Bone marrow mesenchymal stem cell therapy regulates gut microbiota to improve post-stroke neurological function recovery in rats
2022-01-07LinNaZhaoSongWenMaJieXiaoLiJiYangShiXinXuLanZhao
Lin-Na Zhao, Song-Wen Ma, Jie Xiao, Li-Ji Yang, Shi-Xin Xu, Lan Zhao
Lin-Na Zhao, Song-Wen Ma, Jie Xiao, Li-Ji Yang, Shi-Xin Xu, Lan Zhao, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin 300381, China
Lin-Na Zhao, Song-Wen Ma, Jie Xiao, Li-Ji Yang, Shi-Xin Xu, Lan Zhao, National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion, Tianjin 300381, China
Lin-Na Zhao, Shi-Xin Xu, Tianjin Key Laboratory of Translational Research of TCM Prescription and Syndrome, Tianjin 300381, China
Abstract BACKGROUND As a cellular mode of therapy, bone marrow mesenchymal stem cells (BMSCs) are used to treat stroke.However, their mechanisms in stroke treatment have not been established.Recent evidence suggests that regulation of dysregulated gut flora after stroke affects stroke outcomes.AIM To investigate the effects of BMSCs on gut microbiota after ischemic stroke.METHODS A total of 30 Sprague-Dawley rats were randomly divided into three groups, including sham operation control group, transient middle cerebral artery occlusion (MCAO) group, and MCAO with BMSC treatment group.The modified Neurological Severity Score (mNSS), beam walking test, and Morris water maze test were used to evaluate neurological function recovery after BMSC transplantation.Nissl staining was performed to elucidate on the pathology of nerve cells in the hippocampus.Feces from each group of rats were collected and analyzed by 16s rDNA sequencing.RESULTS BMSC transplantation significantly reduced mNSS (P < 0.01).Rats performed better in the beam walking test in the BMSC group than in the MCAO group (P < 0.01).The Morris water maze test revealed that the BMSC treatment group exhibited a significant improvement in learning and memory.Nissl staining for neuronal damage assessment after stroke showed that in the BMSC group, cells were orderly arranged with significantly reduced necrosis.Moreover, BMSCs regulated microbial structure composition.In rats treated with BMSCs, the abundance of potential short-chain fatty acid producing bacteria and Lactobacillus was increased.CONCLUSION BMSC transplantation is a potential therapeutic option for ischemic stroke, and it promotes neurological functions by regulating gut microbiota dysbiosis.
Key Words: Ischemic stroke; Bone marrow mesenchymal stem cells; Neurological function; Gut microbiota
INTRODUCTION
Globally, stroke is a lethal disability-causing disease that affects up to 13 million people annually[1].The latest data from the American Heart Association shows that in the United States, one person suffers a stroke after every 40 s[2].Stroke patients exhibit recurrent attacks, which exerts a huge socio-economic burden on the society and families.Ischemic stroke is the most prevalent stroke type, accounting for 70%-80% of all stroke types[3].Intravenous thrombolysis and endovascular thrombectomy are the primary treatment options for stroke.However, they are associated with time and technical limitations[4,5].Therefore, it is important to develop novel therapeutic approaches for ischemic stroke.
Stem cell transplantation is considered a potential therapeutic strategy for patients after ischemic stroke[6].Bone marrow mesenchymal stem cells (BMSCs) are a group of stem cells with various characteristics, including autologous harvesting, rapid proliferation, easyin vitroculture, and low immunogenicity.Moreover, they are not limited by ethical restrictions.BMSCs have the effects of neuroprotection, modulation of inflammation, immune responses, endogenous neurogenesis, and astrogenesis[7].Specifically, their inflammatory regulatory function has been investigated in various inflammatory diseases.
An estimated 100 trillion microorganisms reside in the human gut.They are closely associated with human health and diseases[8].The understanding of gut microbiota is only at the rudimentary stage; however, studies have confirmed the existence of bidirectional communication in the microbiota-gut-brain axis, which influences stroke treatment and prognosis[9-11].After a stroke, the central nervous system (CNS) is injured, then, as a stress response mechanism, the hypothalamic-pituitary-adrenal axis triggers the release of adrenocorticotropic hormone-releasing factor (CRF) and glucocorticoids[12].Sympathetic and parasympathetic nerves directly affect gastrointestinal functionsviacommunication with the enteric nervous system[10].This induces suppressed gut motility, increased gut permeability, gut microbiota dysbiosis, and immune cell activation.Studies have documented significant microbial diversity changes in feces of stroke patients[13,14].Severe stroke destroys the intestinal barrier, therefore, commensal gut microbiota migrates to other organs; this is the primary cause of systemic infections after stroke[15].A few bacterial species in gut microbiota or their metabolites regulate intestinal immunity, which regulates post-stroke immunity[16].Animal model experiments have established that changing the gut microbiota improves the prognosis of stroke[17,18].Despite the documented efficacy of stem cell therapy in altering the populations of gut microbiota in several inflammatory diseases, it has not determined whether it has a similar effect on ischemic stroke.
