Direct-acting antivirals for chronic hepatitis C treatment: The experience of two tertiary university centers in Brazil
2022-02-12MarianaSandovalLourencoPatriciaMomoyoZitelliMarloneCunhaSilvaArthurIvanOliveiraClaudiaOliveiraTiagoSevPereiraFlairJosCarrilhoMarioPessoaDanielMazo
Mariana Sandoval Lourenco, Patricia Momoyo Y Zitelli, Marlone Cunha-Silva, Arthur Ivan N Oliveira, Claudia P Oliveira, Tiago Sevá-Pereira, Flair José Carrilho, Mario G Pessoa, Daniel F Mazo
Mariana Sandoval Lourenço, Marlone Cunha-Silva, Tiago Sevá-Pereira, Daniel F Mazo, Division of Gastroenterology, Department of Internal Medicine, School of Medical Sciences, University of Campinas, Sao Paulo 13083-878, Brazil
Patricia Momoyo Y Zitelli, Arthur Ivan N Oliveira, Cláudia P Oliveira, Flair José Carrilho, Mario G Pessoa, Daniel F Mazo, Division of Clinical Gastroenterology and Hepatology, Department of Gastroenterology, University of São Paulo School of Medicine, Sao Paulo 05403-900, Brazil
Abstract BACKGROUND Hepatitis C virus (HCV) treatment has undergone major changes in recent years.Previous interferon-based therapies have been replaced by oral direct-acting antivirals (DAA) regimens, with high sustained virologic response (SVR) rates, and a lower incidence of adverse events (AEs).AIM To evaluate the efficacy and safety of DAAs for HCV treatment in subjects from two tertiary university centers in Brazil.METHODS This is a multicenter retrospective cohort study of 532 patients with chronic hepatitis C (CHC), undergoing treatment with interferon-free regimens from November 2015 to November 2019.The therapeutic regimen was defined by the current Brazilian guidelines for HCV management at the time of treatment.Demographic, anthropometric, clinical, and laboratory variables were evaluated.SVRs were assessed at 12 to 24 wk after therapy by intention-to-treat (ITT), and modified ITT (m-ITT) analysis.AEs and serious adverse events (SAEs) were registered.In the statistical analysis, a P value of < 0.05 was considered significant.RESULTS The mean age was 56.88 years, with 415 (78.5%) being HCV genotype 1, followed by genotype 3 (20.1%).Moreover, 306 (57.5%) subjects had cirrhosis, and a third of them had decompensated cirrhosis.Sofosbuvir (SOF) plus daclatasvir ± ribavirin was the most frequently used treatment (66.9%), followed by SOF plus simeprevir (21.2%).The overall ITT SVR was 92.6% (493/532), while the m-ITT SVR was 96.8% (493/509).Variables associated with treatment failure via ITT evaluation were hepatic encephalopathy (OR: 4.320; 95%CI: 1.920-9.721, P = 0.0004), presence of esophageal varices (OR: 2.381; 95%CI: 1.137-4.988, P = 0.0215), previous portal hypertensive bleeding (OR: 2.756; 95%CI: 1.173-6.471, P = 0.02), higher model for end-stage liver disease scores (OR: 1.143, 95%CI: 1.060-1.233, P = 0.0005), lower serum albumin levels (OR: 0.528, 95%CI: 0.322-0.867, P = 0.0115), higher serum creatinine (OR: 1.117, 95%CI: 1.056-1.312, P = 0.0033), and international normalized ratio (INR) levels (OR: 5.542, 95%CI: 2.023-15.182, P = 0.0009).AEs were reported in 41.1% (211/514) of patients, and SAEs in 3.7%.The female gender, higher body mass index, esophageal varices, higher INR values, and longer treatment duration were independently associated with AE occurrence.CONCLUSION Treatment with oral DAAs attains a high SVR rate, with fewer SAEs in a real-life cohort of subjects with CHC, from two tertiary university centers in Brazil.
