heart is met mainly through fatty acid oxidation, which explains the fact that genes required for fatty acid metabolism are upregulated in the differentiated CMs.These genes include acetyl-CoA acyltransferase 2 (ACAA2), NADH dehydrogenase ubiquinone 1, α/β subcomplex 1 (NDUFAB1), protein kinase AMP-activated α-2 catalytic subunit (PRKAA2), and ECI1 enoyl-CoA delta isomerase 1 (DCI).In addition, other genes involved in glucose metabolism are also upregulated in α-MHC+CMs, including protein phosphatase 1 regulatory subunit 3C (PPP1R3C), glycogen phosphorylase, muscle associated (PYGM), enolase 3 (ENO3), phosphoglycerate mutase 2 (PGAM2), amylo-α-1,6-glucosidase 4-α-glucanotransferase (AGL), 6-phosphofructokinase muscle (PFKM), and malate dehydrogenase 1 (MDH1)[191].This is interpreted by the fact that adult cardiac cells are metabolically flexible, being capable of oxidizing other energy sources, such as glucose, lactate, amino acids, and ketone bodies for the production of ATP and non-ATP-producing intermediate metabolites with high biological significance[169].Another example of CMs’ metabolic plasticity is shown byHIF-1expression, which is important for their metabolic adaptation to hypoxic and ischemic conditions[192].
NANOTECHNOLOGY AND CSCs
Since Richard Feynman laid down the foundation of nanotechnology 1959[193], remarkable developments have been witnessed attributed to the novel properties possessed by the materials at the nanoscale, which differ from their bulk forms.A panel of NPs, ranging from soft to inorganic, are used in nanomedicine, depending on their unique property matching the field of interest[194-197] (Figure 5).
The interplay of nanotechnology with SCs has gained increasing interest, whether for differentiating, tracking, imaging, or for therapeutic purposes.Accumulating evidence presents that the small size and bioactive characteristics of NPs could influence SC function.Several engineering techniques have been developed to obtain nano-fibrous scaffolds that facilitate controlling SC proliferation, migration, and differentiation.A diversity of SCs, including ESCs, skeletal myoblasts, BM-MSCs, and CSCs, have been tested to repair acutely or chronically damaged myocardium.However, the optimal cell type, the efficient cell number, the appropriate route for cell delivery, and the ideal time point for cell delivery after MI are still unanswered questions.The biodistribution of SCs and the specific mechanism by which therapeutic cells improve cardiac function remains under investigation[198].Using NPs could solve some of these obstacles either by gene delivery to SCs, enhancing the retention of SCs, facilitating SCs’ proangiogenic effect, or mimicking the ECM[199].
In view of this, we have summarized the impacts of NPs on SCs, especially CSCs, from differentiating, therapeutic, and tracking viewpoints (Figure 5, Table 1).
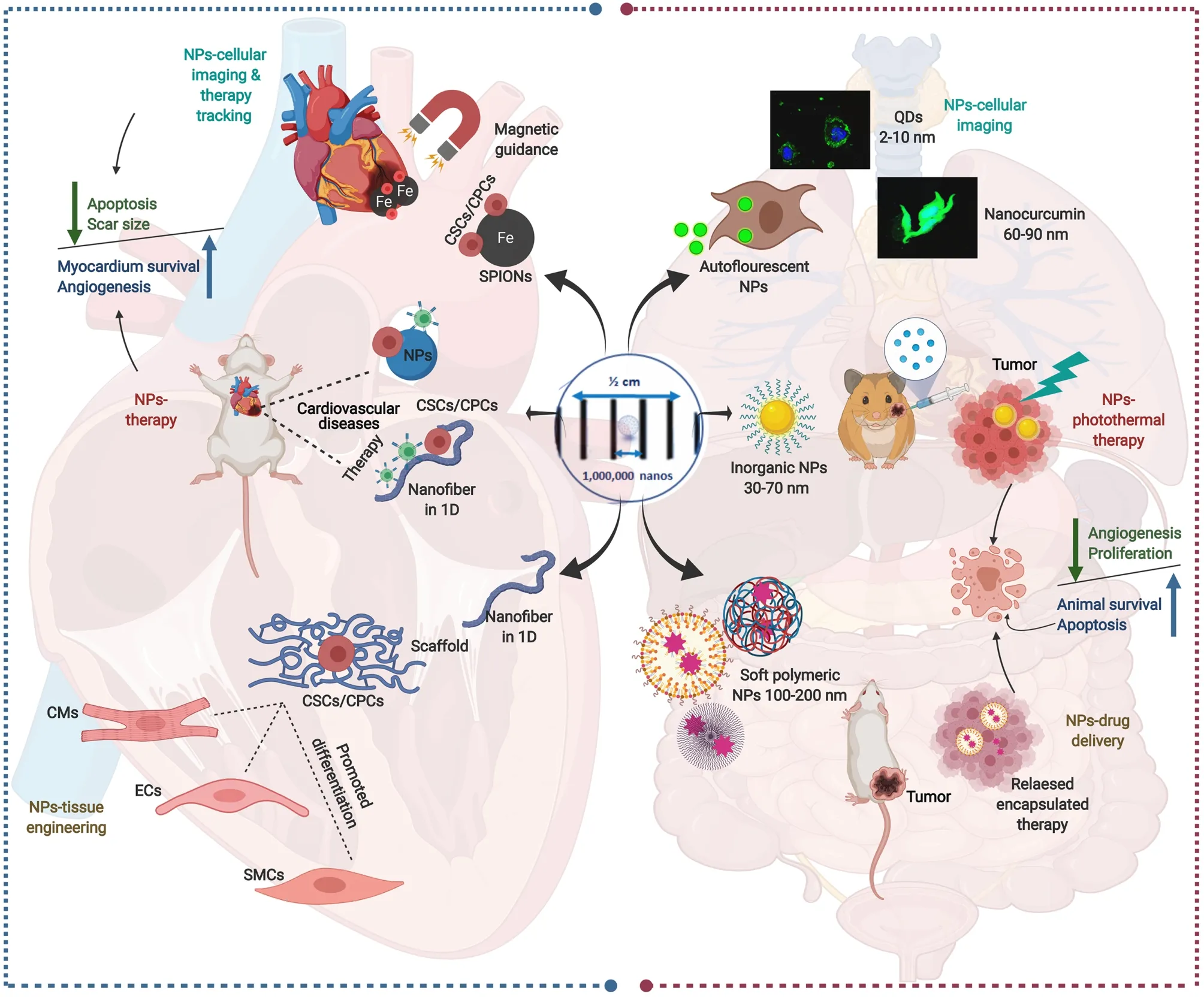
Figure 5 Schematic presentation of multiple nanoparticles-based paradigms in nanomedicine (right side) and their mirror images in nanoparticle-assisted cardiac stem cell interventions (left side).
