Multiple roles of mothers against decapentaplegic homolog 4 in tumorigenesis, stem cells, drug resistance, and cancer therapy
2022-02-12ChuanJingDaiYuTingCaoFangHuangYiGangWang
Chuan-Jing Dai, Yu-Ting Cao, Fang Huang, Yi-Gang Wang
Chuan-Jing Dai, Yu-Ting Cao, Yi-Gang Wang, College of Life Sciences and Medicine, Zhejiang Sci-Tech University, Hangzhou 310018, Zhejiang Province, China
Fang Huang, Department of Pathology, Zhejiang Provincial People’s Hospital of Hangzhou Medical University, Hangzhou 310014, Zhejiang Province, China
Abstract The transforming growth factor (TGF)-β signaling pathway controls many cellular processes, including proliferation, differentiation, and apoptosis.Abnormalities in the TGF-β signaling pathway and its components are closely related to the occurrence of many human diseases, including cancer.Mothers against decapentaplegic homolog 4 (Smad4), also known as deleted in pancreatic cancer locus 4, is a typical tumor suppressor candidate gene locating at q21.1 of human chromosome 18 and the common mediator of the TGF-β/Smad and bone morphogenetic protein/Smad signaling pathways.It is believed that Smad4 inactivation correlates with the development of tumors and stem cell fate decisions.Smad4 also interacts with cytokines, miRNAs, and other signaling pathways, jointly regulating cell behavior.However, the regulatory function of Smad4 in tumorigenesis, stem cells, and drug resistance is currently controversial.In addition, Smad4 represents an attractive therapeutic target for cancer.Elucidating the specific role of Smad4 is important for understanding the mechanism of tumorigenesis and cancer treatment.Here, we review the identification and characterization of Smad4, the canonical TGF-β/Smad pathway, as well as the multiple roles of Smad4 in tumorigenesis, stem cells, and drug resistance.Furthermore, we provide novel insights into the prospects of Smad4-targeted cancer therapy and the challenges that it will face in the future.
Key Words: Cancer therapy; Drug resistance; Mothers against decapentaplegic homolog 4; Stemness; Transforming growth factor-β; Tumorigenesis
INTRODUCTION
The transforming growth factor (TGF)-β signaling pathway controls several cell behaviors, including proliferation, inflammation, differentiation, and apoptosis[1,2].Abnormalities of the TGF-β signaling pathway and its components are related to many human diseases such as fibrosis[3], immune diseases[4], and cancer[5].Although TGFβ signals are mainly transmitted to the nucleus through the TGF-β/mothers against decapentaplegic homolog (Smad) signaling pathway[6], TGF-β superfamily ligands often interact with other signaling pathways, including JNK/p38, PI3K/AKT, ERK/MAPK, and integrin signaling pathways, regulating various cellular responses in a non-Smad-dependent manner[7-9].The function of TGF-β in tumorigenesis is frequently described as a double-edged sword, but the precise mechanism for this phenomenon is still unclear[5,10,11].In addition, TGF-β plays a role in the development of cardiac[12] and kidney[3] fibrosis and interacts with integrins to mediate asthmatic remodeling of the airway[13].Moreover, TGF-β exerts an immunosuppressive function[14].In recent years, studies on TGF-β have been focused on elucidating the regulation and effects of its upstream and downstream components on various diseases, as well as the interaction between different factors and TGF-β[10,15,16].Understanding of these processes may improve the diagnosis and treatment of these diseases in the future.