Key Words: Chronic hepatitis C; Antiviral agents; Hepatitis C virus; Sustained virologic response; Liver cirrhosis; Safety
INTRODUCTION
Hepatitis C represents a global health problem.It is estimated that there are approximately 71 million people on a global basis who are chronically infected with the hepatitis C virus (HCV), with a prevalence of 1.1%[1].However, many carriers are unaware of the infection, and do not receive treatment[2].Despite the rising prevalence of metabolic-dysfunction associated fatty liver disease, HCV is a major cause of cirrhosis, and hepatocellular carcinoma (HCC) worldwide[3].It is estimated that 0.53% of the total Brazilian population has antibodies against HCV, while in 2019, this virus was the leading cause of death for viral hepatitis in Brazil[4,5].
The main objective of therapy is the eradication of the virus, defined as sustained virologic response (SVR), associated with a reduction of liver inflammation and fibrosis, and the incidence of hepatic decompensation and HCC[6].In addition, SVR leads to a decrease in mortality from both hepatic and non-hepatic causes[7,8].
HCV treatment has undergone major changes to date[9].Previous interferon-based therapies with lower SVR rates and several adverse events (AEs)[9] were replaced by oral direct-acting antiviral (DAA) regimens, with SVR rates greater than 90% and a lower incidence of AEs[10-13].Significant advances in the understanding and management of this disease started over twenty years ago[14].These early efforts were recognized by the 2020 Nobel Prize in Physiology or Medicine[15].
In Brazil, acquiring and dispensing of all oral DAA regimens for patients with chronic hepatitis C (CHC) was provided through the Sistema Unico de Saude, the national public healthcare system[16].There are few studies in large centers showing experience with DAAs in patients with chronic HCV infection.So, the aim of this study was to evaluate the efficacy and safety of DAAs for treatment of HCV-infected patients from two tertiary university centers in the southeastern region of the country.
MATERIALS AND METHODS
Study design and patient selection
This is a multicenter retrospective cohort study, carried out at the liver outpatient clinics of the Division of Gastroenterology at the University of Campinas (UNICAMP), and the Department of Gastroenterology at the University of São Paulo School of Medicine (FMUSP) for patients with CHC, who underwent treatment with interferonfree regimens from November 2015 to November 2019.
Inclusion criteria were: (1) age ≥ 18 years and the presence of CHC, defined by HCV RNA positivity through a polymerase chain reaction (PCR) for at least 6 mo, regardless of the HCV genotype; and (2) those treated with oral DAAs.Exclusion criteria were: diagnosis of any other liver disease, human immunodeficiency virus, or hepatitis B virus coinfection, active HCC, liver transplant recipients, previous treatment for HCV with interferon-free regimens, and lack of information on the current HCV treatment.
HCV treatment regimen
The therapeutic regimen was defined by the current Brazilian guidelines for HCV management at the time of treatment[16-18].HCV therapy was composed of the DAAs: sofosbuvir (SOF), daclatasvir (DCV), simeprevir (SMV), ledipasvir, and the combined regimen ombitasvir plus veruprevir/ritonavir plus dasabuvir.Ribavirin (RBV) was also used.Relevant drug-drug interactions were checked prior to use of DAAs.
Variables evaluated
Demographic and anthropometric variables [age, gender, body mass index (BMI)], presence of comorbidities (arterial hypertension, diabetes mellitus, dyslipidemia, hypothyroidism, psychiatric disorders, previous alcohol use), and laboratory variables were evaluated through computerized medical records.Serum biochemical assessment was conducted before treatment, and 12 to 24 wk after the end of treatment, as per routine clinical practice.Serum HCV-RNA levels were assessed with real-time PCR and the Amplicor HCV Monitor 2.0 test (Abbott Molecular, Des Plaines, IL, United States, detection limit: 12 IU/mL).Viral genotyping was performed with Versant®HCV Genotype 2.0 LiPA test (Imunogenetics, Ghent, Belgium).