Therefore, we used a rat model of transient middle cerebral artery occlusion (MCAO) to investigate whether BMSCs can improve abnormal intestinal flora after ischemic stroke.
MATERIALS AND METHODS
Animals
Adult male Sprague-Dawley (SD) rats, 5-6 week old, weighing 220-250 g, were purchased from Beijing Huafukang Biotechnology Company (Beijing, China).The rats were housed in pathogen-free conditions under a 12 h-light/12 h-dark cycle at 25 °C.The Ethics Committee of Tianjin University of Traditional Chinese Medicine approved this study (approval number: TCM-LAEC2019038).
BMSC isolation, culture, and identification
In this study, 4-wk-old SD rats were cervically dislocated.The femur and tibia were isolated and removed under sterile conditions.The Dulbecco's modified Eagle medium (DMEM) was used for flushing the bone marrow cavity, and the bone marrow flush was collected.The isolated cell suspension was sieved through a 200-mesh nylon sieve and then centrifuged (1000 r/min) for 10 min at 4 °C.The supernatant was discarded, and the cells were re-suspended with DMEM containing 10% fetal bovine serum (FBS; BI).The cell density was adjusted to 2 × 106cells into 25 cm2culture flasks and incubated in a cell incubator (37 °C, 5% CO2).The cells were passaged every 3-4 d, and the third-passage cells were used for further experiments.BMSCs were incubated with fluorescence antibodies, including CD90-PE, CD29-APC, CD45-PerCP, and CD31-FITC (1:100, Miltenyi, Germany), to identify the phenotype by flow cytometry (FACS Calibur, BD, San Jose, CA, United States).
Experimental design
Rats were randomly divided into three groups (n= 10 each): Sham operation control group (Sham), transient MCAO group, and MCAO with BMSC treatment group.The Sham and MCAO groups were injected with normal saline (PBS), and the BMSCs group was injected with 1 × 106BMSCs through the tail vein 24 h after reperfusion.Rats were killed after 21 d of reperfusion to collect feces and brain tissue for analysis (Figure 1A).
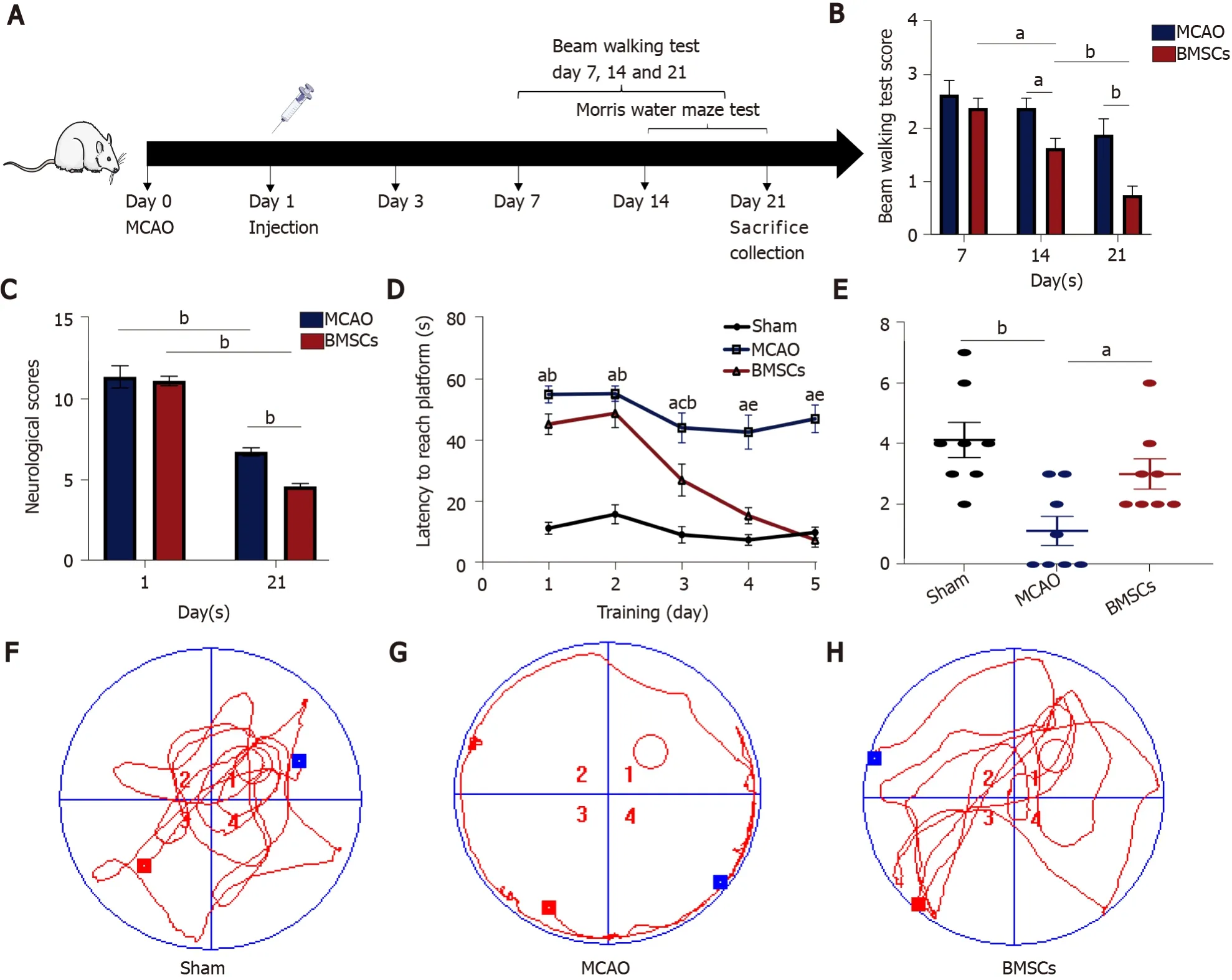
Figure 1 Bone marrow mesenchymal stem cells improve neurological function after stroke.
MCAO