Cancer has become a major threat to human health worldwide.In 2020 alone, 19.3 million new cases were diagnosed, and nearly 10 million people died of cancer[17].Unfortunately, our current understanding of the mechanism of tumorigenesis is not enough to completely overcome cancer[18].Smad4, a key component of the canonical TGF-β signaling pathway, exhibits varying degrees of inactivation among cancers[19], and its expression is significantly correlated with tumor development and prognosis in cancer patients[20,21].Therefore, Smad4 is potently regarded as a tumor suppressor.Moreover, considering the critical role of Smad4 in tumor progression, Smad4 can be an attractive target for cancer treatment[22].Additional novel options for cancer therapy were revealed by demonstrating that many RNAs directly/indirectly targeting Smad4, including miRNAs, circular (circ) RNAs, and long noncoding (lnc) RNAs, are dysregulated during cancer progression[23-25].In the past decade, it has been found that Smad4 seems to play a tumor-promoting role in certain types of cancer, such as hepatocellular carcinoma[26,27].In addition, an increasing number of studies demonstrated a close association of Smad4 with stem cell fate[28] and drug resistance of cancer cells[29].Elucidating the specific role of Smad4 is important for understanding the mechanism of tumorigenesis and cancer treatment.Here, we review the identification and characteristics of Smad4, and the canonical TGF-β signaling pathway, and summarize the multiple regulatory functions of Smad4 in tumorigenesis, stem cell fate, and drug resistance.In addition, we provide new insights into the prospects of Smad4-targeting cancer therapy and its challenges in the future.
IDENTIFICATION AND CHARACTERISTICS OF SMAD4
Smad4, also known as deleted in pancreatic cancer locus 4 (DPC4), was initially found as a tumor suppressor candidate gene in human pancreatic carcinoma in 1996[30].The term “Smad” was a combination of thesmagene ofCaenorhabditis elegansand themadgene ofDrosophila melanogaster[31].Subsequently, Smad4 mutations were found in additional types of tumors, such as gastrointestinal carcinoid[32], prostate cancer[33], squamous cell carcinoma[34], and lung cancer[35].The gene coding for Smad4 is located at human chromosome locus 18q21.1 (Figure 1A) and is composed of 12 exons and 10 introns[36].The 12thexon was named exon 0 because it was discovered after the identification of 11 exons and is located upstream of exon 1[32].
Smad4 protein consists of 552 amino acids and has a molecular weight of 60 kDa[36].It is composed of the N-terminal mad homology domain 1 (MH1), the middle linker region including nuclear export signal and Smad activation domain (SAD), as well as the C-terminal MH2[37].The MH1 domain of Smad4 is involved in DNA binding by recognizing the Smad-binding site of DNA.The SAD of the linker region and MH2 domain are responsible for transcriptional activity.Additionally, the MH2 domain interacts with the MH1 domain of other Smads, enabling the transduction of various signaling pathways, including the TGF-β signaling (Figure 1B).
TGF-β/SMAD AND BMP/SMAD SIGNALING PATHWAYS
The canonical TGF-β signaling pathway is an uncomplicated linear cascade, which involves TGF-β superfamily ligands, receptors, and signal transducers (Figure 2)[38].At present, there are 33 known TGF-β ligands encoded by mammalian genomes, including activin, nodal, TGF-βs, bone morphogenetic proteins (BMPs), and growth differentiation factors (GDFs)[39,40].According to the difference in structure and function, these polypeptides can be subdivided into two families: TGF-βs (TGF-β, GDF, nodal, and activin) and BMPs (BMP2, BMP4, and BMP7).The TGF-β type I and type II receptors (TβRI and TβRII) are composed of several pairs of serine/threonine protein kinases[41,42].
The eight known Smads are divided into three categories, including a common Smad (Co-Smad, Smad4), two inhibitory Smads (I-Smads, Smad6, and Smad7), and receptor-regulated Smads (R-Smads, Smad2/3 transducing TGF-β signaling, and Smad1/5/8 transducing BMP signaling).Co-Smad and Smad4/DPC4 bind to RSmads, forming two different complexes, Smad4/Smad2/3 and Smad4/Smad1/5/8.Subsequently, the heteromeric complexes are translocated into the nucleus, where they interact with transcriptional factors and bind to regulatory elements of target genes, affecting their expression[43].Smad6 and Smad7 can both inhibit the transcriptional activity of target genes, thus blocking TGF-β signal transmission.It must be emphasized that Smad6 acts in a manner that is not always consistent with that of Smad7[44,45].