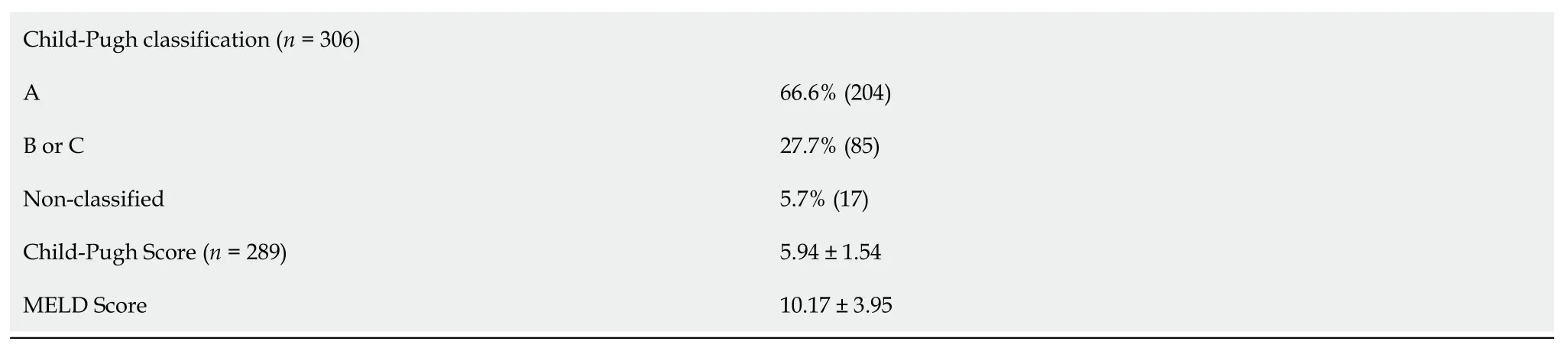
The staging of hepatic fibrosis was assessed prior to treatment with histology, according to the Metavir classification, or use of non-invasive methods (transient elastography, APRI, and FIB-4).In patients with cirrhosis, Child-Pugh and model for end-stage liver disease (MELD) scores were also assessed.
HCV treatment efficacy analysis
SVR was defined as undetectable HCV-RNA at 12 or 24 wk following treatment.An intention-to-treat (ITT) analysis was performed, considering patients who abandoned treatment, were lost to follow-up, or did not have complete information about their medical records, seen as virologic failures.A modified intention-to-treat (m-ITT) analysis was carried out, excluding efficacy for patients lost to follow-up, or who discontinued therapy, or any deaths unrelated to treatment or its adverse events.
Safety assessment
The analysis of AE was classified according to the Common Terminology Criteria for Adverse Events[19].Management of anemia was considered with a drop in hemoglobin (Hb) greater than 3 points or associated symptoms if Hb > 10 g/dL.Anemia was classified into grade 1 (Hb 10-8 g/dL), grade 2 (Hb < 8 g/dL or need for a blood transfusion), grade 3 (risk of death), and grade 4 (death).Serious adverse events (SAEs) were considered: (1) hepatic decompensation; (2) need for hospitalization; (3) need to discontinue treatment; and (4) events resulting in death[20].
Ethical aspects
This study was approved by the Ethics Committee of UNICAMP and Clinics Hospital of FMUSP (Approval No.2042967 and 2670862, respectively).The protocol was conducted in accord with the ethical guidelines of the 2013 World Medical Association Declaration of Helsinki[21].Informed consent was waived for participants.
Statistical analysis
To describe the sample according to the variables under study, frequency tables of categorical variables with absolute frequency (n) and percentage (%) values, as well as descriptive statistics of numerical variables, with mean and standard deviation were used.To assess the relationship between categorical variables, the Chi-square test and, when necessary, Fisher's exact test were used.For numerical variables, the Mann-Whitney test was utilized.To assess factors related to treatment failure and AEs, univariate and multivariate logistic regression was performed whenever methodologically feasible.The selection of variables in the multivariate logistic regression analysis was done in a stepwise manner.Odds ratio (OR) and 95%CI were calculated.APvalue of < 0.05 was considered significant.The Statistical Analysis System (SAS) for Windows software package, version 9.4 (SAS Institute Inc, 2002-2008, Cary, NC, United) was used for statistical analyses by biomedical statisticians from the Statistics Service at the School of Medical Sciences of the University of Campinas.
RESULTS
Baseline characteristics
A total of 532 patients treated with DAAs were included in the study.There was a slight predominance of males, with the mean age of 56.88 years.The mean BMI was 27.01, with most patients having comorbidities, mainly arterial hypertension and diabetes mellitus.There was a predominance of patients with HCV genotype 1 (78.5%), followed by genotype 3 (20.1%).Over 50% of patients were treatmentexperienced.Three hundred six (57.5%) patients had cirrhosis, and a third had decompensated liver disease.The main baseline for demographic, clinical, and laboratory characteristics of the study population are shown in Table 1.
