Hepatitis C: Problems to extinction and residual hepatic and extrahepatic lesions after sustained virological response
2022-02-12SaraCuestaSanchoMercedesrquezCoelloFranciscoIllaneslvarezDenisserquezRuizAnaArizcorretatimaGalnchezNataliaMontielManuelRodriguezIglesiasJosAntonioGirGonzlez
Sara Cuesta-Sancho, Mercedes Márquez-Coello, Francisco Illanes-álvarez, Denisse Márquez-Ruiz, Ana Arizcorreta, Fátima Galán-Sánchez, Natalia Montiel, Manuel Rodriguez-Iglesias, José-Antonio Girón-González
Sara Cuesta-Sancho, Mercedes Márquez-Coello, Francisco Illanes-Álvarez, Denisse Márquez-Ruiz, Ana Arizcorreta, José-Antonio Girón-González, Medicina Interna, Hospital Universitario Puerta del Mar, Facultad de Medicina, Universidad de Cádiz, Instituto para la Investigación e Innovaci ón en Ciencias Biomédicas de Cádiz (INiBICA), Cádiz 11009, Spain
Fátima Galán-Sánchez, Natalia Montiel, Manuel Rodriguez-Iglesias, Microbiología, Hospital Universitario Puerta del Mar, Facultad de Medicina, Universidad de Cádiz, Instituto para la Investigación e Innovación en Ciencias Biomédicas de Cádiz (INiBICA), Cádiz 11009, Spain
Abstract Loss of follow-up or reinfections hinder the expectations of hepatitis C eradication despite the existence of highly effective treatments.Moreover, the elimination of the infection does not imply the reversion of those chronic alterations derived from the previous infection by hepatitis C virus (HCV).This review analyzes the risk factors associated with loss to follow-up in diagnosis or treatment, and the possibility of reinfection.Likewise, it assesses the residual alterations induced by chronic HCV infection considering the liver alterations (inflammation, fibrosis, risk of decompensation, hepatocellular carcinoma, liver transplantation) and, on the other hand, the comorbidities and extrahepatic manifestations (cryoglobulinemia, non-Hodgkin lymphoma, peripheral insulin resistance, and lipid, bone and cognitive alterations).Peculiarities present in subjects coinfected with human immunodeficiency virus are analyzed in each section.
Key Words: Hepatitis C virus; Sustained virological response; Direct antiviral agents; Human immunodeficiency virus; Cirrhosis decompensation; Hepatocarcinoma; Extrahepatic complications
INTRODUCTION
In 2015, 71 million people were estimated to be infected by hepatitis C virus (HCV) worldwide[1].Based on the release of curative treatment for chronic hepatitis C infection[2], the World Health Assembly set the target of a 90% reduction in new infections and a 65% reduction in viral hepatitis related mortality by 2030 as compared to 2015[3].
These expectations should not let us forget some problems that underlie the current situation and that could be classified into the following (Figure 1): (1) Patients whose HCV infection has not been eradicated or those who have been reinfected; and (2) Organic injuries associated with chronic hepatitis C, whether hepatic or extrahepatic, whose normalization is not reached by the elimination of the virus.
