Stem cell-derived biofactors fight against coronavirus infection
2022-01-07MohammadrezaArdalanLeilaChodariSepidehZununiVahedSeyedMahdiHosseiniyanKhatibiAzizEftekhariSoodabehDavaranMagaliCucchiariniLeilaRoshangarElhamAhmadian
Mohammadreza Ardalan, Leila Chodari, Sepideh Zununi Vahed, Seyed Mahdi Hosseiniyan Khatibi, Aziz Eftekhari, Soodabeh Davaran, Magali Cucchiarini, Leila Roshangar, Elham Ahmadian
Mohammadreza Ardalan, Sepideh Zununi Vahed, Seyed Mahdi Hosseiniyan Khatibi, Elham Ahmadian, Kidney Research Center, Tabriz University of Medical Sciences, Tabriz 5166614766, Iran
Leila Chodari, Physiology Department, Faculty of Medicine, Urmia University of Medical Sciences, Urmia 5715799313, Iran
Aziz Eftekhari, Department of Toxicology, Maragheh University of Medical Sciences, Maragheh 3453554, Iran
Soodabeh Davaran, Department of Medicinal Chemistry, Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz 5166614766, Iran
Soodabeh Davaran,Applied Drug Research Center, Tabriz University of Medical Sciences, Tabriz 5166614766, Iran
Magali Cucchiarini, Center of Experimental Orthopaedics, Saarland University Medical Center, Homburg D-66421, Germany
Leila Roshangar, Stem Cell Research Center, Tabriz University of Medical Sciences, Tabriz 5166614766, Iran
Abstract Despite various treatment protocols and newly recognized therapeutics, there are no effective treatment approaches against coronavirus disease.New therapeutic strategies including the use of stem cells-derived secretome as a cell-free therapy have been recommended for patients with critical illness.The pro-regenerative, pro-angiogenic, anti-inflammatory, anti-apoptotic, immunomodulatory, and trophic properties of stem cells-derived secretome, extracellular vesicles (EVs), and bioactive factors have made them suitable candidates for respiratory tract regeneration in coronavirus disease 2019 (COVID-19) patients.EVs including microvesicles and exosomes can be applied for communication at the intercellular level due to their abilities in the long-distance transfer of biological messages such as mRNAs, growth factors, transcription factors, microRNAs, and cytokines, and therefore, simulate the specifications of the parent cell, influencing target cells upon internalization and/or binding.EVs exhibit both anti-inflammatory and tolerogenic immune responses by regulation of proliferation, polarization, activation, and migration of different immune cells.Due to effective immunomodulatory and high safety including a minimum risk of immunogenicity and tumorigenicity, mesenchymal stem cell (MSC)-EVs are more preferable to MSC-based therapies.Thus, as an endogenous repair and inflammation-reducing agent, MSCEVs could be used against COVID-19 induced morbidity and mortality after further mechanistic and preclinical/clinical investigations.This review is focused on the therapeutic perspective of the secretome of stem cells in alleviating the cytokine storm and organ injury in COVID-19 patients.
Key Words: COVID-19; Secretome; Mesenchymal stem cell; Exosome; Stem cell; Biofactors
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is designated as the etiology of coronavirus disease 2019 (COVID-19), which is a pandemic viral disease.The symptoms from death or acute respiratory distress syndrome (ARDS) to mild upper respiratory symptoms[1].Excessive systemic immune activation of patients generates a cytokine storm, which is found in severely ill COVID-19 patients.Recent evidence indicates that the cytokine storm could play a key role in disease progression, resulting in the failure of various organs or even death.Hence, approaches which prevent the cytokine storm may be significant in mitigating COVID-19[2,3].