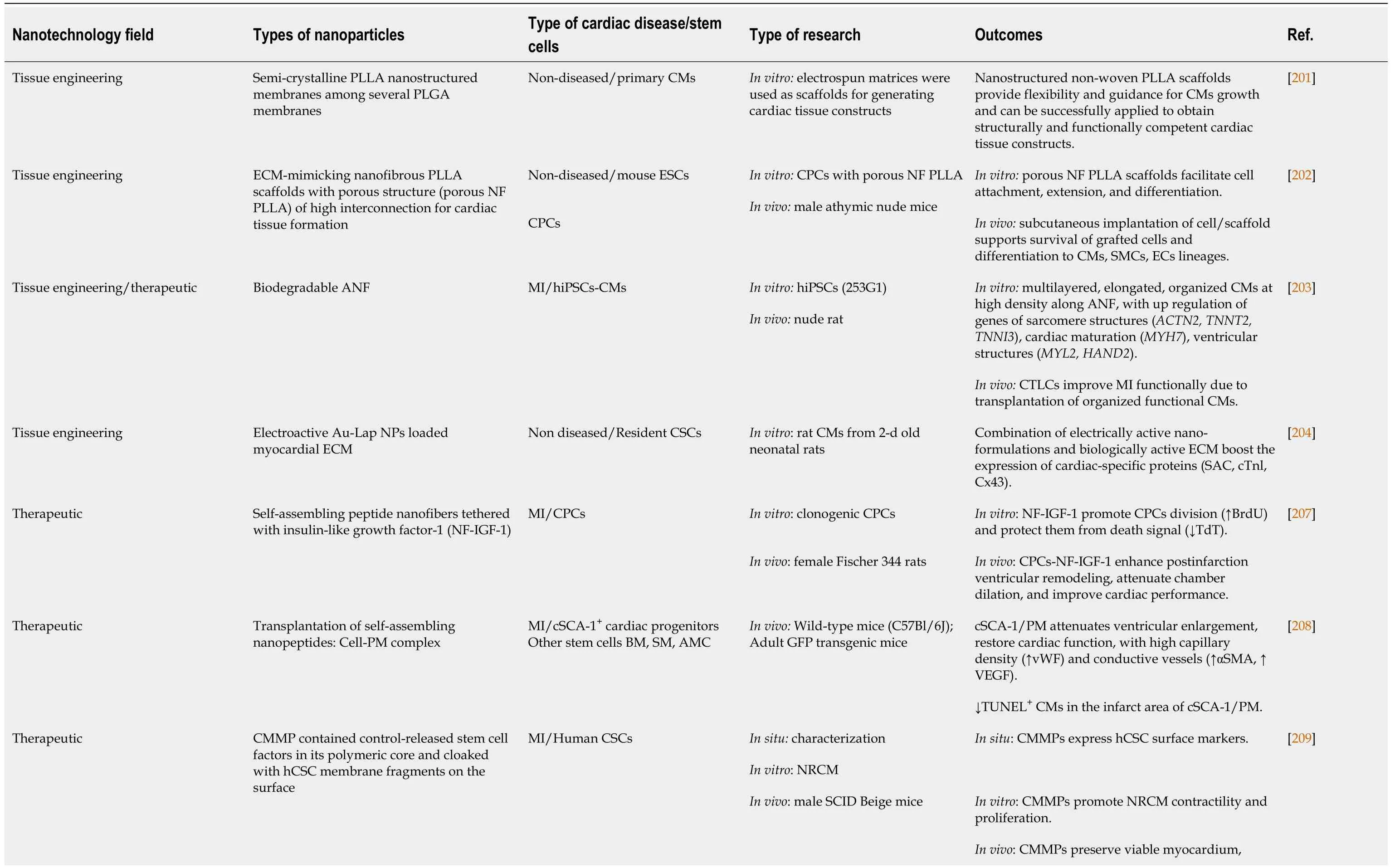
Table 1 Main outcomes of the studies investigated the impact of nanotechnology in cardiac stem cell-based studies
NP-assisted CSC differentiation
Nanotechnology has revolutionized the tissue engineering field and altered the landscape of scaffolds syntheses.In typical tissue engineering, a bio-mimicked scaffold provides adhesive surfaces for the seeded cells, where the SCs deposit their proteins to make the engineered-scaffold more biocompatible.However, improper vascularization, lack of functional cells, the low mechanical strength of engineered cells, immunological incompatibility with the host, and nutrient constraints are the main limitations encountered in tissue engineering.Therefore, synthesis of a biomimetic scaffold at the nanoscale, in a minimum of one dimension, would offer a more effective microenvironment needed for cell growth.Nano-tissue engineering provides the scaffold with a simple substrate for SC adhesion and active agents for their proliferation[200].
In consideration for CSCs, nano- and microstructured electrospun matrices have been used as non-woven scaffolds for the construction of cardiac tissue from primary CMs.Among different nanostructured poly (D, L-lactic-co-glycolic acid; PLGA) membranes, the poly (L-lactide; PLLA) scaffolds superiorly developed mature contractile machinery (sarcomeres).Functional studies (excitability) of CMs tested by optical imaging of electrical activity have confirmed the superior response on PLLA scaffolds compared with other ones[201].
Anin vitroandin vivostudy conducted by Liuet al[202] using porous ECMmimicking nanofibrous PLLA scaffolds (porous NF PLLA) demonstrated cardiac tissue formation from CPCs.The scaffold has facilitated thein vitrodifferentiation of isolated mouse ESCs into CPCs.Thereafter, the transplanted NF PLLA/CPCs integrated successfully with the host tissue, with superior expression of cardiac committed markers cardiac troponin T, smooth muscle MHC, and CD31.
The inductive and therapeutic properties of biodegradable PLGA nanofibers have been testedin vitroandin vivo.Different hiPSCs-CMs have been seeded on aligned PLGA nanofibers to differentiate into high-quality cardiac tissue-like constructs, where cardiac biomarkers and cardiac functions have been upregulated.When utilizedin vivofor treating MI, the cardiac tissue-like constructs have shown more robust results than the two-dimensional conventional control in improving the ejection fraction, the fractional shortening, and left ventricular end-systolic diameter[203].
Recently, an injectable ECM hydrogel loaded with gold (Au)/Laponite (Lap) nanocomposite has been tested on the biological activity of resident CSCs.The electroactive Au/Lap-ECM hydrogel improved cell biocompatibility and phenotypes maturation of cardiac-specific markers (SAC, cardiac troponin 1, and Cx43)[204].
NP-assisted CSC therapy
More than 90 years ago, the Nobel laureate German immunologist Paul Ehrlich proposed the term “magic bullets” to describe the artificial biochemical agents that would transport and release drugs at the desired sites only[205].Since then, drug delivery research has witnessed notable growth due to NPs utilization as “controlled release reservoirs” for drug delivery in order to combat many diseases[206].