MULTIPLE ROLES OF SMAD4 IN TUMORIGENESIS
Due to the multiple interactions of environmental chemicals, genes, and endogenous signals, the process of carcinogenesis is extremely complex[46].As a tumor suppressor gene,Smad4exerts its inhibitory effect on tumor cells primarilyviathe canonical TGF-β signaling pathway[47,48].The mechanism of this inhibition, which prevents carcinogenesis, involves the role of Smad4 in inhibiting the tumor-promoting activity of proinflammatory cytokines, inducing the cell cycle arrest, and promoting apoptosis through activating the TGF-β/BMP/Smad4 axis.However, once theSmad4gene is mutated, TGF-β cannot induce G1 or G2 cell cycle arrest and switch from tumor suppressor to tumor promoter, leading to tumor growth and metastasis[10,49].In addition, Smad4 is indispensable for the tumor suppressor function of TGF-β[50].So far, there are many contradictory results and conclusions about the role of Smad4 in tumorigenesis.Here, we focus on pancreatic cancer and hepatocellular carcinoma (HCC) to discuss the role of Smad4 in cancer progression (Figure 3).
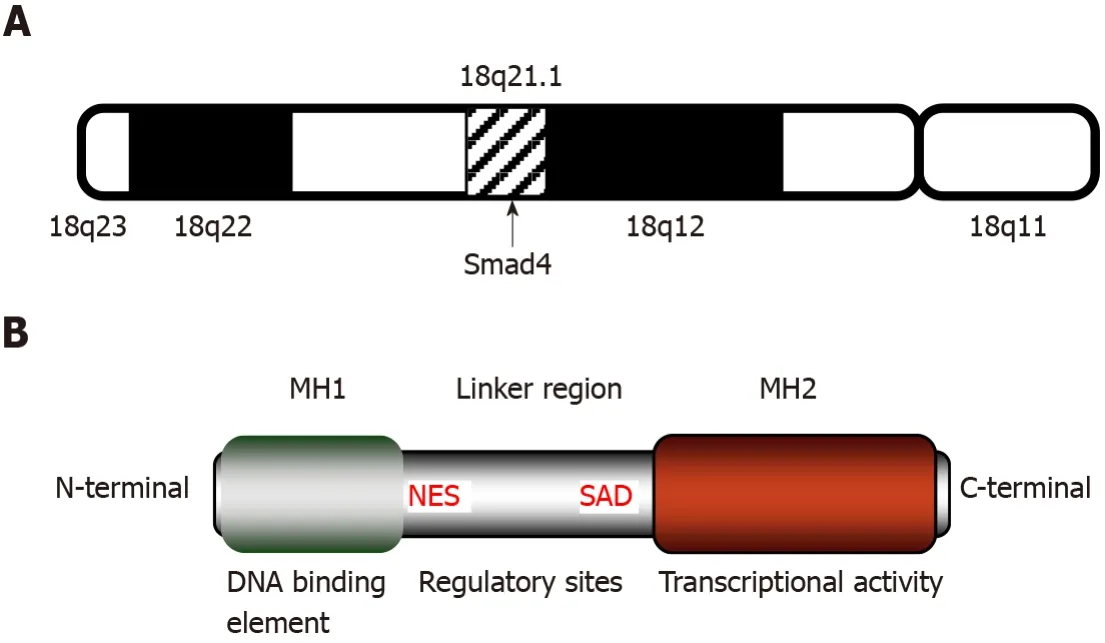
Figure 1 Structure and features of mothers against decapentaplegic homolog 4.