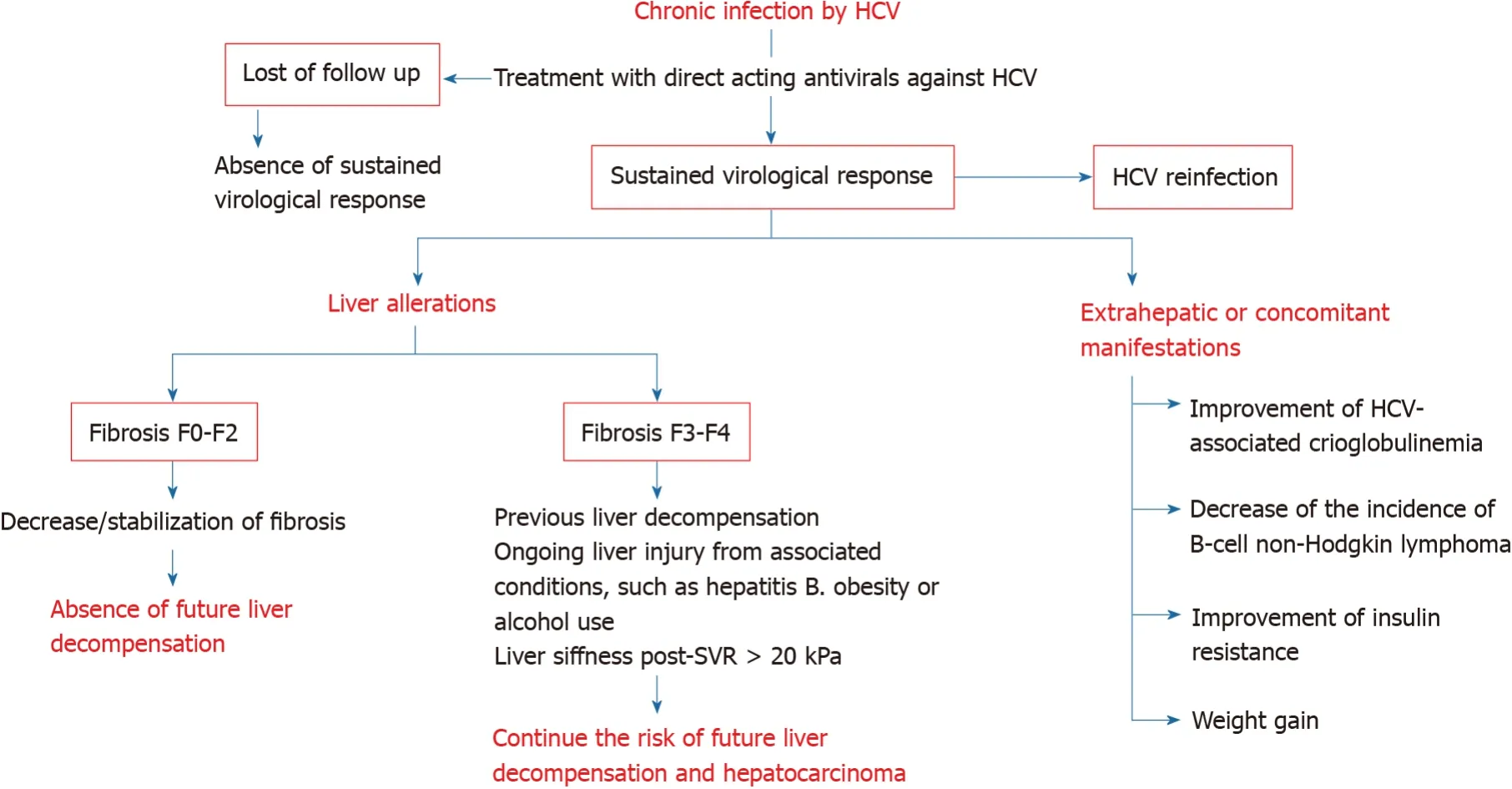
Figure 1 Modification of hepatic and extrahepatic manifestations of chronic hepatitis C after therapy with direct acting agents.
FAILURE TO ERADICATE HCV INFECTION
Both American[4] and European[5] Associations for the Study of Liver Diseases (AASLD and EASL, respectively) recommend combinations of direct acting agents (DAAs) against HCV, such as an NS5A inhibitor with either an NS3/4 protease inhibitor (grazoprevir/elbasvir or glecaprevir/pibrentasvir), or a nucleotide analogue plus an NS5A inhibitor (sofosbuvir/velpatasvir), for eight to twelve weeks.The preferred regimens to simplify HCV therapy are pangenotypics combinations (sofosbuvir/velpatasvir or glecaprevir/pibrentasvir)[6].