In cardiovascular diseases, NP-based drug delivery targeting CSCs would be a successful therapeutic regimen.In anin vivostudy of induced MI, self-assembling peptide nanofibers tethered with NF-IGF-1 positively influenced CPCs in female Fischer rats.The local injection of CPCs loaded on NF, with the prolonged release of
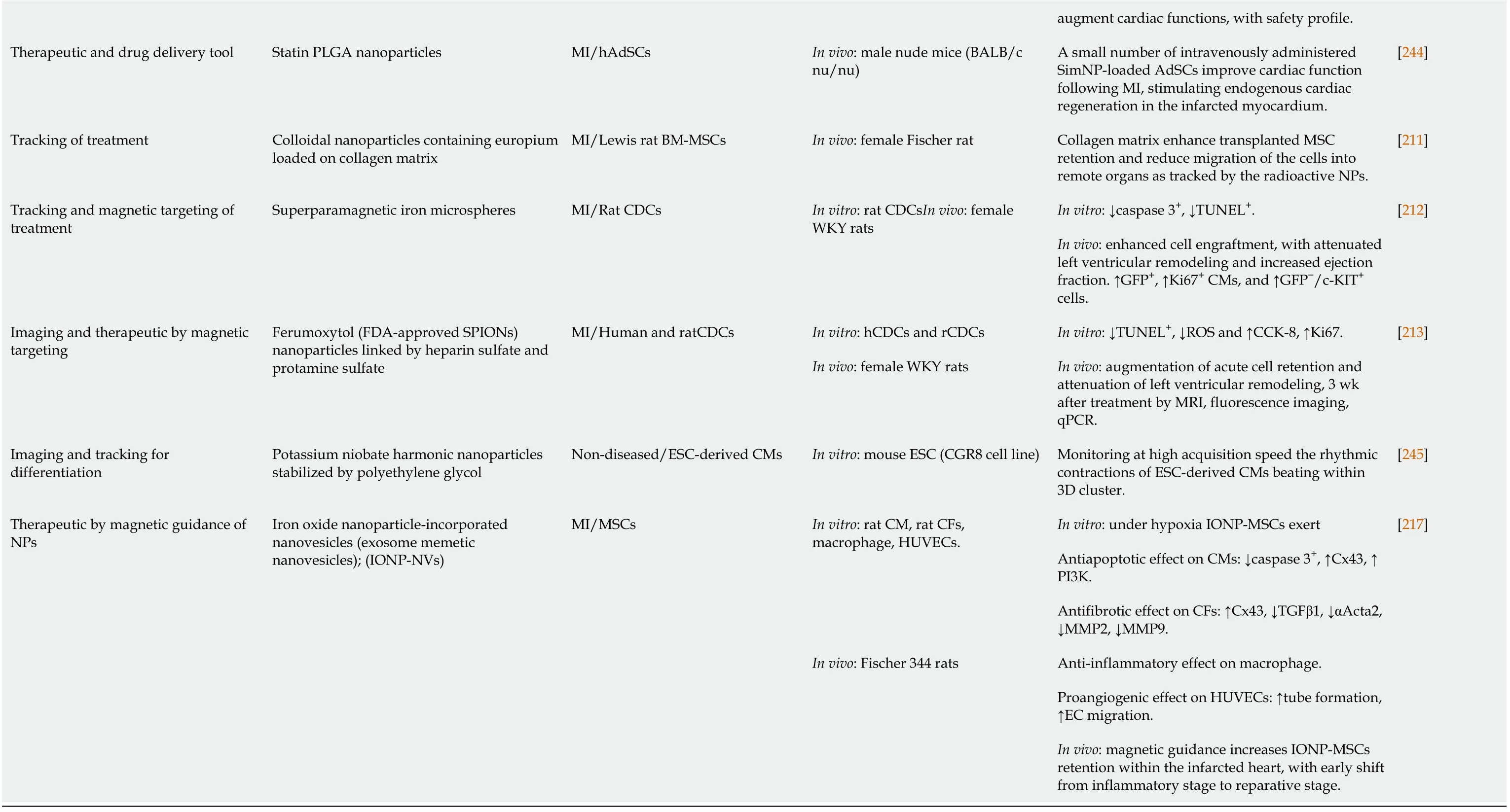
AMC: Adipose tissue-derived mesenchymal cell; ANF: Aligned PLGA nanofibers; Au-Lap NP: Gold and laponite nanoparticle; BrdU: Bromodeoxyuridine cell proliferation assay; CCK-8: Cell counting kit-8; CF: Cardiac fibroblast; CM: Cardiomyocyte; CMMP: Cell-mimicking microparticle; CPC: Cardiac progenitor cell; CTLC: Cardiac tissue-like construct; cTnl: Cardiac troponin 1; FDA: Food and Drug Administration; ECM: Extracellular matrix; ESC: Embryonic stem cell; hAdSC: Human adipose-derived stem cell; HUVEC: Human umbilical cord vein endothelial cell; IONP: Iron oxide nanoparticle; MRI: Magnetic resonance imaging; NF: Nanofibrous; MI: Myocardial infarction; MMP: Matrix metalloproteinase; NRCM: Neonatal rat cardiomyocyte; PLGA: Poly D,L-lactic-co-glycolic acid; PLLA: Poly (L-lactic acid); PM: Puramatrix™; qPCR: Quantitative polymerase chain reaction; SM: Skeletal myoblast; SPION: Superparamagnetic iron oxide NP; TdT: Terminal deoxynucleotidyl transferase apoptotic assay; TUNEL: Terminal deoxynucleotidyl transferase dUTP nick end labeling apoptotic assay; vWF: von-Willebrand factor; WKY: Wistar-Kyoto; αSMA: Alpha smooth muscle actin; 3-D: Three-dimensional.
Other self-assembling nanopeptides loaded with cell-Puramatrix™ complex have revealed promising results in treating MI, especially when targeting clonal SCA-1+CPCs.The infarct area of SCA-1+/PM became smaller than that of other tested SCs.Moreover, SCA-1+/PM have secreted VEGF, enhancing their differentiation potentiality into CMs and vascular SMCs[208].
Interestingly, a synthetic cell-mimicking microparticle (CMMP) has recapitulated CSC function with a safe immunological profile.The core of the CMMP is a PLGA containing human-derived CSC (hCSC) secretome, while the surface has been cloaked with SC membrane fragments.These CD105+and CD90+CMMPs have shown synchronized movement with adjacent beating CMsin vitro.When injected in the MI mouse model, they have shown prolonged retention without eliciting the T-cell immunoreaction that transplanted hCSCs provoked[209].
NP-assisted CSC imaging and tracking
Combining the therapeutic effect of SC-based therapy with concomitantin vitroorin vivovisualization of the SCl is a strategy helped by the auto-luminescent NPs.Furthermore, delivery of the SCs to the infarcted cardiac area could be guided by a magnetic field depending on the unique magnetic properties of iron oxide NPs[210].