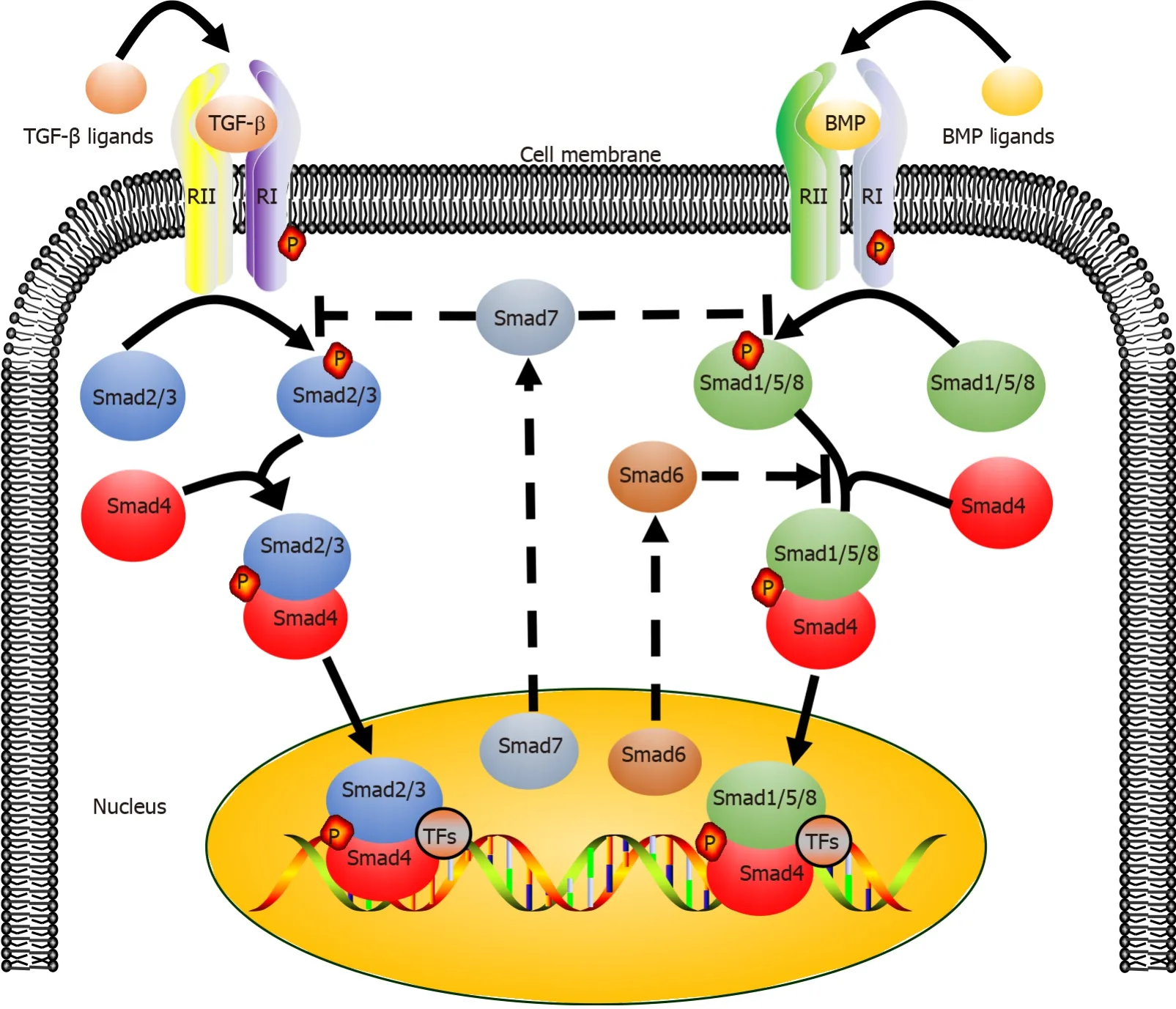
Figure 2 Transforming growth factor-β/mothers against decapentaplegic homolog 4 and bone morphogenetic protein/mothers against decapentaplegic homolog signaling pathways.

Figure 3 Different roles of mothers against decapentaplegic homolog 4 in the development of hepatocellular carcinoma and pancreatic cancer.
It is recognized that Smad4 acts as a tumor suppressor in pancreatic cancer.Smad4mutation or deletion is found in > 50% of pancreatic cancer and is associated with the proliferation and metastasis of tumor cells[51,52].The alterations in theSmad4gene mainly include deletion, frameshift mutation, point mutation, amplification, and translocation[19,22].Furthermore,Smad4gene mutation is associated with stages of pancreatic cancer.A study by Nottaet al[53] showed that the inactivation rate of Smad4 in mid-advanced pancreatic cancer was higher than it in early pancreatic cancer.Therefore, Smad4 dysfunction may be considered an advanced event in pancreatic cancer.Mechanically, the behavior that Smad4 deletion accelerates the progression of pancreatic cancer may be related to the increased expression of HNF4G, PAR-4, and PGK-1[51,54,55].Additionally, Smad4 mutation in mice does not directly contribute to the formation of pancreatic tumors[56], indicating that a single Smad4 mutation is not sufficient to initiate pancreatic carcinogenesis.Thus, mutations inSmad4are likely to cooperate with mutations in other genes in promoting pancreatic cancer progression.For example, Izeradjeneet al[57] revealed that the formation of mucinous cystic neoplasms is induced by synergistic effects of Kras-G12D and Smad4 mutations.The role of Smad4 in other human cancers, such as colorectal cancer (CRC)[58], gastric cancer[59], ovarian cancer[60] and head and neck squamous cell carcinoma (HNSCC)[61], is similar to that in pancreatic cancer.Downregulation of Smad4 is considered an early event of HNSCC, which is different from pancreatic cancer.