Treatment should be offered to all HCV RNA-positive patients.The efficacy of these combinations has been higher than 90%[6].
The proportion of patients who do not achieve a sustained virological response (SVR) is lower than 10%, considering those who have virologic relapse and those who are lost during the follow-up[7,8].The combined therapy with sofosbuvir, velpatasvir and voxilaprevir is recommended for retreating patients with previously failing DAAs regimens[9,10].
There are few contraindications to therapy with DAAs.The use of certain cytochrome P450/P-glycoprotein inducing agents are contraindicated with all regimens, because of the risk of reducing DAAs concentrations.In patients with Child-Pugh B or C decompensated cirrhosis, NS3/4a protease inhibitors are contraindicated due to the increased concentrations of protease inhibitor in these patients and its associated toxicity risk.In patients with a glomerular filtration rate lower than 30 mL/min/1.73 m2, increased serum levels of sofosbuvir are detected[8].Interactions between DAAs and other drugs need to be addressed in patients, mainly in those with human immunodeficiency virus (HIV)/HCV-coinfection and those with central nervous system-acting drugs[11].
A major barrier to HCV elimination is the loss to follow-up, defined as nonattendance to any appointment in the care cascade at any time[12].A review about the loss to follow-up in HCV care has been recently published[13].Factors associated with the loss to follow-up are younger age (< 45 years old)[14], treatment in hospital[15], a history of homelessness[15,16], mental illness[16,17] and injecting drug use, either past[18] or ongoing[17].In contrast, factors associated with retention in care are older age (≥ 60 years old) and HIV coinfection[19].
Several strategies have been proposed to overcome the loss to follow-up in HCV care[20]: (1) Enhancing HCV identification and linkage to care for vulnerable populations (injecting drug users) through intensified outreach screening.A largescale intensified screening initiative across Europa (Hep-Check and Hep-Link) has been started up[21,22]; (2) HCV micro-elimination strategies, focused on collectives with a high prevalence and/or increased risk of loss of follow-up and/or in patients with worst short-term prognosis[23,24]; (3) Reflex testing, a strategy of hepatitis C diagnosis in a single step, based on the detection of HCV RNA or HCV core antigen when the anti-HCV antibody test proves to be positive[25] and referral to an HCV specialist for further evaluation[26]; (4) Use of pan-genotypic HCV drug regimens; and (5) Inclusion of HCV-infected patients who use drugs on opioid agonist therapy programs can reach elevated HCV elimination rates with current DAAs[27].
Reinfection following SVR has been documented in several studies in drug users[28], prisoners[29], and men who have sex with men (MSM)[30].After SVR, the incidence of reinfection is 2 to 6/100 person-years in subjects who inject drugs and 10 to 15/100 person-years in HIV-infected MSM[30-33].Elevated rates of reinfection may compromise the benefits of treatment.
EFFECTS OF SVR
The analysis of the changes in the hepatitis C evolution after SVR will be considered in several sections: (1) Overall survival; (2) Changes in liver disease (liver fibrosis, liver function, decompensation of chronic liver disease, hepatocarcinoma); and (3) Modifications of extrahepatic alterations.In each section and whenever evidence is available, the changes in HIV/HCV-coinfected patients will be discussed.
Global survival
Chronic HCV infection is associated with a substantially impaired overall survival, both by liver-related and extrahepatic causes[34].
Individuals with compensated cirrhosis who reach SVR with interferon-based treatments have an improved long-term outcome[35-37].Real-world cohorts have confirmed this significant reduction in the liver-related death risk after DAAs: In the HEPATHER study, the annual incidence of liver-related mortality in subjects with SVR was 0.36%vs0.96% in non-SVRs; and in individuals with cirrhosis, the respective incidence was 0.64%vs1.57%[38].Even though in other studies[39-41], the risk reduction was more pronounced, the risk still existed.Therefore, a proportion of subjects (small but worrisome) dies because of liver-related disease after viral clearance.