Complications such as cardiovascular system dysfunction, primarily acute myocardial injury, arrhythmia, or heart failure[4], neurological complications[5], gastrointestinal symptoms[6], and acute kidney injury[7] have been identified in a substantial proportion of COVID-19 patients.The unprecedented COVID-19 pandemic demands urgent therapies.Currently, multiple medicines involving anti-viral, antimalarial, and anti-inflammatory agents are being investigated.Regardless of the patient's recovery and survival due to various therapeutics, lung damage in these patients does not fully recover.Recently, promising stem cell therapies and importantly secreted extracellular vesicles (EVs) have been shown to exhibit antiinflammatory effects and attenuate COVID-19-related lung injury.
A new therapeutic approach which involves cellular therapies is promising in treating chronic and acute lung diseases due to their anti-inflammatory, immunomodulatory, regenerative, pro-angiogenic, and anti-fibrotic features.Mesenchymal stem cell (MSC)-secretome (a paracrine mechanism) composed of EVs and free soluble proteins mediate those therapeutic impacts[8].The remarkable properties of exosomes have gained considerable attention as a probable therapeutic option in COVID-19.In vivoandin vitrostudies have been conducted to determine the various therapeutic effects of MSC-secretome in tissue regeneration, heart, and lung diseases.The inflammation suppressing effects of MSC-secretome are due to the prevention of monocyte differentiation into dendritic cells, prohibiting natural killer (NK) cells proliferation and cytotoxicity, stimulating macrophage polarization from the pro-inflammatory (M1) to anti-inflammatory (M2) phenotype, regulating the inflammatory characteristics of T helper cells, and inhibiting T cell proliferation.Growth factors found in the MSC-secretome regenerate the damaged lung tissue by increasing proliferation and reducing apoptosis of resident lung epithelial and endothelial cells.Additionally, antimicrobial peptides (AMPs) have been observed in MSC-secretomes and demonstrate antimicrobial properties, whereas protease inhibitors reduce extra protease function in the lung, preserving the protease/anti-protease equilibrium[9].Exosomes extracted from MSC act as multitargeting agents.Therefore, they diminish the cytokine storm and prevent the inhibition of COVID-19-related anti-viral defenses in hosts[10].Exosomes may hamper the cytokine storm and inflammatory process due to their reparative properties and thus induce endogenous repair.Hence, MSCsecretome might be a valuable cell-free substitute to cell-based therapies alone or in combination with pharmacological agents.In this review, the therapeutic potential of the secretome of stem cells in mitigating COVID-19-induced cytokine storm and organ damage is presented.
STEM CELL-BASED THERAPEUTICS
Evidence has shown the promising role of MSCs in COVID-19 pneumonia treatment.Human umbilical cord MSCs given to a 65-year-old female with severe COVID-19 induced substantial recovery through modulation of the immune system and regeneration of damaged tissue with high safety[11].Every three days, MSCs were administered intravenously by clinicians three times (5 × 107cells each time).Lenget al[12] demonstrated the enhancement of pulmonary function and symptom improvement in seven COVID-19 patients with pneumonia in only two days after administration of MSCs.In their study, only one administration per kilogram of weight containing 1 × 106cells was performed.The authors proposed that the therapeutic impact mainly occurred based on the immunoregulating features of MSCs.Remarkably, MSCs are not virus infectable as they are angiotensin-converting enzyme 2 negative.Hence, for treating seriously ill COVID-19 patients under certain protocols, MSCs can be considered a potential treatment option[13].As the severity of this viral infection is closely associated with the host’s immune response, the immunomodulatory effects of MSCs can efficiently prohibit the cytokine storm and thus treat severe cases of COVID-19.Indeed the outcomes of COVID-19 patients can be enhanced by the transplantation of MSCs using various methods, including “as a result of their immunoregulatory impact, as a result of inducing regeneration and repairing tissue, and as a result of their antimicrobial, antifibrotic, and angiogenic properties”.
All these methods improve lung repair and prevent multiple organs from exaggerated immune response-derived damage.The ongoing COVID-19 clinical trials based on MSCs have been reviewed recently[14].It now seems that MSCs convey their therapeutic effects through the paracrine pathway.As these cells can discharge secretome (active biological substances), therefore they can be potentially addressed as drug stores[15].