The intraluminal filament model was used to induce transient MCAO as described by Jackmanet al[19].Rats were anesthetized with 4% isoflurane and fixed in a supine position, and a longitudinal incision was made 0.3 cm to the right of the midline of their neck.Then, the muscles and tissues were separated to expose the right common carotid artery (CCA), external carotid artery (ECA), and internal carotid artery (ICA).Subsequently, a filament nylon suture was inserted into the right ECA and pushed until the middle cerebral artery (MCA) was obstructed.After 90 min of ischemia, the filament was removed carefully and reperfusion performed.During surgery, the rats were placed on a thermostat system to maintain body temperature.
Neurobehavioral scores
The Longa 5-point scale was used to judge whether MCAO surgery is successful: 4, the animal died; 3, the animal could not walk in a straight line, and its body was tilted to one side; 2, the animal turned to one side during crawling; 1, the animal could not straighten its limbs and was stiff; 0, the animal was normal.If the score was 1-3, the model was considered successful, and the experiment can be carried out later; 0 and 4 were rejected.Animals with a score of 1 to 3 will be grouped for later experiments.
The modified Neurological Severity Score (mNSS) was used to score the neurological function of the rats on days 1 and 21 after reperfusion, which included motor, sensory, reflex, and balance tests with a total score of 18[20]; the higher scores mean more severe injuries.
Behavioral analysis
Two blinded investigators observed all behavioral tests at regular times of the day.The apparatus was washed with 70% ethanol after each animal was tested to eliminate olfactory cues.
Beam walking test:For detecting motor coordination and balance, the beam walking test was evaluated at 7, 14, and 21 d after reperfusion.The rats were placed on a balance beam that was 1 m long, 2.5 cm wide, and 20 cm high from the ground.A soft cushion was placed under the balance beam to prevent the mouse from falling.Every mouse was scored according to the following rules: (1) If the rat crossed the balance beam smoothly without the hind limbs slipping; (2) If the rat gripped the edge of the balance beam, but the hind limbs did not dangle; (3) If the rat clutched the balance beam, and one limb dropped from the balance beam; (4) If the rat clutched the balance beam, and two limbs dropped from the balance beam or rotated on the balance beam (> 60 s); (5) If the rat tried to balance on the balance beam but fell (> 40 s); (6) If the rat tried to balance on the balance beam but failed (> 20 s); and (7) If the rats fell and did not attempt to balance on the beam (< 20 s).