Symptoms and mortality from severe extrahepatic manifestations, such as cryoglobulinemic vasculitis, renal-related effects[42,43] and some lymphoproliferative disorders[44,45] decrease with HCV eradication as well.Moreover, subjects with SVR have a better physical and emotional health and an improved life quality[46].
In HIV/HCV coinfected patients with compensated cirrhosis, benefits from SVR due to interferon-based regimens in the incidence of liver-related decompensation, some extrahepatic manifestations and the overall mortality, have been demonstrated[47,48].In addition, decreases of HIV reservoirs[49] and HIV progression[48] have been observed after HCV eradication.
Hepatic modifications after SVR
Inflammation, fibrosis, and liver function previous to treatment against HCV affect the prognosis of chronic liver disease[50].
Liver inflammation and fibrosis:Several factors contribute to liver inflammation, acting on macrophage receptors.They include viral particles; pathogen-associated molecular patterns, such as lipopolysaccharides, which can translocate from the intestine into the circulation because of increased intestinal permeability; and damageassociated molecular patterns released by hepatocytes[51].These factors induce and amplify hepatic inflammation by activating macrophages[52].Macrophage activation promotes hepatic stellate cell activation and extracellular matrix accumulation[53].After antiviral therapy of chronic hepatitis C, macrophage activation is diminished, as indicated by biomarkers[54] or aminotransferase levels[55], in parallel with the amelioration of hepatic inflammation observed in liver biopsies[56].Only patients who achieve a SVR solve inflammation in liver biopsies[57].
Biopsy-proven fibrosis regression is developed when SVR is achieved[58].In patients treated with interferon-based regimens, a 39%-73% of subjects who reached SVR had decreased liver fibrosis and necrosis, as assessed by liver biopsy[55].Likewise, several studies have reported a significant diminution in liver stiffness after treatment, either by interferon- or DAAs-based schemes[59-64].
HCV-induced fibrosis progresses more rapidly in HIV-coinfected patients than in monoinfected individuals[65].The impact of SVR on liver fibrosis (biopsy-proven) or liver stiffness within HIV/HCV patients treated with interferon- or DAAs-based regimens has been also proved[66].
Liver function:In routine clinical practice, Child-Pugh and Model for End-stage Liver Disease (MELD) scores are often used for the evaluation of liver function.Although improvement of liver function is not uniformly demonstrated in studies with HCVinduced liver cirrhosis, a Child-Pugh decrease ≥ 1 and/or MELD decrease ≥ 2 between baseline and SVR has been demonstrated in a 56%-57% of DAAs-treated patients.Factors independently related with liver function improvement are male gender, bilirubin < 1.2 mg/dL and international normalized rate < 1.3 at baseline[67].
Short-term outcomes after DAAs in individuals with decompensated cirrhosis showed a decrease of the MELD score in the majority, while it did not change in 17%, and worsened in 25% of them[68-73].Subjects with low MELD scores can even be removed from liver transplantation lists[74-76], although the clinical improvement may not necessarily persist, and they may be still in risk of relisting on the transplant list or even death[76-78].
A more detailed analysis of liver function changes can be obtained by using other methods[79].Thus, it has been demonstrated that amelioration of inflammation improves the ureagenesis[80].
Decompensation of liver cirrhosis:Decompensated cirrhosis is characterized by the development of new ascites, spontaneous bacterial peritonitis, hepatic encephalopathy, bleeding gastro-esophageal varices, hepato-renal syndrome or hepatopulmonary syndrome[81].
With DAAs, subjects with compensated cirrhosis achieve SVR rates over 95%[82,83].Among subject with ongoing or previous decompensation, SVR rates of approximately 80% are reached with DAAs treatment[69,73,84].