Isotopic colloidal NPs tagged with europium have successfully tracked the retention of BM-MSCs in the infarcted area.The NP-labeled MSCs loaded on a collagen matrix have shown reduced relocation of MSCs to remote organs.However, delivering the NP-MSCsviacollagen has failed to improve cardiac function[211].Further retention and magnetic targeting of CDCs have been retrieved using superparamagnetic microspheres (SPM).Quantitative polymerase chain reaction and optical imaging have confirmed the magnetic targeting and the increased cardiac retention of transplanted cells, with reduced lung migration in a rat model of ischemia/reperfusion injury.Moreover, the prolonged survival of SPM-labelled CDCs by cell counting kit-8 and Western blot has proved the safety profile of SPM[212].
The success of magnetic cell delivery in various preclinical studies potentiates the translation into clinical ones encouraged with the Food and Drug Administrationapproved superparamagnetic iron oxide NP ferumoxytol.A thorough investigation of ferumoxytol-labeled (FHP) human and rat CDCs offered the potential for rapid clinical translation of the magnetically targeted cell delivery to an ischemic heart.Thein vitrostudy proved that FHP nanocomplex is not toxic to hCDCs, where a panel of cytotoxicity assays have revealed prolonged survival, potentiated differentiation, and genetic stability of FHP-hCDCs.Furthermore,in vivotracking of FHP-rCDCs by magnetic resonance imaging (MRI), fluorescence imaging, and quantitative polymerase chain reaction have shown that magnetic targeting increased cardiac retention without eliciting cardiac inflammation or causing iron overload.The histological assessment revealed enhancement of angiogenesis and cell engraftment in the hearts of the magnetic targeting group[213].
NP-based cardiac therapy limitations and prospects
Despite the promising results of NP-assisted SC interventions, the reported nanotoxicity is considered a major obstacle for clinical translation of these preclinical trials[214].Besides, most of thein vitroandin vivoNP-based SC trials focused on the shortterm effect of the NP interventions.The long-term safety profile of the injected NPs/CSCs or NPs/scaffolds with host interactions needs large scale investigations.These shortcomings have directed the search for natural cell-derived immune compatible nanostructures.
Exosomes attracted the attention as therapeutic cellular-derived NPs.However, the small quantity of these exosomes secreted from SCs is considered the main limitation to its therapeutic implementation.A novel exosome-mimetic extracellular nanovesicles (NVs) have bypassed this obstacle[215].The large-scaled mechanically synthesized NVs from ESCs, by Jo and his colleagues[216], have conserved both RNA and protein profiles of the ESCs.Furthermore, treatment of MSCs with NVs has promoted cellular proliferation, which has been comparable with or even superior to the positive MSCs control treated with silica nanobeads that are well known for their ability to stimulate proliferationviaactivation of the MAPK pathway.
Recently, NVs derived from iron oxide NPs (IONPs) incorporated MSCs (IONPMSCs) have co-cultured with different primary cell lines to investigate their physical and biological characteristics.IONPs-MSCs have revealed cardioprotective effectsviaPI3K/AKT activation.Underin vitrohypoxic conditions, IONPs-MSCs have upregulated Cx43, an electrical coupling molecule, whose reduction is responsible for arrhythmia and cardiac cell death in hypoxia.Furthermore, the NVs inhibited cardiac fibrosis by inhibiting the differentiation of cardiac fibroblasts into cardiac myofibroblasts after hypoxia.Magnetically targeting an induced MI with IONPs-MSCs have attenuated apoptosis, reduced inflammation, and increased blood vessel density, with increased retention of IONPs-MSCs in the infarcted myocardium, improving left ventricular remodeling[217].
Designer exosomes is another hope, boosting the exosomal theragnostic potential independently of its low yielding.Kojima and coworkers[218] genetically engineered the producer cells with the three genes responsible for potentiating exosome production: Six-Transmembrane Epithelial Antigen of Prostate 3 for exosome biogenesis, syndecan-4 for budding of endosomal membranes, and the fragment of Laspartate oxidase for cellular metabolism.These exosome production boosters have been further genetically upgraded to RNA packaging device and cytosolic delivery helper.Thereafter, they have used this collectively EXOtic device to deliver cargo messenger RNA to the mice brain, attenuating neurotoxicity in neuroinflammatory disease by enhancing cell-to-cell communication without the need for exosomes concentration.These results demonstrate the usefulness of designer exosomes for therapeutic RNA delivery that can be applied to CPC/CDC exosomes to enhance their efficacy.
CLINICAL TRIALS
The rationale of clinical studies testing CSC therapy on human subjects has been based on the conclusions from the promising results of experimental studies utilizing SCs[219,220].Clinical trials using CSCs, CPCs, and CDCs have been and are still targeting variable cardiac diseases, namely ischemic heart diseases, non-ischemic cardiomyopathy, heart failure, congenital heart diseases, and pulmonary atrial hypertension (Figure 6, Tables 2 and 3).
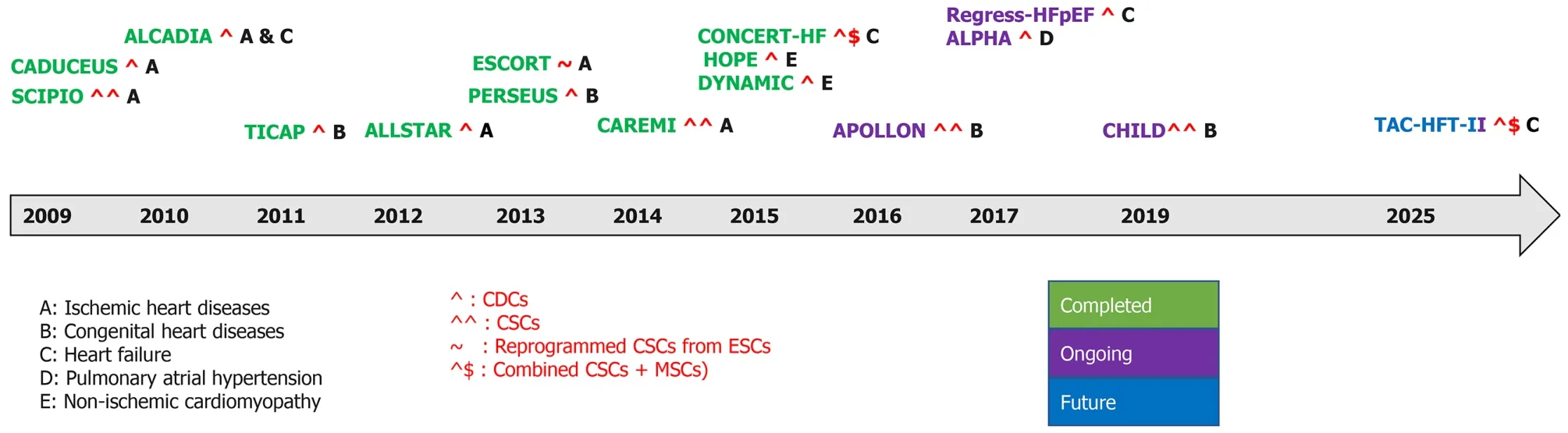
Figure 6 Timeline of completed, ongoing, and future clinical trials in cardiovascular diseases.