Morris water maze test:The Morris water maze test was performed 14 d after surgery for six consecutive days to test rats' spatial memory ability.The water maze was a circular black pool (Shanghai Xinsoft Information Technology Co., Ltd.), 150 cm in diameter, 50 cm high, and 25 cm deep, with the water temperature maintained at 20 ± 1 °C.The pool was divided into four quadrants (1, 2, 3, and 4), and the circular platform was located in quadrant 1, 2 cm below the water surface.The rats were tested twice daily for 60 s for the first 5 d and were allowed to remain on the platform for 10 s after each test.On day 6, a probe trial was performed by removing the platform and allowing the rat to swim freely in the pool for the 60 s.The time and route taken by the rats to complete the task were recorded.Finally, the data were exported and analyzed using Morris water maze analysis software.
Histological analysis of rat brain
The rats were fixed by perfusion in 4% paraformaldehyde (PFA).The brains were quickly removed and fixed in 4% PFA at 4 °C for 24 h.After dehydration, they were embedded in paraffin and serially sectioned into 4 μm tissue sections for histological analysis.Nissl staining was performed to evaluate neuron damage.The histopathology of the hippocampus of brain tissues was observed with a microscope (BX43; Olympus).
Microbiome 16S rDNA sequencing and analysis
The rat feces from each group were collected into 2 mL sterile freezing tubes on day 21 and stored at -80 °C until the bacterial DNA was extracted.Total bacterial DNA was extracted using DNA Extraction Kit (QIAGEN, Germany) following the manufacturer’s instructions.To ensure the quality and quantity of DNA, extracted DNA was detected by agarose gel electrophoresis and stored at -20 °C until further processing.The diluted DNA was used as the template for PCR amplification of bacterial 16s rRNA genes with the barcoded primers (V3-V4 regions) and Takara Ex Taq (Takara).The PCR product was purified with AMPure XP beads (Beckman Coulter Genomics, United States) and quantified using a Qubit dsDNA assay kit (Life Technologies, United States).According to the standard protocols, equal amounts of purified amplicon were sequenced using the Illumina Miseq sequencer PE250 (Illumina, United States).The raw data were processed sequentially with the software Trimmomatic (version 0.35), Flash (version 1.2.11), QIIME (version 1.8.0), and UCHIME (version 2.4.2) to get the operational taxonomic units (OTUs).The valid tags were classified at a 97% similarity cutoff to analyze the gut microbiota diversity.
α-diversity is a measure of the abundance and diversity of microbial communities in a sample.In this paper, the Shannon index and Chao index were used to represent αdiversity[21,22].The Shannon index is an alpha diversity statistic for estimating the index of microbial diversity in a sample.A higher value indicates that the community is more diverse.The Chao index assesses the number of OTUs in a sample.The larger the Chao index, the higher the number of OTUs, indicating that the number of species in the sample is more numerous.The functional pathways of microbial communities for each sample were inferred using Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) software[23].The PICRUSt software predicts the metabolic function of microorganisms by comparing the resulting 16S sequencing data with a genomic reference database of microorganisms with known metabolic functions.
Statistical analysis
The results are expressed as the mean ± SEM.The data were analyzed using one-way analysis of variance (ANOVA) andt-test.The difference was considered significant atP< 0.05.
RESULTS
BMSCs improve neurological function after ischemic stroke
The mNSS, beam walking test, and Morris water maze test were used to estimate the neurological function after ischemic stroke.The neurological deficit scores of each group of rats were evaluated at 1 and 21 d after ischemia-reperfusion (Figure 1B).Compared with the MCAO group, the BMSCs group had significantly improved neurological function.The mNSS scores of both the MCAO and BMSCs groups were substantially lower at 21 d than on the first day (P< 0.01).However, the BMSCs group had a more significant decrease in mNSS scores at day 21 than the MCAO group (P< 0.01).Beam walking test showed that rats subjected to BMSCs transplantation presented a larger motor functional improvement (14 d,P< 0.05; 21 d,P< 0.01; Figure 1C)
To assess the spatial learning and memory capacity of BMSC-treated rats after stroke, the Morris water maze test was used to detect the escape latency of a random search for the hidden platform during the first 5 d.Compared to the MCAO group, the BMSCs group showed a significantly shorter duration of escaping latency (P< 0.05; Figure 1D).After removing the hidden platform at 6 d, rats of the BMSCs group were easier to find the previous location of the platform site compared to those of the MCAO group, which passed over the platform site more times (P< 0.05; Figure 1E).The typical swimming tracks of each group (Figure 1F-H) also indicated that rats treated with BMSCs had significantly improved spatial memory.