An interesting fact regarding HCC is that the role of Smad4 in HCC differs significantly from that in cancers mentioned above.The nuclear level of Smad4 in liver cancer tissue is markedly higher than that in the adjacent noncancerous tissue[26].The ability of liver cancer cells to form colonies and migrate was significantly reduced afterSmad4gene knockout in mouse models.Yuanet al[62] showed that ubiquitin-specific proteases promote HCC cell migration and invasion by deubiquitinating and stabilizing Smad4 protein.These findings seem to indicate that Smad4 plays a tumorpromoting rather than tumor-suppressing role in HCC.However, the underlying mechanism of Smad4 in the pathogenesis of HCC remains elusive.We propose that this difference may be explained by the fact that the cellular behaviors of the TGF-β/Smad4 signaling pathway varies with types of cells, their extracellular matrix, TGF-β concentration, and tumor microenvironment.These possibilities warrant further investigation.It is certain that Smad4 plays multiple regulatory roles in carcinogenesis, and whether it has a tumor-promoting or tumor-suppressing function may depend on the microenvironment of tumor cells and surrounding stromal cells.
SMAD4 IN STEM CELLS
Besides tumorigenesis, Smad4 is involved in the self-renewal and pluripotency of human stem cells.It has been reported that several miRNAs targeting Smad4 negatively regulate the differentiation of human mesenchymal stem cells (hMSCs)[63,64], but the underlying mechanisms of stem cell differentiation are poorly understood.The Smad4/TAZ axis might be involved in this process.TAZ protein, a transcriptional coactivator with PDZ-binding motif, can bind to Smad4 protein and translocate to the nucleus, where it enhances the expression of osteogenic genes, promoting the osteogenic differentiation of hMSCs[65].These findings suggest that Smad4 is essential for stem cell differentiation.However, Averyet al[28] demonstrated that human embryonic stem cells (hESCs) remain undifferentiated afterSmad4gene knockdown, indicating that the single event of Smad4 inactivation does not induce stem cell differentiation.Similarly, Smad4 inactivation does not directly induce self-renewal of stem cells.For instance, Smad4 mutation has no effect on hESC self-renewal but is essential for their differentiation into cardiac mesodermal precursors[66].
BMP signaling is also indispensable for regulation of the proliferation of stem cells and maintenance of metabolic homeostasis[67].BMP binds with leukemia inhibitory factor to maintain the self-renewal of ESCs[68].Subsequent studies have documented that stem cell fate decisions induced by BMP may be related to Smad4.The BMP/Smad axis regulates the proliferation and differentiation of alveolar stem cells.BMP suppresses proliferation of alveolar type 2 epithelial cells (AT2s), while antagonists promoted the self-renewal of AT2s at the expense of differentiation[67].BMP restricts the self-renewal of intestinal Lgr5+stem cells by Smad4-mediated transcriptional repression and thus prevents excessive proliferation of epithelial cells[69].Therefore, the inactivation of Smad4 may counteract the inhibitory effect of BMP on stem cell proliferation, contributing in this manner to the occurrence of cancer cells.