THE SECRETOME OF STEM CELLS: A CELL-FREE ALTERNATIVE TO CELL-BASED THERAPEUTICS
The secretome is defined as a stem cell secretion composed of regulatory factors and various soluble molecules, including AMPs, angiogenic growth factors, lipid mediators, and anti-inflammatory cytokines.Evidence has shown that these molecules are packed into EVs, also known as cell-secreted vesicles[16,17].
Common secretory mechanisms are involved in the excretion of secretomes by stem cells (Figure 1).Following the administration of the secretome or the culture medium in patients, through a paracrine signaling pathway, neighboring cells assimilate them[18].Two important secreted EVs, are exosomes and MVs, also known as microvesicles alongside the apoptotic bodies secreted by stem cells[19].The fusing of plasma and multi-vesicular bodies facilitates exosome (30-100 nm) elicitation.However, cellular membrane budding generates MVs (100-1000 nm), which possess cellular cytoplasm.EVs are discharged into the extracellular microenvironment and act like soluble components and through endocrine and paracrine methods, they express their biological effects.In a more general definition, MSC-secretome contains all the secreted bioactive factors of MSCs, with both extravesicular and the soluble elements[20,21].
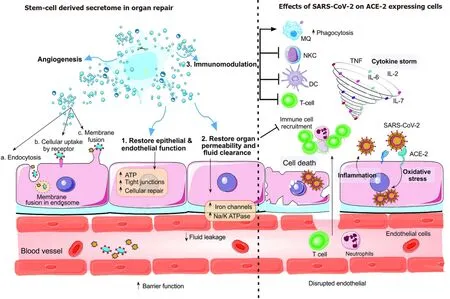
Figure 1 The stem cell secretome.
MSC-DERIVED EVS
Upon secretion, proteins, and EVs, through ligand-receptor interactions or internalization, engage with the target cells and regulate cellular responses.The secretome stimulates endogenous stem cells and progenitor cells, prevents apoptosis, attenuates the inflammatory response, triggers extracellular matrix remodeling and angiogenesis, prevents fibrosis, and regulates chemoattraction.It was revealed that following the MSCs' functional mitochondria or mitochondrial DNA transfer to target cells, they protect cellular aerobic respiration with non-healthy mitochondria or modulate T cell functions[21].By resembling their parent cells, EVs from MSCs have characteristics such as immunoregulatory, anti-oxidative, progenerative, pro-metabolic, antiapoptotic, and anti-inflammatory, in the microenvironment of damaged tissue.In MSC-based therapy, extracted EVs from MSCs are considered a substantial alternative to cell-based treatments[22].Their efficacy is presently underin vitroexamination for lung damage treatment utilizing MSC-derived EVs in various preclinical experiments[22].EVs extracted from MSCs showed efficacy in lung injury due to influenza in a pig model.Studies have revealed that extracted EVs from MSCs are available 12 h after virus infection and diminish the levels of pro-inflammatory cytokines as well as viral replication[23].Ang-1 mRNA (an angiogenic trophic factor) is found in EVs from MSCs.Due to this factor's role in limiting leukocytes and vessel endothelial cells' interaction and sustaining the vascular barrier integrity, Ang-1 mRNA is considered substantial in endothelial cell stabilization during the injury process.The impact of EVs extracted from MSCs on lipopolysaccharide-induced acute lung damage in an experimental mouse model sheds light on the contributions of Ang-1 mRNA transfer by EVs to restore pulmonary capillary permeability[24].Furthermore, EVs extracted from MSCs influence inflammation by inhibiting the expression of tumor necrosis factor alpha (TNF-α) and stimulating interleukin (IL)-10 secretion.Upon Ang-1 mRNA transfer and the internalization of EVs extracted from MSCs into injured endothelium cells, within the damaged lung microvascular endothelium, Ang-1 mRNA partially preserves protein permeability[25].EVs are increasingly regarded as a substantial alternative to cell-based therapy.