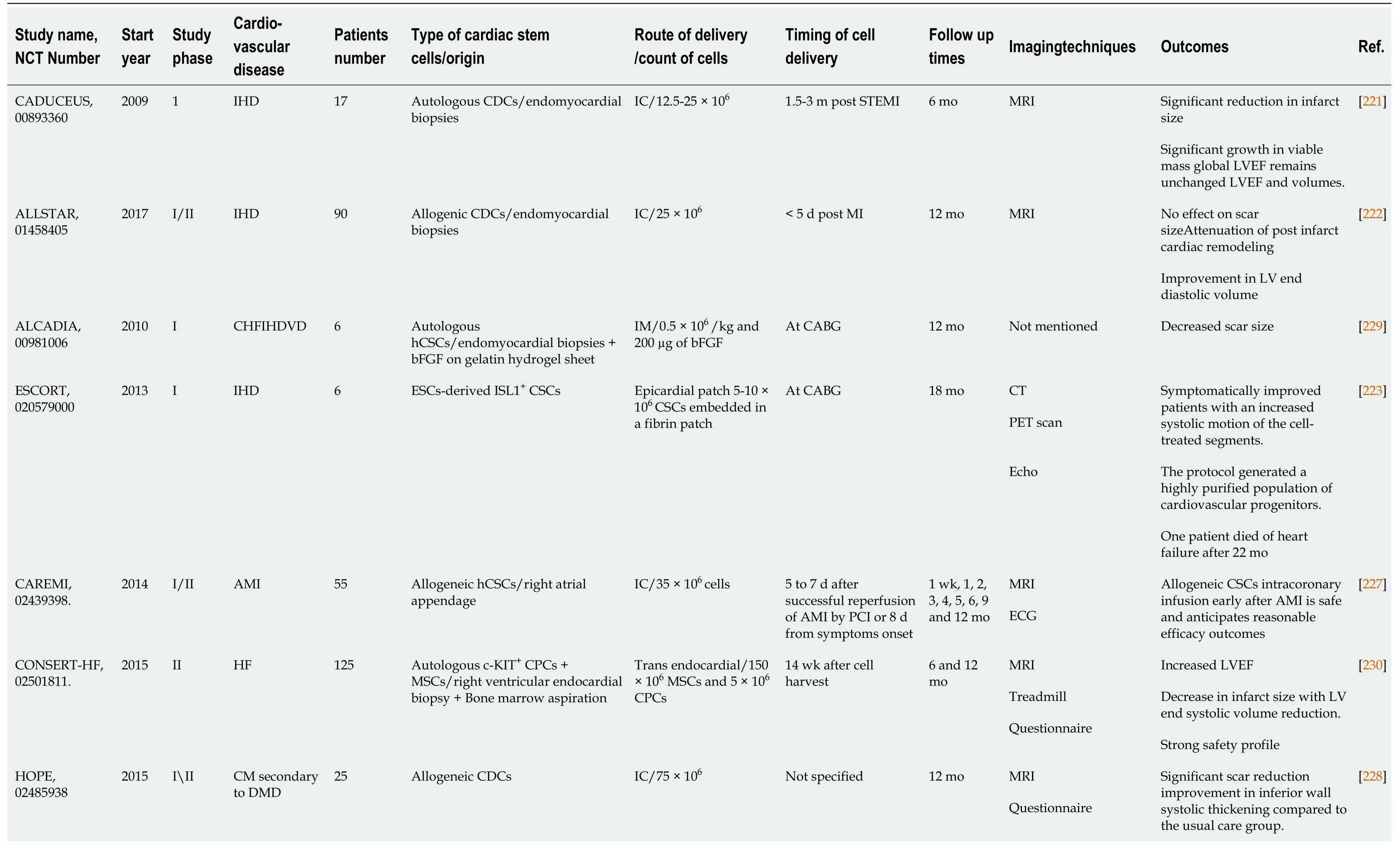
Table 2 Completed clinical trials reporting on the use of cardiac derived stem cells in cardiovascular disorders
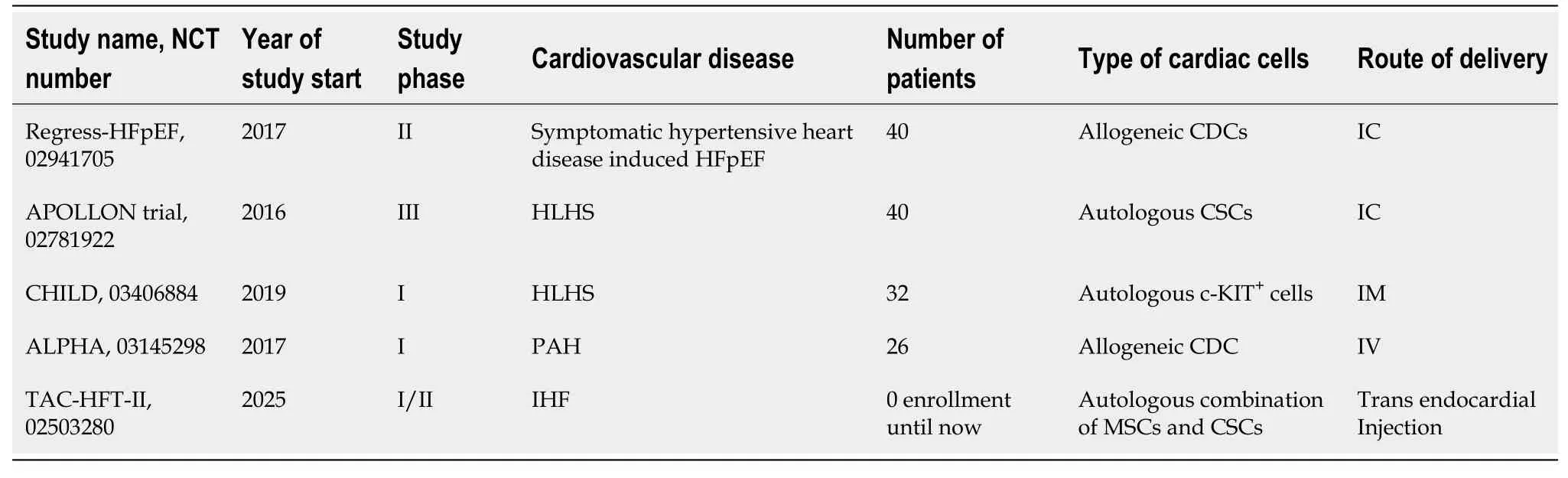
Table 3 Ongoing and future expected clinical trials
Ischemic heart diseases
There have been numerous ischemic heart diseases (IHD) trials.Cardiosphere-derived autologous stem cells to reverse ventricular dysfunction (CAUDEUS) and allogeneic heart stem cells to achieve myocardial regeneration (ALLSTAR) are two complementary studies in which CAUDEUS is the phase I and ALLSTAR is the phase II of clinical trial of intracoronary delivery of autologous CDCs in patients with IHD and left ventricular dysfunction.Firstly, CAUDEUS, with 6 mo and 12-mo follow-up of patients has shown significant reduction in the scar size (-7.7%, -11.1% respectively), increased viable myocardium (+13 g, +22.6 g respectively), and improvement of the regional function of infarcted myocardium[221].Then, ALLSTAR results showed attenuation of the post infarct cardiac remodeling and improvement in the left ventricular diastolic volume, but it has failed to achieve the results of CAUDEUS regarding the reduction of scar size; hence ALLSTAR was dismissed[222].