Hepatic venous pressure gradient (HVPG) improves shortly after DAAs therapy in patients with HCV-related cirrhosis[85-87].However, an HVPG more elevated at baseline is linked with smaller reductions in portal pressure and practically all of those with an HVPG ≥ 16 mmHg remain with clinically significant portal hypertension after SVR[85,88,89].These findings contribute to explain the persistence of risk of decompensations due to portal hypertension in patients with decompensated liver cirrhosis after SVR.
The severity of liver disease prior to the therapy is a predictor of liver decompensation after SVR.The Scottish real-world study[39] informed that the risk of decompensation decreased by 86% compared to non-SVR in patients with compensated cirrhosis.In those patients with previously decompensated cirrhosis, viral clearance was linked to a lower incidence rate of decompensations[73].In the Krassenburg’s series, the cumulative 2-year event-free survival was 89.0% for those with Child-Pugh class A cirrhosis compared to 45.2% for those with Child-Pugh class B/C cirrhosis.Furthermore, while SVR was independently associated with an improved event-free survival in patients with Child-Pugh class A cirrhosis, it did not in patients with Child-Pugh class B/C cirrhosis[90], although controversial results have been published[41].
Attending to these contradictory findings in patients with decompensated cirrhosis, in a retrospective study of patients with Child-Pugh class B or C, El-Sherifet al[77] analyzed those factors related with the reduction of Child-Pugh score to class A (implicating the absence of new decompensations) after DAAs.During a follow-up of 255 d, 31.6% of subjects with baseline Child-Pugh class B cirrhosis and 12.3% of subjects with Child-Pugh class C cirrhosis met the primary study end point.The presence of complications such as ascites or encephalopathy, serum concentration of albumin < 3.5 g/dL or alanine aminotransferase < 60 U/L, and body mass index (BMI) > 25 kg/m2were related to a higher risk of not achieving a decrease in Child-Pugh to class A, regardless of SVR[77].
Bleeding from esophageal varices is uncommon after SVR[91].However, subjects with compensated cirrhosis who achieve SVR should follow on receiving endoscopic surveillance for esophageal varices, according to the AASLD guidance[92]: (1) In those without known varices, surveillance endoscopy is indicated every 2 years if there are associated conditions, such as obesity or alcohol use; and every 3 years if liver injury is suppressed, such as after alcohol abstinence; and (2) In those with known varices, surveillance endoscopy is indicated every 12 mo if there is proof of present liver injury from associated conditions and every 24 mo if liver injury is quiescent[92].However, in our opinion, these recommendations could be modulated by the knowledge of liver stiffness, as will be analyzed later on.
In individuals with compensated cirrhosis, HIV coinfection was not related with an increased probability of liver complications after viral eradication than those HCVmonoinfected[93,94].In the series of Corma-Gómezet al[95], the likelihood of staying free of hepatic complications or transplant at 12 and 24 mo was 99% and 96% in HCVmonoinfected patients and 99 and 98% in HIV/HCV coinfected patients with predominantly (> 95% of individuals) Child-Pugh class A cirrhosis (P= 0.648).In the multivariate analysis of the overall population, liver decompensation before SVR, drug use as the risk factor for HCV infection -reduced healthcare adherence and ongoing use of toxics may underlie this finding- and liver stiffness at SVR were independently linked to the presence of a hepatic complication or requiring a liver transplant[96].
The importance of the liver stiffness at SVR has been remarked.Post-treatment liver stiffness > 20 kPa is significantly associated with developing cirrhosis decompensation, either ascites, variceal bleeding or hepatic encephalopathy (Hazard ratio 8.04)[97].This cutoff point has been supported by other authors[98].
Furthermore, liver stiffness-based strategies recognize subjects with reduced risk of developing esophageal variceal bleeding episodes, in whom esophagogastroduodenoscopy screening can be unnecessary[95,99-102].Corma-Gómezet al[95] demonstrated that subjects with a liver stiffness < 30 kPa and a platelet count > 110000/mm3after SVR are not at risk of variceal bleeding[102].