Secretome administration is associated with multiple advantages in comparison with complete MSCs therapy.The secretome lacks self-replication and is not involved in endogenous tumor development, as it has low immunogenicity, and upon intravenous injection, it contributes to low emboli formation.Therefore, the secretome is deemed more advantageous than cells[26].The MSC-secretome can be altered and preserved more conveniently than cells with fewer costs regarding technological advances.It also suits emergency interventions as the product is ready-to-use[27].With regard to monoclonal antibody therapy, the costs of the MSC-secretome are lower, which is vital in the management of a pandemic.Nonetheless, many concerns regarding EVs should be resolved before clinical application, and the delivery route (intravenous or inhalation), purification, bio-distribution, production, and characterization should be determined.
EXOSOMES
Exosomes are nanoparticles (40-150 nm) which have various bioactive components, including proteins, growth factors, lipids, microRNAs (miRNA), long noncoding RNAs, and transfer RNAs.The lipid contents of exosomes provide the platform for their infusion with neighboring cells and plasma membrane[28].Following internalization of the secretome components, neighboring cells alter several downstream pathways, such as fibrosis inhibition, immunoregulation, damaged tissue remodeling, and apoptosis suppression[8].
With regard to exosome isolation and production, MSCs discharge exosomes under circumstances such as cytokine treatment, serum starvation, or hypoxia[29].Purification and the introduction of exosomes into the body can then take place.It has been shown that exosomes derived from MSCs generate an impact resembling that of MSCs[30].The multiple proteins, miRNAs, and mRNAs transported from secretory cells to the exosomes' target cells exhibit anti-inflammatory traits[31].Exosomes can stimulate regulatory cytokines, decrease the production of inflammatory cytokines, and prevent inflammation[32].Impeding NK cells, CD4+ and CD8+ T cells can occur with MSC-exosomes[33].They induce T cell IL-7 expression and stimulate the expression of IL-10 by regulatory cells, which are implicated in the suppression of inflammation.Furthermore, MSC-exosomes, by secreting transforming growth factor β (TGF-β) prevent CD4+ and CD8+ T cell differentiation and suppress inflammationin vivo[34].MSC-exosomes treatment inhibits the activation and proliferation of NK cells[35].MSC-exosomes engage in prohibiting pro-inflammatory states by shifting M1 macrophages to M2 phenotypes[36].Moreover, MSC-exosomes prevent the secretion of pro-inflammatory factors including IL-17, interferon (IFN)-γ, IL-1β, TNF-α, and IL-6[37], and stimulate the secretion of anti-inflammatory factors including TGF-β, IL-4, and IL-10[38].Also, their function decreases the serum chemokine level[39].The immunoregulatory features of MSC-exosomes are associated with their anti-inflammatory components, including PD-L1, HLA-G, Galectin-1, and IDO[40-42].Furthermore, MSC-exosomes, by escalating ATP levels in alveolar type II cells, increase their survivability[43].In addition, exosomes possess adhesion molecules which accurately guide them to the damaged site.The exosome components then cross the blood-brain barrier.They are low-cost and do not undergo independent self-renewal processes.Thus, they impede serious consequences involving tumor development and other adversities.
THE ADVANTAGEOUS THERAPEUTIC IMPACT OF STEM CELL-DERIVED SECRETOME
The therapeutic effects of MSC-derived EVs on lung and heart injuries have been demonstrated.There are also studies on the impact of MSC-EVs on hemagglutination of swine, avian, and human influenza viruses[23].In addition, MSC-exosomes reduced mortality in H7N9 patients with no concomitant toxic complications during the followup period[44].One study revealed that S proteins within exosomes can be considered a novel vaccine for countering SARS-CoV infections[45].In a test of S-containing exosomes immunogenicity in mice, the results showed amplified titers of neutralizing antibody.Moreover, with regard to economics, MSC-exosomes therapy was a less expensive treatment than maintaining and extending individualized clonal cell populations[46].In the following section, we provide a general review of the present findings on stem-cell extracted secretomes in preclinical studies for lung and heart injuries (the organs most damaged by SARS-CoV-2).