HCV therapeutic regimens and efficacy analysis
The combination of SOF plus DCV, associated with RBV, was the most frequently used treatment (66.9%), followed by SOF plus SMV (21.2%).Table 2 shows HCV treatment regimens in the study population.The overall ITT SVR rate was 92.6% (493/532), and the m-ITT SVR rate was 96.8% (493/509).Twenty-three patients were lost to follow-up, with no conclusive SVR data at the end of treatment (Table 3).In the ITT analysis, pretreatment variables related to treatment failure were a higher MELD score (P= 0.001), higher serum levels of aspartate aminotransferase (AST) and international normalized ratio (INR) (P= 0.043 andP= 0.023, respectively), lower serum albumin levels (P= 0.032), and a higher frequency of liver-related complications [hepatic encephalopathy (P= 0.001), esophageal varices (P= 0.018), and previous portal hypertensive bleeding (P= 0.039)], as shown in Table 4.
When assessing m-ITT SVR, the presence of cirrhosis (P= 0.049), higher serum values of AST (P= 0.030), higher MELD scores (P= 0.004), presence of hepatic encephalopathy (P= 0.006), and male gender (P= 0.049) negatively impacted SVR achievement.Genotype 3 HCV patients had numerically lower SVR rates than nongenotype 3 subjects (93%vs97.7%-100%), almost reaching statistical significance (P= 0.055).In the univariate logistic regression analysis, baseline variables associated with treatment failure by ITT evaluation were: hepatic encephalopathy (OR: 4.320; 95%CI: 1.920-9.721,P= 0.0004), presence of esophageal varices (OR: 2.381; 95%CI: 1.137-4.988,P= 0.0215), previous portal hypertensive bleeding (OR: 2.756; 95%CI: 1.173-6.471,P= 0.02), higher MELD scores (OR: 1.143, 95%CI: 1.060-1.233,P= 0.0005), lower serum albumin levels (OR: 0.528, 95%CI: 0.322-0.867,P= 0.0115), higher serum creatinine (OR: 1.117, 95%CI: 1.056-1.312,P= 0.0033), and INR levels (OR: 5.542, 95%CI: 2.023-15.182,P= 0.0009), shown in Table 5.It was not possible to perform multivariate logistic regression analysis, due to the low occurrence of non-responders and missing data.

Table 1 Characteristics of patients with hepatitis C virus (n = 532)
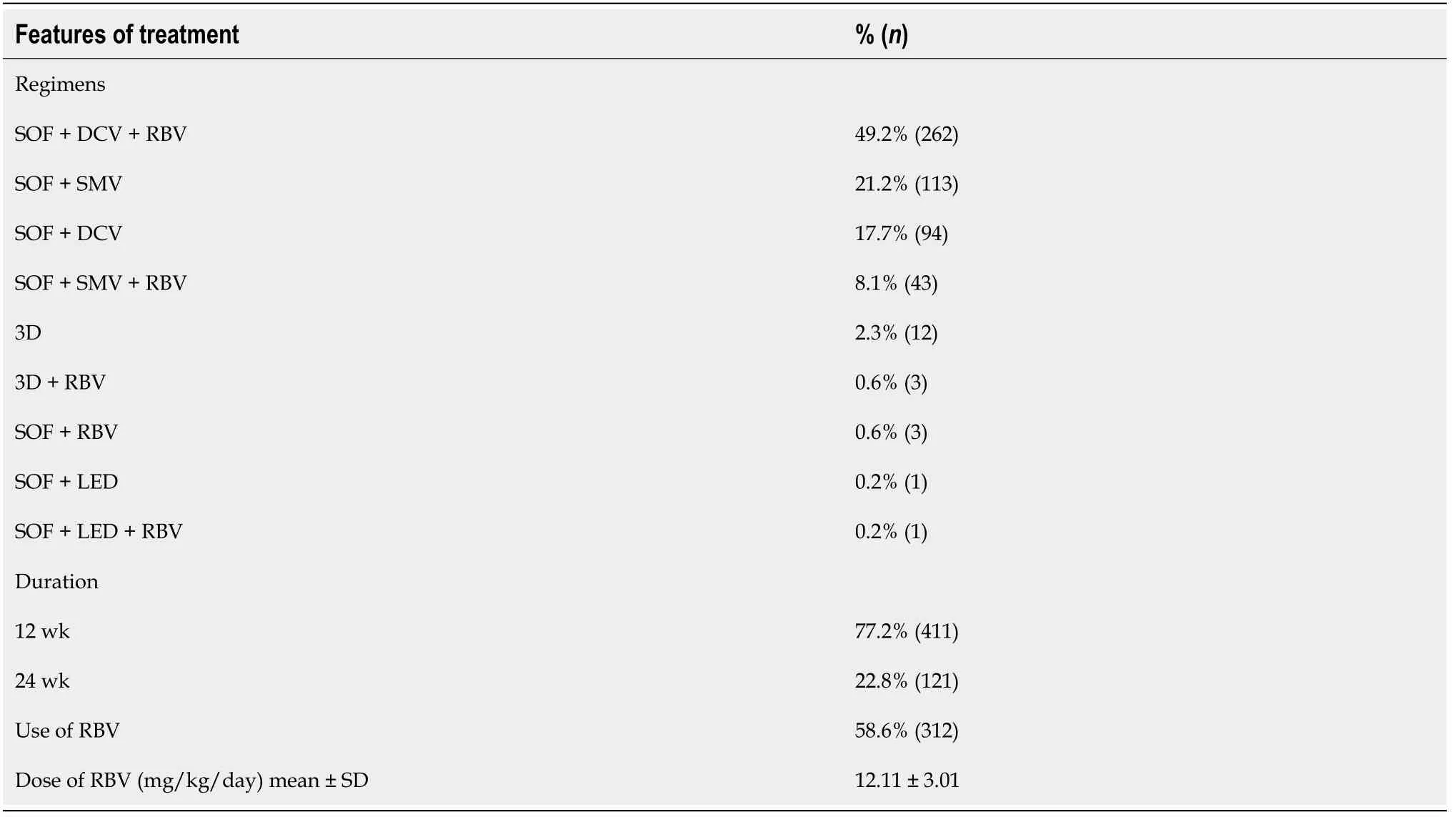
Table 2 Hepatitis C virus therapeutic regimens (n = 532)
Safety analysis