BMSCs alleviate neuronal loss in the hippocampus after ischemic stroke
Nissl staining demonstrated no significant changes in neurons in the hippocampal CA1 area of the brain in the Sham group on day 21.In the MCAO group, the boundaries of the hippocampal CA1 area were irregular, the number of Nissl bodies was reduced, and a large number of neurons underwent necrosis.Compared with the MCAO group, the rat hippocampal neurons in the group treated with BMSCs were arranged in an orderly manner, and necrotic cells were significantly reduced (Figure 2).These results suggest that stroke causes severe neuronal damage in rats and that BMSC treatment can effectively protect neurons and prevent neuronal loss.
Effect of BMSCs on microbial α-diversity and structure after ischemic stroke
To identify whether treatment with BMSCs influences the gut microbiota after ischemic stroke, we analyzed differences in species complexity and bacterial communities between populations based on OTUS and species annotation results.We obtained a total of 1494295 quality filtered 16s rRNA gene sequences from three groups of 30 samples, with an average of 49810 ± 1281 reads per sample.We compared microbial α-diversity between the Sham, MCAO, and BMSCs groups, and both Shannon and Chao index results showed no statistical difference between the three groups (Figure 3A and B).
We calculated inter-sample distances between the three groups to analyze the differences in community species composition among individual samples within each group.We demonstrate the nonmetric multi-dimensional scaling (nMDS) plot, and the principle co-ordinates analysis (PCoA) plots in Figure 3C and D.Different groups are presented in different colors in the figure, and samples from the same group are clustered together.The nMDS analysis and PCoA showed that MCAO and BMSCs could alter the microbiota composition significantly compared to the Sham group.However, there was no significant difference in microbiota structure between the two groups of MCAO and BMSCs.To further investigate the variability of microbial communities between the two groups, the ANOSIM test was used to test both Bray-Curtis and Unweighted Unifrac algorithms (Bray-Curtis,r= 0.0769,P= 0.042; Unweighted Unifrac,r= 0.0679,P= 0.0415, respectively).The results showed significant differences in the microbial communities between the two groups.
BMSCs modulate gut microbiota after ischemic stroke
We next sought to explore the effect of treatment with BMSCs on the composition of the microbial structure.Figure 4A shows the abundance of microorganisms in the three groups, in which Bacteroidetes, Firmicutes, Proteobacteria, and Epsilonbacteraeota were the most significant contributors at the microbial phylum level.Compared with the Sham group, MCAO and BMSC increased the relative abundance of Proteobacteria, suggesting significant differences in the gut microbiota structure after stroke.Furthermore, we analyzed the differences in the relative abundance of microorganisms between the three groups at the level of genus (Figure 4B).The data showed that the relative abundance ofRuminococcaceae_UCG-005,Mycoplasma,Ruminiclostridium_5,Oceanimonas, andMarvinbryantiawas significantly decreased, and the relative abundance ofEscherichia-Shigella,Alloprevotella,Butyricimonas,ASF356, andEnterococcuswas increased in the MCAO group compared with the Sham group.BMSC treatment increased the relative abundance ofRuminiclostridium_5and decreasedButyricimonasandASF356at the species level.The dominant bacteria of MCAO and BMSCs are shown separately at the species level in Figure 4C and D.We concluded that species enriched in the BH group includedClostridiumspp andLachno-spiraceaespp, which are the potential species to produce short-chain fatty acid (SCFA).A comparison of potential SCFA producing bacteria in the feces revealed that depletion occurred in the MCAO group (Figure 4E).Additionally, it was observed that the relative abundance ofLactobacilluswas significantly increased at the genus level after BMSC treatment (Figure 4F).