STEM CELL-DERIVED SECRETOME IN THE PATHOGENESIS OF ORGANS
EVs extracted from MSCs have been shown to have an impressive impact on ARDS and acute lung injury.This is the result of the immunoregulatory and anti-inflammatory features of MSC-EVs[24], which induces shrinkage of the permeability of the endothelium and epithelium of alveoli[25], enhancing alveolar fluid clearance[43], macrophage phagocytosis improvement[47], and direct mitochondrial transfer with host cells promoting tissue repair.
Secretome in the blood has impressive stability, subsequent to MSC intravenous administration, and reaches the lungsviablood flow.It is then distributed in the tissues and promotes bacterial clearance, resolution of inflammation, enhances immune regulation, and preserves capillary barrier function[19].The soluble components of MSCs inhibit inflammation, and EVs induce tissue repair.EVs secreted from MSCs, in particular, lung injuries, provide metabolites, DNA, miRNA, mRNA, and proteins to cells thus enhancing lung repair, and restoring and regenerating lung function[48].
Lung accumulation of MSCs occurs after systematic administration.Following secretion of their components, they enhance the pulmonary microenvironment, preserve the epithelial cells of alveoli, inhibit pulmonary fibrosis, and strengthen lung function[13].Furthermore, distant affected organs (for example, the cardiovascular system) can take advantage of MSCs due to the secretory characteristics of these cells.Various studies have focused on the circulation of the cellular cargo, and preclinical trials revealed their capacity to manipulate diverse pathways to promote cellular communication.miRNA (a composition of exosomes) has been demonstrated to have a significant role in physiological functions, including immune modulation, development, epigenetic modifications, and so on[49].The manufacture and isolation of EVs could be a beneficial therapy in pulmonary injuries[50].
An experimental mouse model of neonatal hyperoxia showed that MSCs from human bone marrow and Wharton's jelly inhibited lung fibrosis, enhanced pulmonary vascular remodeling, and stimulated lung development in bronchopulmonary dysplasia.MSC-derived exosomes exhibited anti-inflammatory activity and altered the pro-inflammatory M1 pulmonary macrophages to anti-inflammatory M2 macrophages followed by inhibition of lung inflammation and the immune response facilitating organ development[51].Exosomes derived from MSCs have demonstrated mitigating effects in an asthma and ARDS model of lung damage[52].The potential role of exosomes in alveoli fluid clearance was identified during anex vivoexperiment involving human donor lung (not suitable for transplantation) perfusion.This was assisted by exosome CD-44 which was involved in the internalization mechanism in damaged host cells[53].In addition, exosomes extracted from MSCs have been indicated in the direct inhibition of viral multiplication.
Overall, exosomes extracted from MSCs show promising effects on decreasing pulmonary edema and protein permeability, reversing lung inflammation, the proliferation of lung epithelium, and the polarization of lung macrophages[54].Additionally, MSC-derived exosomes are effective in the treatment of cardiovascular[55] and renal disease[56].
The therapeutic impact of MSC-EVs on acute myocardial infarction has been reported.This has been shown to involve the following underlying mechanisms: Reduction of the inflammatory response[29], reduction of cardiac fibrosis, mitigation of cardiomyocyte apoptosis[57], induction of angiogenesis[58] and promotion of cardiomyocyte autophagy[59].It was also shown that, fibroblast growth factor, composed of MSC-secretome (derived from adipose tissue), inhibited viral replication processes[60].
STEM CELL-DERIVED EXOSOMES; A NANO-PLATFORM FOR COMBATING COVID-19
COVID-19 patients may develop multiorgan damage.In the initial phases of infection, mainly pneumocyte type II cells are infected, and other target cells may be bronchial cells, monocytes, macrophages, and enteric cells.Moreover, the principal SARS-CoV-2 cardiovascular complication is acute myocardial injury[61].Heart tissue biopsies from COVID-19 patients revealed mononuclear inflammatory infiltration, more commonly found in cardiomyocyte necrosis sites[62].Applying stem cell-derived secretome to organs damaged by COVID-19 is possible, according to extracted data.Also, the survival rate of septic mice increased following MSC-derived exosome treatment[63].With regard to MSC-derived exosomes as supportive therapy in the current pandemic, this can be beneficial in inhibiting the effects of COVID-19 [42] and healing organ damage.