AEs were reported in 41.1% (211/514) of patients.The most frequent AE was fatigue,present in 140 patients (27.7%), followed by anemia in 87 patients (17.2%) and headache in 47 patients (9.3%).Anemia or a fall in hemoglobin ≥ 2 g/dL points occurred in 87 patients (17.2%).Of these, only one patient did not use RBV, and already had a hemoglobin of 11.2 g/dL before treatment.Of 312 patients who used RBV, 86 (27.5%) had a drop in hemoglobin during treatment, 61 required a dose reduction, and 15 had RBV suspended.Five patients required treatment with erythropoietin, and 4 required blood transfusion.SAE occurred in 20 patients (3.7%), as 14 had decompensation of their liver disease, with 7 hospitalized.Three patients died during the study evaluation.
ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; BMI: Body mass index; HCV: Hepatitis C virus; INR: International normalized ratio; MELD: Model for end-stage liver disease; Peg-IFN: Pegylated-interferon; PI: Protease inhibitor (boceprevir or telaprevir); RBV: Ribavirin.
In the univariate logistic regression analysis, baseline factors associated with the occurrence of AE were female gender (OR: 1.718, 95%CI: 1.205-2.450,P= 0.0028), higher BMI (OR: 1.060, 95%CI: 1.019-1.102,P= 0.0040), presence of cirrhosis (OR: 2.127, 95%CI: 1.476-3.065,P< 0.0001), liver-related complications [ascites (OR: 2.187, 95%CI: 1.352-3.536,P= 0.0014), hepatic encephalopathy (OR: 3.524, 95%CI: 1.762-7.044,P= 0.0004), and the presence of esophageal varices (OR: 2.795, 95%CI: 1.874-4.169,P≤ 0.0001)], higher MELD (OR: 1.071, 95%CI: 1.019-1.126,P= 0.0073) and Child-Pugh scores (OR: 1.196, 95%CI: 1.014-1.410,P= 0.0332), lower serum albumin (OR: 0.432, 95%CI: 0.314-0.595,P< 0.0001), higher bilirubin (OR: 1.283, 95%CI: 1.052-1.564,P= 0.0138) and INR values (OR: 3.835, 95%CI: 1.539-9.560,P= 0.0039), higher RBV daily dose (OR: 1.249, 95%CI: 1.132-1.379,P< 0.0001), and longer treatment duration (OR:1.071, 95%CI: 1.034-1.109,P= 0.0001), as shown in Table 6.Factors independently associated with AE occurrence were female gender (OR: 2.191, 95%CI: 1.145-1.192,P= 0.0178), higher BMI (OR: 1.107, 95%CI: 1.038-1.180,P= 0.0020), presence of esophageal varices (OR: 3.463, 95%CI: 1.688-7.105,P= 0.0007), higher INR values (OR: 3.748, 95%CI: 1.060-13.251,P= 0.0403), and longer treatment duration (OR: 1.062, 95%CI: 1.003-1.125,P= 0.0406).