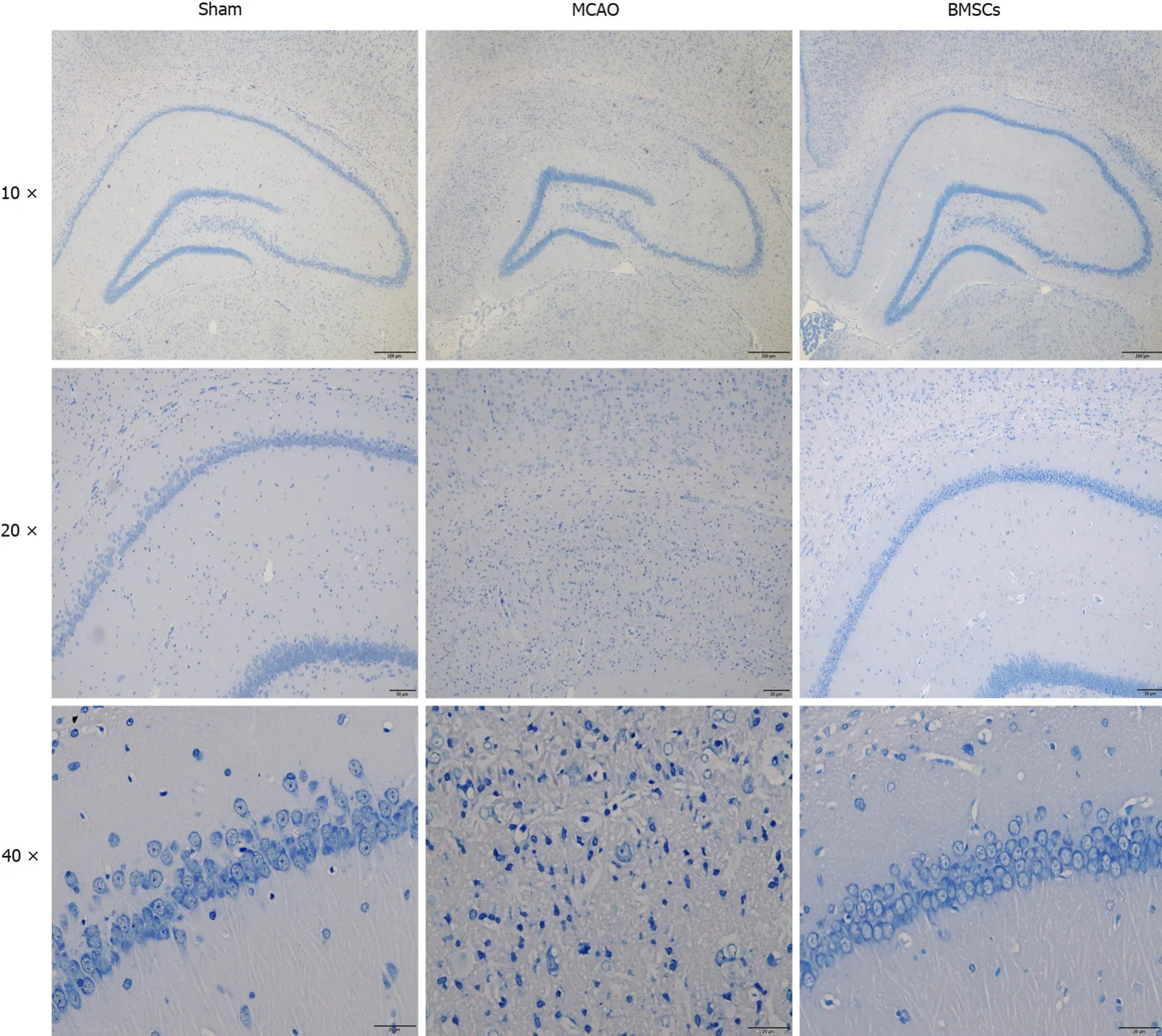
Figure 2 Histopathological changes in brain tissue of rats.
Predictive analysis of gut microbiota function
PICRUSt functional prediction analysis was based on 16S sequencing data annotated in the Greengenes database.Using PICRUSt software can predict the composition of known microbial gene functions and thus statistically different functions between groups.In this study, the Kyoto Encyclopedia of Genes and Genomes (KEGG) was used to assess microbial function, and 25 differentially KEGG functional pathways were identified between MCAO and BMSCs (Figure 5).The gut microbiota of BMSCs influenced the pathways of metabolism, including “Carbohydrate Metabolism”, “Biosynthesis of Other Secondary Metabolites”, “Glycan Biosynthesis and Metabolism”, “Lipid Metabolism”, “Metabolism of Cofactors and Vitamins”, “Metabolism of Other Amino Acids”, and “Xenobiotics Biodegradation and Metabolism”.We also found that BMSCs-enriched function pathways were associated with “Membrane Transport”, “Signaling Molecules and Interaction”, “Transport and Catabolism”, and “Transcription”.
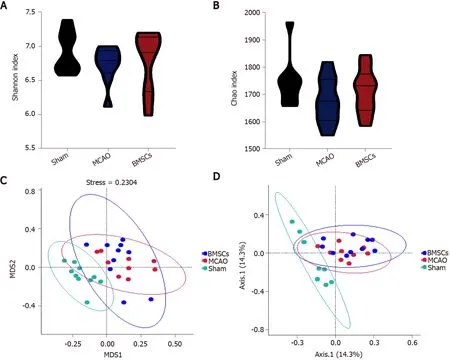
Figure 3 Abundance and structures analysis of intestinal microecology.