Experimental studies on the biological activity of MSC secretomes have demonstrated the possibility of applying MSC-derived secretomes for seriously ill COVID-19 patients as a cell-free therapy.EVs and proteins contained in MSCs affect endogenous stem and progenitor lung cells.They promote cell differentiation and proliferation, inhibit the inflammatory response, prevent apoptosis, reduce fibrosis, and recover capillary barrier function.Due to their similarity to parental MSCs, they are also effective in the management of chronic and acute lung injuries[64].Exosomes (ExoFloTM) derived from MSCs of allogeneic bone marrow have been proposed as a treatment for seriously ill COVID-19 patients according to the first prospective nonrandomized open-cohort study conducted.ExoFlo is considered to be a hopeful therapeutic candidate for COVID-19 due to its capacity to restore oxygenation, immunity reconstitution, safety traits, and downregulation of the cytokine storm[10].This study included twenty-four patients suffering from ARDS with severe and moderate-to-severe symptoms.Exoflo was administered intravenously in a single dose, with no harmful effects identified 72 h after administration.The study showed an 83% survival rate, cytokine storm downregulation, substantial hypoxia recovery, and immune restoration.Exosome-involved COVID-19 clinical trials are ongoing in the United States, China, Turkey, and Russia (NCT04276987, NCT04493242, NCT04491240, ChiCTR2000030484, ChiCTR2000030261, NCT04384445, NCT04389385).
The advantage of MSC-derived secretome therapy is its two forms (inhalable and injectable formulation) for potential clinical applications[9].Both forms exist as freezedried powder and can be used in patients with a severe COVID-19 lung infection.An examination of the inhalable secretome form for COVID-19 pneumonia was conducted in a clinical trial (NCT04276987) in China, and its tolerance was examined in healthy individuals (NCT04313647)[8].Assessment of its therapeutic efficacy demands further randomized controlled trials with comprehensive delivery of the exosomes.Moreover, in addition to MSC-derived secretomes, the secretome of oral tissue stem cells is also considered to have a therapeutic impact in infected cases due to their immunoregulatory and anti-inflammatory characteristics.Non-invasive therapy is superior to invasive therapy in prophylaxis and results in minimum risk of the treatment process, and prevents COVID-19, offering a novel immunoregulatory pathway for COVID-19 therapy[65].
The potential role of exosomes in treating COVID-19 can be classified into three general divisions.First, instead of cell therapy, the exosomes derived from multiple MSCs are utilized.Second, particular mRNAs and miRNAs are incorporated into the exosomes.Third, exosomes could be used as drug carriers in the treatment of COVID-19[66].The efficacy of stem cell-derived secretome therapies is the main focus of continuing clinical trials.Nonetheless, the effectiveness, safety, and long-term consequences of these therapies require further study.
CHALLENGES IN TREATING COVID-19 USING STEM CELL-DERIVED SECRETOME
Administering stem-cell EVs as a possible treatment for COVID-19 is supported by initial examinations.However, for the sake of scientific rationale, further understanding and justification of MSC-EVs and other EV treatment effects on COVID-19 are required.MSCs have demonstrated promising effects on COVID-19 pathogenesis.However, their resemblance to exosomes is uncertain.Regulation of the immune response is the main impact of MSC-EVs, rather than impeding it (Figure 2).By regulating the response, they moderate it.They also strengthen tolerance and improve homeostasis[67].Although stimulating tolerance in graft-vs-host and other noninfectious diseases is effective, it sometimes has an adverse effect on replicating pathogens.Albeit in selected models,Escherichia coliand influenza infection did not escalate but other replicating bacteria and viruses can experience augmented uncontrollable infection due to tolerance stimulation[68].