The clinical trial transplantation of human embryonic stem cell-derived progenitors in severe heart failure (ESCORT) was developed to assess the regenerative effects of human ESC-derived CPCs.At the beginning of coronary artery bypass grafting, patients received a fibrin gel implanted with the hESC derived CPCs.The main conclusion from this trial demonstrated the technical feasibility of producing a clinically operational product.This product is hESCs derived CPCs that can be safely transplanted to patients with severe ischemic left ventricle (LV) dysfunction.It also supports their short- and medium-term safety after transplantation in patients with severe post-infarction LV dysfunction[223].Direct engraftment of CPCs into cardiac tissue along with the paracrine effects of CPCs are the main milieu of cardiac regeneration[224,225].Additionally, fibrin gel has been used as a delivering synthetic biomaterial to improve the long term cell engraftment in the ischemic environment[226].
Safety and efficacy evaluation of intracoronary infusion of allogeneic human cardiac stem cells in patients with AMI (CAREMI), a phase I/II placebo-controlled clinical trial, has been designed to evaluate the safety, practicability, and efficiency of intracoronary transport of allogeneic adult CSC in patients with large ST segment elevation secondary to myocardial infarction, LV dysfunction at risk of developing heart failure.CAREMI has intended to interfere with allogeneic cells immediately after the initial ischemic insult (dodging the aggressive part of the first 5 d) and before myocardial scar starts to form (within 7 d after percutaneous infusion).CAREMI trial is documented proof that allogeneic CSCs intracoronary infusion early after acute MI is safe with rational efficacy outcomes and is promising hope for future clinical trials with Allo-CSCs[227].
Non-ischemic cardiomyopathy
Halt cardiomyopathy progression in Duchenne (HOPE) and dilated cardiomyopathy intervention with allogenic myocardially-regenerative cells (DYNAMIC) studies aim to assess safety and to discover the usefulness of CDCs in patients with non-ischemic cardiomyopathy.HOPE was targeted specifically to explore efficacy of CDCs in patients with advanced stages of Duchenne muscular dystrophy.The results have shown significant and sustained improvements in cardiac structure, function, and significant reduction in cardiac scarring as compared with the control group[228].Meanwhile, the DYNAMIC study does not have any published data so far.
Heart failure
In autologous human cardiac-derived stem cell to treat ischemic cardiomyopathy (ALCADIA), intramyocardial delivery of CDCs with controlled releases of basic FGF in a biodegradable gelatin hydrogel sheet to patients with ischemic heart failure was done.This was at the time of coronary artery bypass grafting.After 6 mo, it was reported that small improvements in regional but not global function,i.e.LVEF (+ 9%-12%), had been noted as well as decreased scar sizes 3.3% at 6-mo follow-upvsbaseline[229].A randomized, double-blinded phase II clinical trial started in 2015 to study the efficacy of combinatorial therapy in treatment of cardiac ischemic disorders.Combination of mesenchymal and c-KIT+CSCs as regenerative therapy for heart failure (CONCERT-HF) utilized autologous endomyocardial CSCs combined with MSCs for heart failure patients.After trans-endocardial injection of this combined therapy, the LVEF increased to 89%, with about 4.5% decrease in infarct size and LV end systolic volume.This is the first clinical trial to assess the benefits of cell combination therapy in humans, which addresses the therapeutic potential for cardiac ischemic patients[230].In addition, according to the ClinicalTrials.gov Identifier (NCT02503280), there is an expected clinical study (TAC-HFT-II) that might start in 2025 to study the safety and effectiveness of trans-endocardial injection of combination of MSCs and CSCs in patients with post-MI HF.Moreover, another ongoing randomized, double blind, placebo-controlled phase II clinical trial for regression of fibrosis and reversal of diastolic dysfunction in heart failure with preserved ejection fraction patients treated with allogeneic CDCs (Regress-HFpEF, NCT02941705) was conducted to determine whether treatment with intracoronary allogeneic CDCs will affect clinical functional status of HFpEF patients, regarding regression of fibrosis and reversal of diastolic dysfunction.
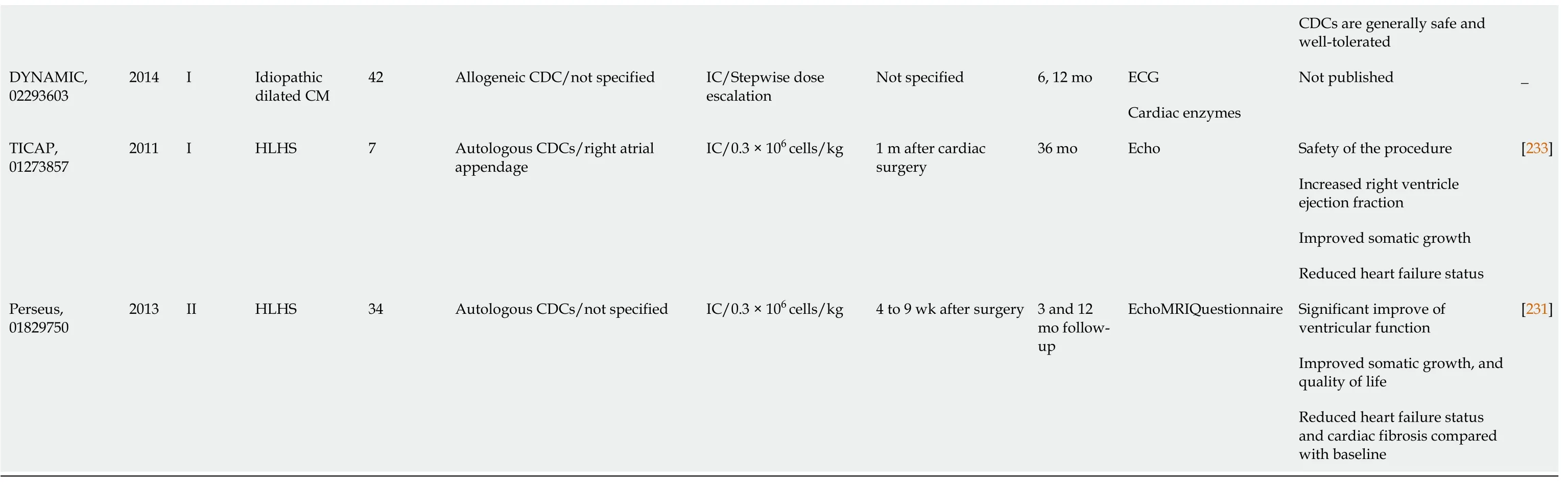
AMI: Acute myocardial infarction; bFGF: Basic fibroblast growth factor; CABG: Coronary artery by bass graft; CHF: Congestive heart failure; CM: Cardiomyopathy; CSC: Cardiac stem cell; DMD: Duchenne muscle dystrophy; hCSC: Human-derived cardiac stem cell; HLHS: Hypoplastic left heart syndrome; IC: Intracoronary; IHD: Ischemic heart disease; IHF: Ischemic heart failure; IM: Intramyocardial; MRI: Magnetic resonance imaging; NCT: National clinical trial; PCI: Percutaneous infusion; STEMI: ST segment elevation after MI; VD: Ventricular dysfunction.