Table 3 Sustained virologic response rates according to therapeutic regimens, hepatitis C virus genotypes and cirrhosis

Table 4 Variables associated with sustained virologic response by intention-to-treat analysis (n = 532)

Table 5 Factors associated with failure to achieve sustained virologic response by intention-to-treat analysis

Table 6 Factors associated with the occurrence of adverse events during treatment
DISCUSSION
This study evaluated the efficacy and safety of all oral DAAs for CHC treatment in a cohort of 532 patients, followed at two Brazilian tertiary university centers.Most patients were HCV genotype 1, with a slight predominance of males, similar to that found in other studies carried out in our country[22-24].
Our results show the high effectiveness of DAAs in this real-life cohort, reaching global SVR rates of 92.6% in the ITT analysis, and 96.8% in the m-ITT evaluation.However, lower rates of m-ITT SVR were observed in patients with cirrhosis.Advanced liver disease negatively impacts response to treatment, especially in those with decompensated disease, who are considered a more difficult group to treat[25-27].Although SOF plus DCV was the most common regimen in the present study, it is interesting to cite that other effective treatments have been made available, such as SOF plus velpatasvir, elbasvir plus grazoprevir and glecaprevir plus pibrentasvir, for both naïve and DAA experienced patients[6].SOF plus velpatasvir plus RBV was effective in patients with decompensated cirrhosis[6].Treatments with drug combinations might be important to ultimately control the emergence of resistance-associated substitutions, and as rescue therapy for non-responders[6,28].
In the m-ITT SVR analysis, HCV genotype 3 individuals had an almost significant lower SVR rate in comparison to non-genotype 3 patients.Published data show that HCV genotype 3, previously considered an “easy to treat” genotype in the interferon era, with cure rates of up to 70%, turned out to be more challenging in the DAA era,with lower SVR rates compared to other HCV genotypes[26,27].HCV genotype 3 interferes with the metabolism of lipids and glucose, and is associated with an increased risk of progressing to cirrhosis and HCC, which may negatively impact SVR rates[29,30].SOF plus pegylated-interferon and RBV for 12 wk is also a treatment option in HCV genotype 3 patients[31-33].

aP value < 0.05.Chi-square test, Fisher's exact test, and Mann-Whitney test.ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; BMI: Body mass index; HCV: Hepatitis C virus; INR: International normalized ratio; ITT: Intention-to-treat; Peg-IFN: Pegylated-interferon; PI: Protease inhibitor (boceprevir or telaprevir); RBV: Ribavirin; SVR: Sustained virologic response.
In the m-ITT SVR analysis, we observed that HCV therapeutic failure was linked to male gender, higher MELD scores and AST values, presence of cirrhosis and hepatic encephalopathy.In the ITT analysis, lower serum albumin, higher creatinine and INR levels, history of hepatic encephalopathy, esophageal varices, and upper gastrointestinal bleeding were related to lower SVR rates.Several of these factors reflect advanced liver disease.Less severe disease, with lower Child-Pugh scores and serum bilirubin values and higher albumin levels, were associated with a greater chance of achieving SVR[34,35].A large Spanish cohort of over 3000 patients showed that high values of transient elastography, cirrhosis, serum levels of bilirubin, and albumin values < 3.5 g/dL were significantly associated with therapeutic failure[36].In Brazil, a 2018 study of 527 patients showed that in those with cirrhosis, the factors associated with SVR were lower MELD scores, higher albumin values, and glomerular filtration rates[37].