Hepatocellular carcinoma:Hepatocellular carcinoma (HCC) incidence has been growing over the last two decades and is expected to rise until 2030 in several countries[103].HCV-infected patients have a lifetime risk of approximately 5%.HCC is expected to occur 30 years after infection.In patients with hepatitis C, HCC is almost invariably present in the setting of cirrhosis[104].
Antiviral treatment of chronic HCV infections significantly reduces the risk of HCC[104-109].Meta-analyses have shown that DAAs therapy is associated with a decrease ofde novoHCC incidence close to 80%, similar to that achieved with interferon-based therapies[108,109].However, it is the most frequent liver-related event after SVR[98,106].The incidence rate of HCC after SVR is 1.1-1.9/100 patient-years[94,98].
A Spanish series including 1035 HCV-infected patients, of which 667 (64%) were coinfected with HIV, has demonstrated that HIV-coinfection appears to be associated with an inferior risk of HCC occurrence among patients with HCV and advanced fibrosis who reach SVR due to DAA[110], although these data are controversial[94].HIV/HCV coinfected patients have an earlier onset and aggressive HCC, with associated higher mortality risk[93].
Post-SVR surveillance by liver imaging and alpha-fetoprotein (AFP) tests every six months after SVR is recommended in cirrhotic population by international guidelines[111,112].This recommendation has also been extended to patients with advanced fibrosis (F3) by EASL guidelines[113].Unless they are affected by liver comorbidities, patients with lower stages of fibrosis may be discharged from specialized care.Age, male sex, lower baseline albumin or higher bilirrubin levels, a FIB-4 score > 3.25, hepatitis B coinfection or a liver stiffness post-SVR ≥ 20 kPa have been associated with a higher risk of developing HCC[94,98,114].A scheme of the factors that could influence the need of surveillance of HCC is shown in Table 1.

Table 1 Factors that influence the surveillance of hepatocarcinoma
A low sensitivity of usual screening methods of HCC occurrence has been observed.Ultrasonography has been reported to have 60% sensitivity and 97% specificity as a screening method of HCC in cirrhotic patients; it has been proved to be cost-effective[115].The performance of ultrasound surveillance of HCC is even worse in HIVcoinfected patients with cirrhosis[116].AFP by itself is not adequate for screening purposes: A low sensitivity (40%-75%), as well as a high false positive rate in active hepatitis, precludes its use as screening method[117].
Liver transplantation:Liver transplantation is an appropriate treatment option for individuals with acute liver failure, end-stage liver disease, and primary hepatic malignancy.Patients with cirrhosis are typically candidates for liver transplantation once MELD score is ≥ 15[118].
A decrease of MELD score is expected in a proportion of patients with cirrhosis after SVR[90].However, although a clinically significant decrease in MELD score is achieved by a 25% of DAAs-treated patients across a short follow-up, after a longer period (median follow-up of 4 years), the average MELD variations are not significant[78].Yet, these data suggest that certain patients with decompensated cirrhosis may benefit from therapy with DAAs.
Both International Liver Transplantation Society Consensus Statement[113] and EASL guidelines[114] only advise against antivirals when MELD score > 20, based on the ELITA study[74].Treatment of subjects with more elevated scores could make MELD score improves at such point that they could no longer be eligible for liver transplantation, but they would still be at risk of fatal complications and/or low life quality.Below this threshold, the recommendations suggest offering antiviral therapy with the hope of a stable improvement in liver function.
Nowadays, the 5-year overall survival after liver transplantation in individuals with chronic HCV infection is expected to be approximately 75%, since HCV recurrence does not limit anymore the liver transplantation outcome because of possibility of SVR after DAAs therapies[119,120].