Cancer stem cells (CSCs) have many characteristics similar to stem cells.However, in contrast to stem cells, they exhibit tumorigenicity and invasiveness[70].The gradual accumulation of genetic mutations in stem cells during the life of the organism is associated with the development of cancer[71], and results in tumor heterogeneity[72].Therefore, elucidating the relationship between Smad4 and CSC fate may provide a clinical diagnostic index or potential therapeutic target for cancer.Xiaet al[73] found that cyclin D1 interacts with Smad2/3 and Smad4, activating the cyclin D1/Smad pathway and upregulating the expression of stemness genes in liver CSCs.This result implies that the use of Smad inhibitors may be an effective strategy for targeting liver CSCs.Wenet al[74] showed that blocking the TGF-β/Smad/EMT pathway inhibited the self-renewal and metastasis of ovarian CSCs.In another study[75], Smad4 mutation was introduced into organoids derived from intestinal epithelium through the CRISPR-Cas9 system.Upon injection of these organoids into the spleen of mice, they formed micrometastases containing invading tumor cells, but they could not colonize the liver, suggesting that Smad4 mutation must be combined with additional factors to promote tumor invasion.
SMAD4 IN DRUG RESISTANCE
Drug resistance is the main cause of chemotherapy failure and tumor recurrence in cancer patients[76].Drug resistance has been shown to be related to the activation of autophagy[77], a highly conserved catabolic process in which large cellular structures are degraded.Autophagy contributes to cell survival by recycling constituents of cellular structures[78].Activation of autophagy not only mediates the resistance to chemotherapy but also induces autophagy-mediated cell death, which helps to eliminate tumor cells[52,79].For instance, autophagy protects breast cancer cells from epirubicin-induced apoptosis and promotes the development of epirubicin resistance[80].Peptidylarginine deiminase IV, a protein involved in many pathological processes, induces the resistance of HCC to chemotherapy by activating autophagy[81].Autophagy may promote the development of multidrug resistance.Fortunately, inducing autophagy-mediated cell death can help overcome the resistance to chemotherapy, and there are many clinically available drugs that can regulate autophagy, such as chloroquine or hydroxychloroquine[82].These autophagy regulators inhibit autophagy mainly by reducing autolysosome fusion[83].In addition, TGF-β activates autophagy activation by both Smad and non-Smad pathways, promoting the survival of cancer cells[84,85].Thus, Smad4 plays an indispensable role in TGF-β-induced autophagy and drug resistance (Figure 4).