AEs were present in 41.1% of the study population, the main ones being fatigue, anemia, and headache; while only 3.7% subjects had SAEs.Our results reinforce the safety of DAAs for the treatment of hepatitis C in the Brazilian population.However, our AE rates were lower than the rates reported in other studies in our country, in which AEs are described in up to 90% of treated patients[38], and SAEs in up to 8.5% of cases[39].The retrospective study design and possible underreport of AEs on medical charts, could justify the lower AE rate in the present cohort.In addition, more than half of our study population included patients without cirrhosis or with compensated liver disease, in which treatment is safer[39,40].In our study, the main factors associated with the occurrence of AEs in the univariate logistic regression analysis were female gender, higher BMI, higher Child-Pugh and MELD scores, presence of cirrhosis and its complications, lower values of albumin, higher values of bilirubin and INR, use of ribavirin, and longer treatment.Patients with decompensated cirrhosis, in addition to having lower SVR rates, were also more likely to experience treatment-related AEs, which leads to earlier discontinuation of therapy and, in part, justifies its lower efficacy[41].In Brazil, a study with 214 patients showed that the factors of HCV treatment discontinuation were advanced age, multiple comorbidities, higher MELD score, higher fibrosis index, and lower hemoglobin[39].
This study has some limitations.Despite the large number of patients, it is a retrospective study, relying on data from medical records.In addition, it was carried out in two public reference centers for hepatology and liver transplantation, which may incur a selection bias for more severe patients.Yet, this study evaluated a large Brazilian cohort of patients with decompensated cirrhosis.
CONCLUSION
In conclusion, in this cohort of patients with CHC followed at two public healthcare facilities in the southeastern region of Brazil, treatment with DAAs proved to be effective, with global SVR rates above 92%, and safe, with a low occurrence of SAEs.
ARTICLE HIGHLIGHTS
Research background
Hepatitis C represents a global health problem and a major cause of cirrhosis, and hepatocellular carcinoma.Hepatitis C virus (HCV) treatment has undergone major changes in recent years with the advent of direct-acting antivirals (DAA) regimens.
Research motivation
In Brazil, acquiring and dispensing of all oral DAA regimens for patients with chronic hepatitis C (CHC) is provided through the national public healthcare system.However, there are few studies in large centers showing experience with DAAs in patients with chronic HCV infection.
Research objectives
We aimed to evaluate the efficacy and safety of DAAs for HCV treatment in subjects from two tertiary public university centers in the southeastern region of Brazil.
Research methods
We evaluated 532 adult patients with CHC who underwent treatment with interferonfree regimens from November 2015 to November 2019.Demographic, anthropometric, clinical, and laboratory variables were evaluated.Sustained virologic response (SVR) rates were assessed at 12 to 24 wk after therapy by intention-to-treat (ITT), and modified ITT (m-ITT) analysis.Adverse events (AEs) and serious adverse events (SAEs) were registered.
Research results
Sofosbuvir (SOF) plus daclatasvir ± ribavirin was the most frequently used treatment (66.9%), followed by SOF plus simeprevir (21.2%).The overall ITT SVR was 92.6% (493/532), while the m-ITT SVR was 96.8% (493/509).Variables associated with treatment failureviaITT evaluation were hepatic encephalopathy, presence of esophageal varices, previous portal hypertensive bleeding, higher model for end-stage liver disease scores, lower serum albumin levels, and higher serum creatinine and international normalized ratio (INR) levels.AEs were reported in 41.1% (211/514) of patients, and SAEs in 3.7%.The female gender, higher body mass index, esophageal varices, higher INR values, and longer treatment duration were independently associated with AE occurrence.
Research conclusions
Treatment with oral DAAs attains a high SVR rate, with fewer SAEs in a real-life cohort of subjects with CHC, from two tertiary university centers in Brazil.
Research perspectives
Long-term follow-up studies of patients after successful HCV eradication are important.
杂志排行
World Journal of Hepatology的其它文章
- Hepatitis C virus: A critical approach to who really needs treatment
- Current aspects of renal dysfunction after liver transplantation
- Hepatitis C: Problems to extinction and residual hepatic and extrahepatic lesions after sustained virological response
- Metabolic and nutritional triggers associated with increased risk of liver complications in SARS-CoV-2
- Recent updates on progressive familial intrahepatic cholestasis types 1, 2 and 3: Outcome and therapeutic strategies
- Targets of immunotherapy for hepatocellular carcinoma: An update