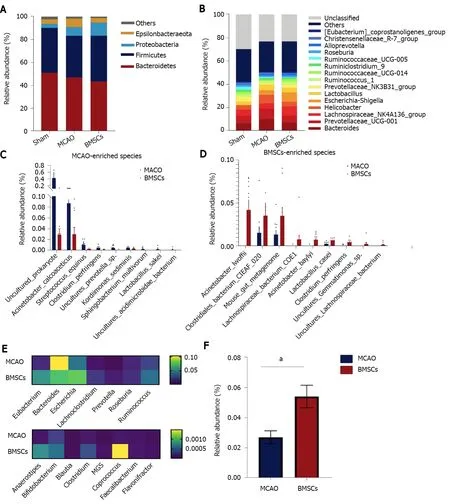
Figure 4 Bone marrow mesenchymal stem cells modulate the composition of gut microbiota.
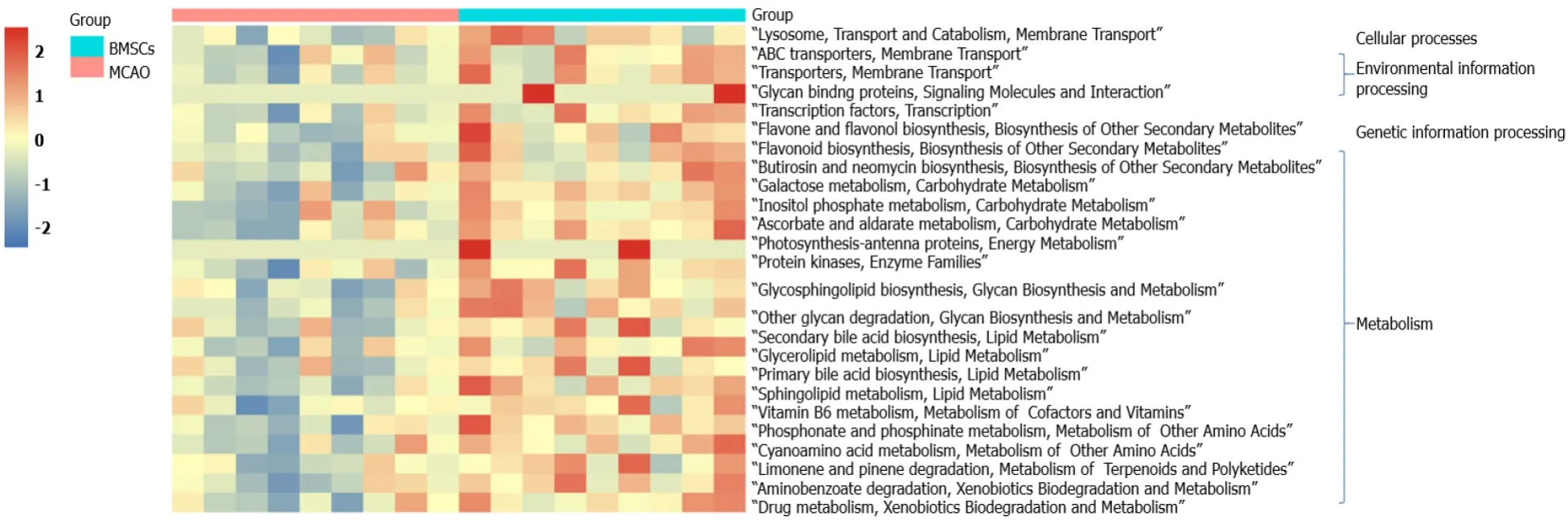
Figure 5 Alterations of microbial function.
DISCUSSION
For the first time, this study showed changes in gut microbiota after ischemic stroke treatment using BMSCs.BMSCs disrupted the composition and structure of gut microbiota, thereby affecting metabolic pathways in ischemic stroke.
Evidence from basic and clinical studies show that BMSCs can effectively treat patients with ischemic stroke[24].Transplantation of BMSCs significantly enhances neurological functions after stroke[25], consistent with our results.We established that treatment with BMSCs significantly reduced mNSS scores and enhanced balance, coordination abilities, and learning memory in rats.Notably, cerebral ischemia caused neuronal damage in the hippocampus, striatum, thalamus, and cerebral and cerebellar cortices, with the CA1 region of the hippocampus being one of the most sensitive brain regions.Nissl staining revealed serious neuronal damage in rats after ischemic stroke, which explains memory impairment in the Morris water maze test.In contrast, BMSCs effectively protected the nerve cells.