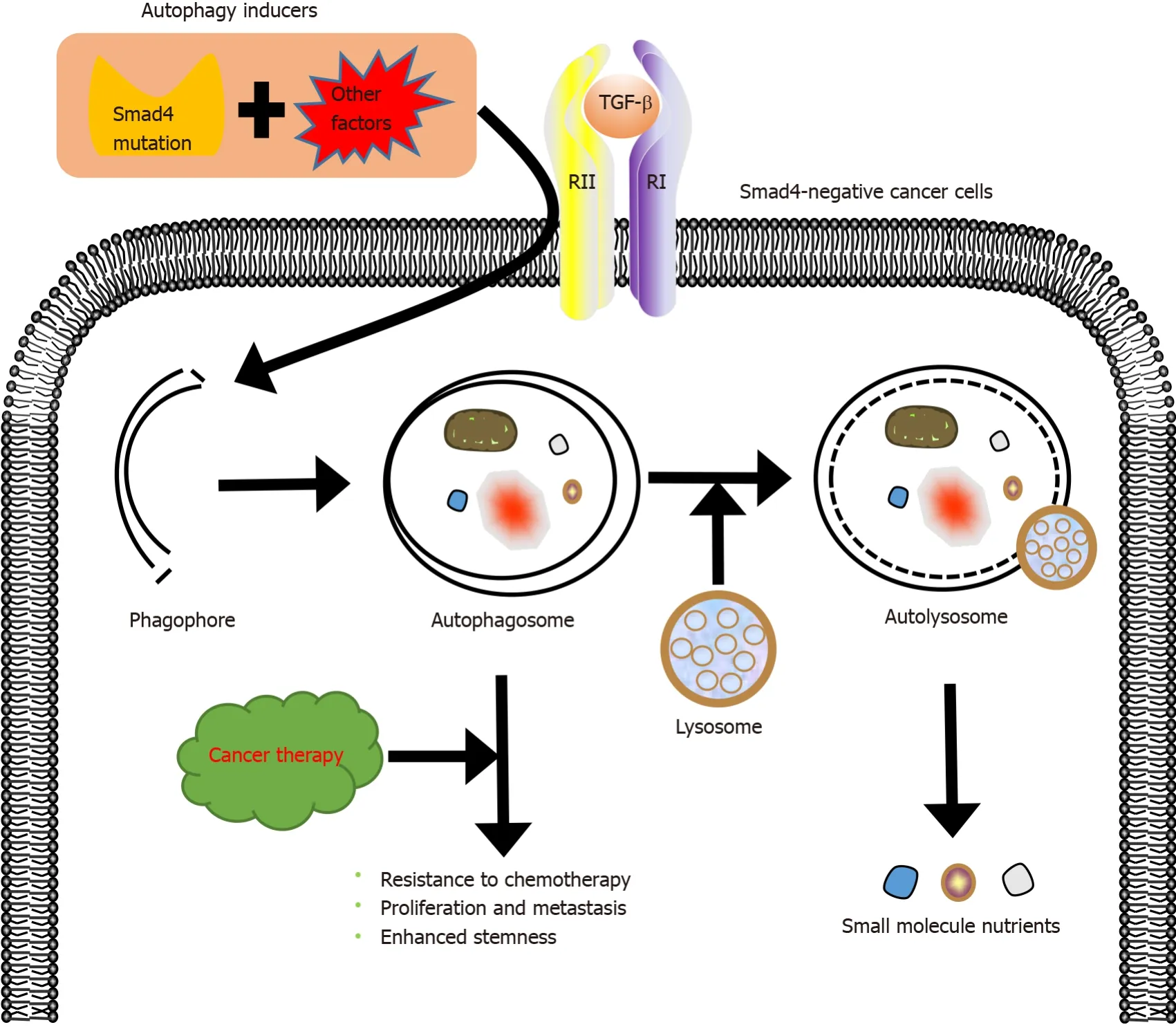
Figure 4 Role of mothers against decapentaplegic homolog 4 in autophagy and drug resistance induced by transforming growth factor-β.
Smad4 inactivation not only involves the development of drug resistance in cancer but also contributes to the crosstalk between TGF-β and other signaling pathways, accelerating this process.Zhanget al[86] demonstrated that Smad4 deletion induces the resistance of colon cancer cells to 5-fluorouracil (5-FU)-based therapy by activating the AKT pathway.Moreover, Smad4 deficiency inhibits 5-FU-mediated apoptosis[87].Besides, the inactivation of Smad4 makes tumors resistant to other chemotherapeutic drugs.Smad4 mutation contributes to platinum resistance in non-small cell lung carcinoma[35] and to cetuximab resistance in HNSCC[29].Targeting the rapamycininsensitive companion of mTOR, a component of mammalian target of rapamycin complex 2, increases the sensitivity of Smad4-negative colon cancer to irinotecan[88].Recent studies have demonstrated that Smad4 deficiency correlates with the development of resistance to chemotherapy and radiotherapy[89].Therefore, Smad4 modifications may be a critical marker predicting the resistance of tumor cells to chemoradiation therapy[90].In our view, elucidating the specific role of Smad4 in resistance of tumors to drugs and developing rational therapeutic applications of autophagy will help to improve the outcomes of treatment of drug-resistant tumors.
SMAD4 IN CANCER THERAPY
Given the key role of Smad4 in tumorigenesis, Smad4 is expected to be an attractive therapeutic target for tumors resistant to radiotherapy and chemotherapy.Many miRNAs, such as miR-224[91], miR-34a[92], and miR-205[93], are essential regulators of TGF-β-induced tumor suppression by affecting the TGF-β/Smad signaling pathway.Therefore, Smad4-targeting miRNAs may become novel therapeutic agents for cancer treatment.Other RNAs, including circRNAs[94] and lncRNAs[95], regulate the level of Smad4 protein by targeting Smad4-targeting miRNAs directly or indirectly.Proteins, such as ALK[96] and tripartite motif 47[97] that promote, respectively, Smad4 phosphorylation and ubiquitination, diminish the tumor suppressor effect of Smad4, indicating that suppressor molecules or enzymes that inhibit Smad4 activity may be a new option for the treatment of Smad4-negative cancers.