Extrahepatic modifications
Two-thirds of patients with chronic hepatitis C present extrahepatic manifestations[75].These include autoimmune and lymphoproliferative disorders, ranging from cryoglobulinemia vasculitis to malignant B-cell lymphoma[42-45], cutaneous, metabolic, cardiovascular, neurological, and bone conditions[40,78,105,106].
Cryoglobulinemia and B cell non-Hodgkin lymphoma:There is evidence that SVR after treatment with peginterferon α and ribavirin is related to improvements in cryoglobulinemia associated to HCV infection and possible regression of B-cell non-Hodgkin lymphoma.In Cacoub’s meta-analysis, SVR was confirmed to be linked to notably more elevated proportion of complete remissions in subjects with cryoglobulinemia vasculitis [odds ratio (OR) 20.76] and objective response in those with malignant B-cell lymphoproliferative diseases (OR 6.49)[121].
Some data with DAAs therapy in the scenario of vasculitis end-organ disease related to cryoglobulinemia, including renal disease, have demonstrated responses in 20% to 90% of subjects[122,123].Notwithstanding, subjects with severe end-organ disease are likely to still need plasmapheresis and/or rituximab[123].
Regression of marginal zone lymphomas in HCV-infected individuals after interferon-based therapies has been noticed[124].In addition, HCV infection treatmentdiminishes the incidence of lymphomas in HCV-monoinfected individuals[45].HCV treatment with interferon does not change the incidence of lymphomas in patients coinfected with HIV[125].
There are few data about the effects of an SVR achieved with DAAs therapy on extrahepatic diseases apart from potential regression of cryoglobulinemia and B-cell non-Hodgkin lymphoma[126,127].
Extrahepatic dermatologic manifestations:Approximately 50% of individuals with porphyria cutanea tarda present HCV infection[128].Amelioration of this metabolic condition during interferon-based therapy has been described repeatedly[129].Currently, there are not enough data to ascertain the effect of DAA therapy on porphyria cutanea tarda.
Between 10% and 40% of patients with lichen planus present HCV antibodies[130,131].Contradictory data have been reported about resolution of lichen planus with interferon-based regimens[130,131], but promising perspectives with DAAs are present[131].
Comorbidities: Liver steatosis:Metabolic dysfunction-associated fatty liver disease (MAFLD)[132] is the main chronic liver disorder[133].Subjects with chronic HCV infection and MAFLD display accelerated liver fibrosis progression, and a higher risk of developing HCC[134,135].HCV clearance can lead to amelioration (and even to regression) of liver steatosis, at least when directly related to HCV genotype 3 infection[135,136].However, a meaningful percentage of patients with SVR may still have continued to have steatosis not related to HCV, but to other factors associated with it, especially overweight/obesity[135].
An additional problem in patients with hepatitis C is the weight gain after SVR.In a prospective study on more than 11000 patients, 52.6% gained weight and 19.8% gained excess weight (defined as at least 9 kg gain after 24 mo).SVR was an independent weight gain predictor[137,138].The mechanisms behind body weight modifications could involve neuropsychiatric alterations or diminished circulating levels of inflammatory cytokines[137].Increased BMI after viral clearance has clinical impact on fibrosis evolution of HCV-infected patients.The long-term evaluation of the German HCV-contaminated anti-D cohort demonstrated that a 6% of patients with SVR after DAAs developed advanced liver fibrosis after 35 years from infection; BMI and viral clearance independently predicted the evolution to cirrhosis[138].
Comorbidities: Insulin resistance and diabetes mellitus, lipid alterations:HCV perturbs glucose metabolism inducing insulin resistance, which may progress to type 2 diabetes[135].Furthermore, type 2 diabetes is one of the main risk factors of progression to chronic hepatitis C[134,135].