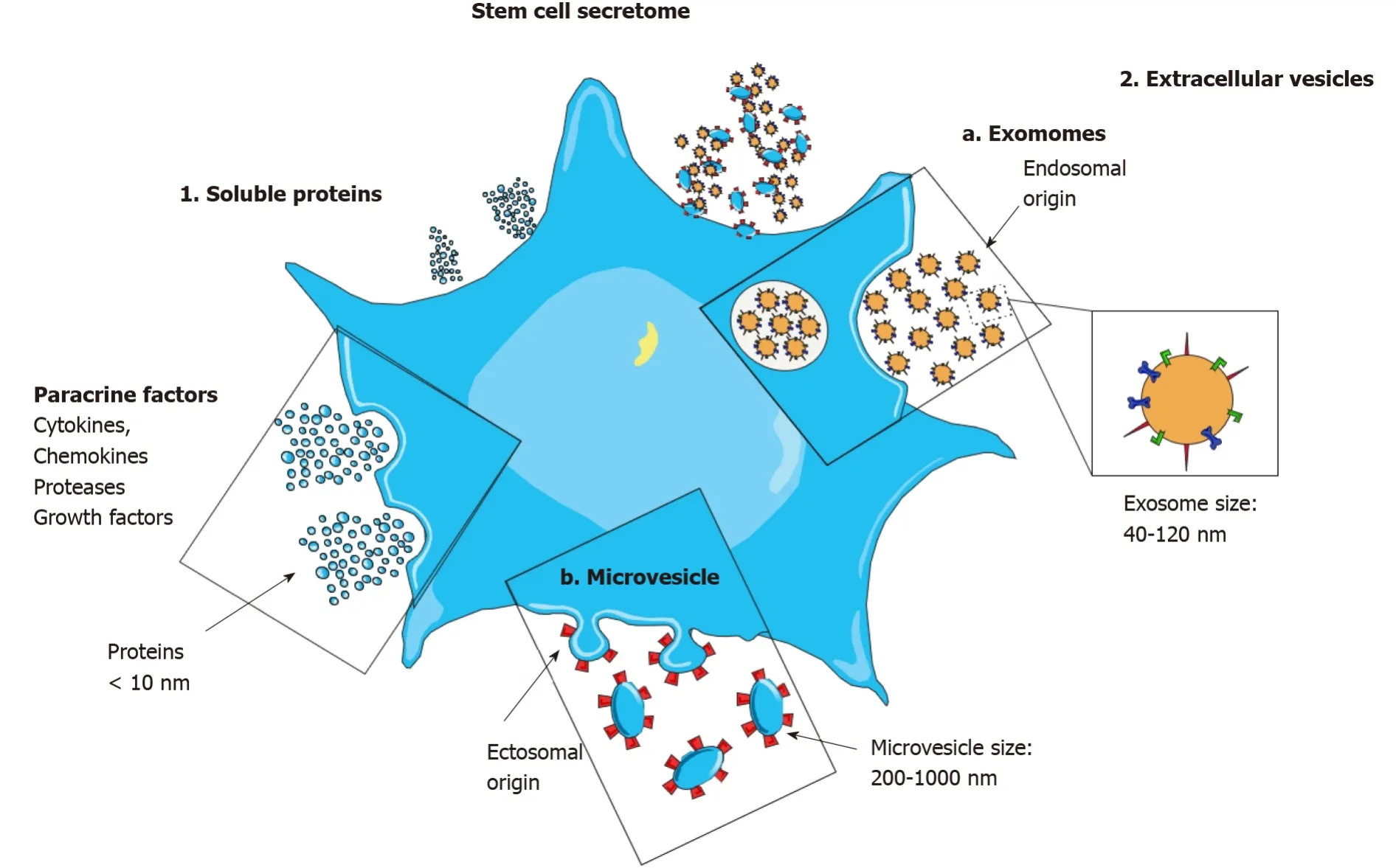
Figure 2 Immunomodulation by the stem cell secretome.