Many inhibitors targeting the TGF-β signaling pathway are being developed clinically[98].Molecules such as TGF-β antibodies, antisense oligonucleotides, and small molecule inhibitors of TGF-β receptor kinase activity show immense clinical potential[99].Although inhibition of the TGF-β signaling pathway is one of the strategies for cancer treatment, the clinical outcomes of targeting TGF-β signaling and its superfamily members are not satisfactory, suggesting that single target-based therapies are not sufficient to inhibit tumors[10].
Combination therapy is an increasingly important part of anticancer therapies, and has more advantages than single-drug therapy[100-102].The combination of Smad4 targeting and traditional/nontraditional therapies may become a novel choice for anticancer treatment.Mariathasanet al[103] showed that the combined application of TGF-β inhibitors together with anti-PD-L1 antibodies decreased the TGF-β level and promoted the infiltration of T-cells into tumor cells, thereby enhancing antitumor immune response and leading to tumor apoptosis.Kassardjian and Wang[104] found that Smad4-positive tumors had a better response to neoadjuvant therapy, and the lymph node metastasis rate of Smad4-positive tumors was significantly lower, suggesting that Smad4 plays an important role in neoadjuvant therapy.In our previous research[105], we combined oncolytic virus therapy and targeted gene therapy to design a new oncolytic adenovirus CD55-Smad4, which can stably produce Smad4 proteinin vitroandin vivo.CD55-Smad4 significantly inhibited proliferation, metastasis, and stemness of CRC cells.All these show that the combination therapy targeting Smad4 has clinical potential.Therefore, we believe that combination of Smad4-targeted therapy with traditional therapies of surgery, radiotherapy, and chemotherapy, and with emerging treatments such as PD-LI inhibitors, chimeric antigen receptor cell therapy, as well as our targeting gene virotherapy will significantly improve the outcomes of anticancer therapies.
CONCLUSION
Targeting abnormal signal transduction or abnormal metabolic pathway has always been the focus of anticancer research.Smad4gene mutations and its abnormal expression have been confirmed to dysregulate the TGF-β signaling pathway, transforming its function from a tumor suppressor to a tumor promoter[106].This signaling disorder accelerates tumor progression, but the precise mechanism by which Smad4 affects tumor development remains unclear.An increasing number of studies have shown that targeting oncogenic miRNAs, circRNAs, and other RNAs can inhibit TGF-β-induced tumorigenesis by directly or indirectly acting on Smad4, increasing susceptibility of tumors to chemoradiation and improving the survival rate of cancer patients.Although the possibility of regulating tumor progression by these RNAs shows clinical potential, the underlying mechanisms are poorly understood.
Fortunately, the vigorous development of genome editing technologies such as CRISPR-Cas9 systems and AAV gene vectors has enabled the transformation of Smad4-targeted therapies into clinical reality[107,108].Furthermore, it is asserted that the presence of Smad4 protein promotes the cell-to-cell spread of the vaccinia virus, which enables combiningSmad4gene therapy with oncolytic virus therapy to effectively eliminate tumors[109].In summary, although the specific mechanisms by which Smad4 affects carcinogenesis, metastasis, drug resistance, and other malignant features of tumors have not been completely clarified, Smad4-targeted therapies coupled with traditional therapies and emerging anticancer treatments have achieved good antitumor effects in animal models.Smad4 combination therapy shows potential for future clinical applications.
杂志排行
World Journal of Stem Cells的其它文章
- Cardiac stem cells: Current knowledge and future prospects
- Modified mesenchymal stem cells in cancer therapy: A smart weapon requiring upgrades for wider clinical applications
- Molecular mechanism of therapeutic approaches for human gastric cancer stem cells
- Epigenetic regulation by long noncoding RNAs in osteo-/adipogenic differentiation of mesenchymal stromal cells and degenerative bone diseases
- Therapeutic effects of menstrual blood-derived endometrial stem cells on mouse models of streptozotocin-induced type 1 diabetes
- Stem cell therapy applied for digestive anastomosis: Current state and future perspectives