Studies have confirmed complex interactions between gut microbiota and stroke.Xiaet al[26] reported thatParabacteroides,Oscillospira, andEnterobacteriaceaeamong others were enriched in stroke patients, whereasPrevotella,Roseburia, andFecalibacteriumwere enriched in healthy individuals[26].In stroke patients, dysbiosis is closely associated with metabolism and inflammation.Besides, a specific genus of gut microbiota and associated metabolites are used as potential indicators for stroke prediction and prognosis[13,27].In stroke animal models, similar alterations in gut microbiota have been detected.Singhet al[9] found that the most abundant phyla ofFirmicutes,Bacteroidetes, andActinobacteriaovergrew in MCAO mice[9].Chenet al[28] reported that after stroke, rats exhibited an increase in the abundance of opportunistic pathogens, includingAlistipes,Bacteroides,Klebsiella,Shuttleworthia,Haemophilus,Fusobacterium,Faecalibacterium,Proteus, andPapillibacter[28].After transplantation of BMSCs, we analyzed the changes in gut microbiota to investigate the role of gut microbiota in post-stroke rats.We found that BMSCs did not alter the α-diversity and structure of gut microbiota after stroke.Further assessments of the composition of microbiota structure suggested that BMSCs significantly increased the abundance of potential SCFA-producing bacteria.
LachnospiraceaeandClostridiumare the main groups of SCFA-producing bacteria[29].For mammals, SCFA is a critical gut microbial metabolite.It can be used as a substrate for the metabolism of cholesterol, glucose, and lipids, which provide nearly 10% of daily caloric requirements[30].Besides, it achieves its anti-inflammatory effects by activating G protein-coupled receptors (GPCR) to regulate T cells[31].Additionally, SCFA protects and repairs the intestinal mucosal barrier by secreting mucus and stimulating tight junction protein expression[32].
The abundance ofLactobacillushas been shown to be significantly increased in cerebral infarction patients[33].Interestingly, we found a significantly high abundance ofLactobacillusin the fecal matter of the BMSCs group.Bourriaudet al[34] realized that butyrate-producing bacteria ferment lactic acid to produce butyrate, which reduces inflammatory responses, thereby protecting the injured brain[34].Given that BMSCs increase the abundance of potential SCFA-producing bacteria, an increase inLactobacillusleads to the production of more lactic acid to be fermented to butyrate, thereby improving neuroinflammation during stroke.
CONCLUSION
This is the first study to elucidate on alterations in gut microbiota after BMSC treatment in an ischemic stroke condition.We found that BMSCs potentially improve neurological damage after stroke by regulating gut microbiota.This provides a basis for future research into the role of BMSCs from the perspective of the "gut-brain axis".
ARTICLE HIGHLIGHTS
Research background
Ischemic stroke is a highly lethal and disabling disease that has a severe impact on the quality of life of patients.Gut microbiota is closely related to the treatment and prognosis of stroke.The improvement of neurological function by bone marrow mesenchymal stem cells (BMSCs) may be related to the regulation of gut microbiota.
Research motivation
Many studies have shown that gut microbiota plays an important role in immunity after stroke through the gut-brain axis.
Research objectives
To observe the regulation of gut microbiota after BMSC treatment.
Research methods
Rats were divided into three groups [Sham, middle cerebral artery occlusion (MCAO),and BMSCs].Recovery of neurological function in rats after BMSC transplantation was observed by the modified Neurological Severity Scores (mNSS), beam walking test,and Morris water maze test.Pathological observation of hippocampal neuronal cells was conducted by Nissl staining.16S rDNA sequencing was used to analyze the composition of gut microbiota.
Research results
Transplantation of BMSCs significantly reduced mNSS scores (P< 0.01), and improved balance and coordination (P< 0.01), learning, and memory in rats.The structure of the C1 region of the hippocampus was clear and necrotic cells were significantly reduced after the intervention of BMSCs.Compared with the MCAO group, BMSCs effectively increased the relative abundance of short-chain fatty acid-producing bacteria andLactobacillusin feces.
Research conclusions
Transplantation of BMSCs can regulate gut microbiota, which provides a potential therapeutic mechanism for stroke treatment.
Research perspectives
We demonstrated the modulatory effect of BMSCs on the gut microbiota after stroke, which provided an experimental basis for elucidating the gut-brain axis.
杂志排行
World Journal of Stem Cells的其它文章
- Stem cell-derived biofactors fight against coronavirus infection
- Application of mesenchymal stem cells derived from human pluripotent stem cells in regenerative medicine
- Strategies to improve regenerative potential of mesenchymal stem cells
- Dental mesenchymal stromal/stem cells in different microenvironments— implications in regenerative therapy
- Regulating the fate of stem cells for regenerating the intervertebral disc degeneration
- SmartFlareTM is a reliable method for assessing mRNA expression in single neural stem cells