SVR has been shown to improve insulin resistance, as measured by the Homeostatic Model Assessment of Insulin Resistance score; it provides a significant protective effect on the incidence of diabetes[122,139].However, patients with diabetes mellitus type 2 diagnosed previously to DAAs remain diabetic despite to SVR, although the doses of antidiabetic drugs could be smaller[139].Moreover, antiviral therapy may reduce renal and cardiovascular (ischemic stroke, acute coronary syndrome) complications in HCV-infected patients with established diabetes, as has been demonstrated in a prospective cohort[140].
Type 2 diabetes mellitus could continue affecting progression of hepatitis C after SVR[134].Pre-treatment diabetes has been linked to a higher risk of cirrhosis, liver decompensation and HCC in a population of 33000 patients without baseline cirrhosis, treated with DAA and followed up for 3 years.The effect of diabetes mellitus was independent of the attainment of SVR[141].
DAAs increase triglyceride and cholesterol release through very low-density lipoproteins, thus normalizing hepatic lipid homeostasis[142].An increase in total cholesterol and low- and high-density lipoproteins is observed during treatment and after treatment completion[143].
Comorbidities: Osteoporosis:Viral hepatitis has been linked to decreased bone mineral density (BMD).Diverse factors have been hypothesized to contribute to it: elevated serum levels of inflammatory cytokines, decreased hepatic hydroxylation of vitamin D, altered hepatic production of insulin-like growth factor 1 and osteoprotegerin, and hypogonadism[144].Osteoporosis and bone fractures are usual among individuals with liver cirrhosis, especially in those with other risk factors[145].
The knowledge about the effects of SVR on BMD in HCV-infected subjects is limited.Studies have included usually samples of less than 50 patients treated with peginterferon plus ribavirin regimens.Although limitations of these studies are evident, they have demonstrated that BMD values at the lumbar spine and the femoral neck improve after the treatment[146,147], but controversial data have been also reported[148].
In HIV coinfected patients, a higher risk of osteoporosis and bone fractures has been communicated.Meaningful modifications in BMD and bone remodeling biomarkers plasma levels have not been observed after HCV eradication[149].
Comorbidities: Cognitive alterations:Improvements of neurocognitive dysfunction are observed after interferon-based SVR[150,151], including self-reported mood outcomes[152].This finding is corroborated by DAAs-induced improvements in brain magnetic resonance spectroscopy[153].However, controversial data have been published[154].These discrepancies could be due to the distinct methods used to assess cognitive dysfunction and the timing of the tests.
CONCLUSION
The excellent HCV response to DAAs treatment should not obviate certain obstacles to eradicate this pathology, especially the loss of follow-up and the possibility of reinfections.
Apart from the above, chronic hepatitis C determines several alterations whose normalization is not expected despite the elimination of HCV, especially in subjects treated in advanced stages of the disease.These include persistent liver dysfunction and continued risk of decompensation and HCC, although certainly less frequently than in individuals without SVR.Furthermore, weight gain after SVR may favor MAFLD in these patients increasing the risk of progression of liver disease.In HIV coinfected patients, SVR is not associated with a higher probability of liver complications than that of those HCV-monoinfected.
To summarize, DAAs administration reduces the death risk by cirrhosis and HCC and also reduces common comorbidities among people with HCV.Nevertheless, we are still far from eradicating the disease, but the World Health Organization goal by 2030 (a 90% reduction in new infections and a 65% reduction in viral hepatitis related mortality as compared to 2015)[3] is hopefully feasible.
杂志排行
World Journal of Hepatology的其它文章
- Hepatitis C virus: A critical approach to who really needs treatment
- Current aspects of renal dysfunction after liver transplantation
- Metabolic and nutritional triggers associated with increased risk of liver complications in SARS-CoV-2
- Recent updates on progressive familial intrahepatic cholestasis types 1, 2 and 3: Outcome and therapeutic strategies
- Targets of immunotherapy for hepatocellular carcinoma: An update
- Redefining non-alcoholic fatty liver disease to metabolic associated fatty liver disease: Is this plausible?