Congenital heart diseases
There are reports on phase I, II, and III clinical trials assessing the use of autologous CDCs in pediatric patients.One of the early clinical trials in children is the transcoronary infusion of CPCs in patients with single ventricle physiology (TICAP) trial.It has confirmed the practicality of intracoronary delivery of CDCs in post palliative single ventricle physiology patients.In this study, autologous CDCs have been isolated, expanded, and then finally administeredviaintracoronary delivery 4-5 wk after the stage II/III palliative surgery.TICAP has proved that the methodology is safe and achievable for improving cardiac function after 18 mo.The safety of the CDC therapy has been also analyzed at 36 mo post-transplantation.Neither tumor formation nor arrhythmias have been reported, and the initial observed benefits have enhanced, with attenuation of ventricular stiffness and improvement of ventriculoarterial coupling[231].
Following the TICAP trial, the cardiac progenitor cell infusion to treat univentricular heart disease (PERSEUS) trial, a phase II randomized controlled trial has been planned to follow-up on the TICAP trial.CDCs have been givenviathe coronary arteries after stage II or III palliative operations[232].The main result is the change in EF from baseline to 3 mo that has been measured by cardiac MRI and echocardiography.The CDC treated group showed greater improvement in right ventricle (RV) function at 3 mo than controls (+6.4%vs+1.3%) and at 1 year continued to exhibit augmented RV function.Follow-up analysis at 36 mo demonstrated an improvement in RVEF among patients receiving CDCs (+8.0%vs+2.2%) even at this late time point.Furthermore, the therapy is currently being tested in a phase III trial cardiac stem/progenitor cell infusion in univentricular physiology (APOLLON).The phase III trial is an extension of the TICAP and PERSEUS trials exploiting autologous CDCs administered by intracoronary injection in single ventricle patients, for which results are still awaiting[233].In addition, there is another ongoing phase I clinical trial (CHILD, NCT03406884) for examining the safety and efficacy of intramyocardial injection of c-KIT CSCs injection in patients with hypoplastic left heart syndrome.
Pulmonary atrial hypertension
Pulmonary arterial hypertension (PAH) is a progressive condition for which there is no cure.As reported in PAH archives and with the pharmacologic developments in the current treatment scope, survival remains poor.Preclinical studies suggest that the transplantation of allogeneic CDCs could reduce unfavorable PAH related arteriolar remodeling.Until now, there are no completed studies on PAH, though, there is an ongoing phase I clinical trial [(ALPHA), NCT03145298] exploring the potential beneficial effects of central intravenous delivery of allogeneic human CDCs.
THERAPEUTIC POTENTIAL OF COMBINED CELLULAR THERAPY
Despite the great differentiation potential of CSCs and their ability to restore the injured area, it is influenced by many factors, such as oxidative stress and the aging process, which diminish its function.Thus, the administration of mixtures of SCs can exhibit synergistic effects to get the maximum benefit from the transplanted cells in cardiac regeneration.Cell combination therapies are classified into various entities; the most commonly performed is combination of CSCs with MSCs either applied from autologous or allogeneic sources.The most recent combinatorial approaches are CardioChimerias (CCs) and CardioClusters.Merging several cell types harmonized efficiently cardiomyoplasty in preclinical cardiac ischemic models[234,235].
The mechanism behind the effect of MSCs when combined with CSCs has been clarified in a study conducted by Hatzistergoset al[236], where transendocardial injection of MSCs derived from allogenic male bone marrow in a reperfused MI swine model led to myocardial repair by stimulating and increasing the differentiation potential of endogenous CSCs to regenerate myocardium.Remarkably, 2 wk after MSC injection, the MSC-treated hearts showed chimeric collections containing both exogenous immature MSCs and endogenous CSCs with a 20-fold increase in c-KIT+cells, indicating cardiac lineage commitment.In addition, to confirm the origin of CSCs, porcine endomyocardial biopsies have been isolated and plated as explants with or without MSC feeder layers, signifying that the MSCs have stimulated the expansion of c-KIT+CSCs.The MSC co-culture derived CSCs have been more than 90% positive for Nkx2-5, which is considered an embryonic heart phenotype.MSCs have facilitated cardiac repair through stimulating a succession of secondary endogenous responses that triggered considerable amounts of adult CMs and immature CSCs to multiply and repair the injured areas with CMs, vascular SMCs, and ECs of host origin.
From this point of view, a study conducted by Williamset al[237] to assess the combination of CSCs and MSCs isolated from a human cardiac tissue and iliac crest, respectively, where 1 × 106human CSCs and 200 × 106human MSCs were injected intra-myocardially in the infarcted area in immunosuppressed pigs after 2 wk of MI induction.All cell-based cardiomyoplasty groups showed a decrease in the infarct size 4 wk following cell injection with a 2-fold greater reduction in scar mass with combined SCs in comparison with each therapy alone.The results have been evaluated by cardiac MRI and conductance catheterization hemodynamics, revealing the improvement of cardiac response to MI functionally and structurally with returning of EF values to baseline, where the combined cell therapy enhanced the LV performance during systole and diastole.
In addition to cardiomyogenic repair in acute MI, the combination therapy enhances cardiac performance, both functionally and structurally, in chronic ischemic cardiomyopathies.The hypothesis was adopted by Karantaliset al[238].The autologous CSCs isolated from the septal wall of the right ventricle immediately after MI induction and the autologous MSCs obtained from the tibial cavity were injected trans-endocardially in the same non-immunosuppressed pigs that undergo ischemic injury for 3 mo.The favorable effects of combinational therapy occurred in chronic MI models, where the size of the infarcted area diminished with improvement of EF, diastolic strain, and cardiac output in comparison with single therapy with MSCs.
In agreement with previous studies to explore the effect of allogeneic combination therapy in non-immunosuppressed swine chronic ischemic cardiomyopathy model, Natsumedaet al[239] addressed the advantage of such therapy from allogeneic sources to reduce the evoked immune response to administrated CSCs alone.The MSCs inhibited the rejection of the immune system to allo-CSCs, improving the retention of the combined cell therapy in the affected area to maintain the regenerative capacity.Moreover, in comparison with the autologous combination approach, angiogenesis increased with improvement of perfusion to the previously infarcted area.Furthermore, the added CSCs to allo-MSCs augmented the contractile function of cardiac muscle by enhancing CM proliferation.This denotes that allogeneic combination cell therapy exerts cardiomyogenic regeneration in chronic ischemic conditions without immune rejection from the host.
The enumerated promising findings demonstrate the privileges of combinational therapy to attain the desirable effects of the differentiation potentiality of CSCs with the supporting and paracrine effects of MSCs in cardiac regeneration.However, the effectiveness of this therapy has been compromised by certain factors such as the persistence and long-term cardiogenic repair.Additionally, optimization of the dose of each cell type in the combination mixture is undefined.Therefore, newin vitrocell engineering techniques[240-243] have emerged to unify the maximum favorable effects of this therapy and eliminate the limitations concerning cardiac repair.
A novel modality has been aroused by Zanget al[240] to enhance the engraftment and angiogenesis properties of c-KIT+CSCs to precondition the cells with exosomes- derived MSCs, resulting in increased proliferation, formation of angiotube, and enhanced migrationin vitro.Moreover, in a rat MI model after 28 d, the CSCs combined with exosomes decreased the infarct size with neovascularization and increased the capillary density with higher EF relative to treated groups with CSCs only.The beneficial effect of exosomes on CSCs was evaluated by miRNA network analysis that exhibit influences on Wnt, VEGF, and PTEN/PI3K/Akt signaling pathways.These effects have enhanced CSCs potentials in cardiac repair by adding new properties to CSCs such as increased proliferation, differentiation, and angiogenesis to the pre infarcted area with defense against oxidative stress.
Quijadaet al[241] is the first study conducted to administer a new cardiac hybrid murine MI model created byin vitroviral fusion between CPCs and MSCs with further clonal expansion known as CCs in order to improve and boost combinatorial cell delivery approaches.In this study, two variable clones were selected from 18 clones after 1 mo of fluorescent activated cell sorting of fused fluorescent-tagged CPCs (mcherry) and MSCs (enhanced green fluorescent protein) with an inactivated RNA Sendai virus to accomplish artificial cell fusion.It has been found that CCs exhibit augmented proliferation and survival properties in addition to opposing cell death (CC1 and CC2) compared with individual cell and combination therapies in a MI mouse model.The CCs injected into the acutely damaged heart have recurred structural integrity with functional improvement up to 18 wk with reduction of infarct size at 12 wk and earlier correction of EF at 6 wk.Moreover, they are not subjected to cell aging relative to combinational and single cell therapy.The tremendous effect of CCs was presented in enhanced engraftment in the border zone of infarcted area, which denotes the selectivity of this hybrid in addition to the already retained properties of CSCs and MSCs in cardiomyogenic repair.Notably, in comparison with mixed dual cell therapy the preservation of CM size and induction of c-KIT+cells have occurred at 12 wk, with enhanced capillary density maintaining a longstanding cardiac vasculature.
In agreement with reparative potentials of CCs, Firouziet al[242] applied the principle of cell fusion between c-KIT+cardiac interstitial cells (cCICs) and MSCs in a 2:1 ratio with inactivated Sendai virus.The cells were isolated from human cardiac samples removed during left ventricular assisted device implantation, then were allowed for clonal expansion to study the phenotypic features and function as compared to the parent cells.It was been found that the survival of human CCs is prolonged more than parent cells in addition to the increased survival of CMs when co-cultured with serum deprived neonatal rat CMs.Compared to CCs originating from a mouse model, its ability to resist oxidative stress is increased, thus enhancing survival potential and inheriting the same karyotype of the parent cells with diploid DNA content.However, it has not yet been tested in a MI model to assess the degree of retention or the cardiomyogenic repair potentials.
The evolution of next generation applications to improve the efficacy of combinatorial cell therapy in heart failure have been accomplished by Monsantoet al[243] who have implemented a 3D structure composed of MSCs in the center surrounded by cCICs, while endothelial progenitor cells (EPCs) inhabited the outer layer.These isolated autologous SCs in a ratio 3:2:1 of EPCs, cCICs, and MSCs, respectively, have been co-cultured to enhance the interactions between the cells before injection intramyocardially in a murine MI model.Moreover, this scaffold has inherited the same efficiency of each cell type.The EPCs protected the cCICs from oxidative stress and cell death with neovascularization to the infarcted area.Thus, the power of cCIC differentiation was increased in addition to the supporting milieu exerted by MSCs.The beneficiary outcomes were evidenced by the improved cardiac function in the murine model initiated from the first week post MI injection and sustained up to 20 wk, with greater improvement in EF, end systolic, and diastolic volume.Due to the administered distinct cell ratios and the controllable 3D structure size, which minimize cell loss, the CCs were retained for a long time and hence provided maximum effects of cell combination therapies in cardiomyogenic repair[240].
CONCLUSION
CSCs/CPCs carry a great therapeutic potential in different cardiac diseases owing to their numerous cell population, rich secretome and interplay with other cells.Conducted preclinical studies and clinical trials had demonstrated promising results in regards to the improvement of cardiac function.Studies using these cells targeting variable cardiac diseases are still ongoing aiming to discover more of their regenerative power and thus decrease the burden of morbidity and mortality due to cardiac tissue loss.
杂志排行
World Journal of Stem Cells的其它文章
- Multiple roles of mothers against decapentaplegic homolog 4 in tumorigenesis, stem cells, drug resistance, and cancer therapy
- Modified mesenchymal stem cells in cancer therapy: A smart weapon requiring upgrades for wider clinical applications
- Molecular mechanism of therapeutic approaches for human gastric cancer stem cells
- Epigenetic regulation by long noncoding RNAs in osteo-/adipogenic differentiation of mesenchymal stromal cells and degenerative bone diseases
- Therapeutic effects of menstrual blood-derived endometrial stem cells on mouse models of streptozotocin-induced type 1 diabetes
- Stem cell therapy applied for digestive anastomosis: Current state and future perspectives