Prior to MSC-EVs application in COVID-19 patients, particular concerns must be resolved.The EV isolation method, purification, and characterization must be determined.These have a meaningful impact on the examination results, and in clinical trials can generate obscure conclusions.These parameters involve the origin of MSC-EVs.MSCs (as a heterogeneous cell essence) are derivable from various tissues.Even derivatives of the same tissue vary in inter-individual and clone-specific functions[69].In fact, comparing four MSC-EV samples harvested from separate donor-derived bone marrow placed in conditioned media revealed substantial cytokine component differences[70].Moreover, in one study MSCs from young individuals (suffering from acute lung injury), but not elderly individuals, attenuated lipopolysaccharide-induced acute lung injury[71].
The problem of EV heterogeneity from dissimilar resources, preparations, and other issues can be solved by manufacturing immortal MSC colonies that can be deliberately examined for potency and production of EVs[72].Besides the problem of derived MSC-EVs from different origins, another consideration is their various responses to different disease conditions.Therefore, it is unclear whether the divergent immune response regulation of exosomes is due to tissue specificity.Regardless of the immunoregulatory properties, MSC-EVs seem to influence additional biological mechanisms with therapeutic functions[73] and other probable unanticipated effects.Recently conducted studies demonstrated that adipose-derived MSC-EVs had higher thrombogenic traits than bone marrow-derived MSC-EVs[74].Accordingly, the origin of parental cells can potentially result in a higher thrombosis risk.The complement pathway stimulation accompanied by the procoagulant condition in a fraction of serious COVID-19 cases can result in the devastating microvascular injury syndrome[49], and the application of MSC-EVs may be ineffective.
A further hurdle is sustaining their stability and productivity with time[75].Exosomes, from MSCs at -80°C, are viable for an extended period.Nevertheless, exosome clustering appears after storage due to freeze-thaw cycles.Moreover, preservation at low temperature during transportation and handling contributes to translational application impediments[76].
The manufacture of EVs requires living cells that are cultured under GMPcompliant (good manufacturing practice-compliant) processes to conserve safety and quality standards criteria.Hence, EV manufacture resembles the ethical and scientific guidelines used for MSCs.Also, basing any therapy on EVs extracted from MSCs requires the approval of national regulatory agencies to ensure their safety and productivity.The International Society for Cellular and Gene Therapies and the International Society for Extracellular Vesicles decrease the risk of critical side effects by deliberately weighing the probable benefits and risks of MSC-EVs for COVID-19 and have already provided preclinical data in connection with animal models and relevant MSC clinical trial-derived data.Additionally, they urge deliberate EV use evaluation by rational clinical trial design, applying well-characterized EV preparations generated according to strict GMP conditions and under proper regulatory oversight[77].
CONCLUSION
MSC-derived secretome demonstrates beneficial results as a cell-free therapy for acute and chronic lung diseases.It exhibits immunoregulatory, pro-angiogenic, anti-inflammatory, regenerative, and anti-protease characteristics.Due to the prominent role of MSC-derived exosomes in inhibiting the inflammatory response and in injured tissue regeneration, they may be a valuable therapeutic option in COVID-19-related pneumonia.Additionally, exosomes are considered potential nanocarriers, biomarkers, and vaccines for COVID-19 treatment.Regarding the concerns on their outcome, it is important to assess the risk when utilizing MSC-exosomes for COVID-19 by determining applicable preclinical findingsin vivomodels.
Upon reaching the lungs, the inhalable administered secretome encounters three main hurdles (anatomical, pathological, and immunological) and exerts their therapeutic effects.Although, the advantages of cell-free therapy are significant, it is considered a novel approach and requires secretome optimization and standardizationviaa comprehensive investigation of its components, dosing conditions, quality control, formulation and preparation process, and long-term storage strategies.Further data on the therapeutic mechanism, new formulation strategies, scalable and GMP-compliant isolation processes, and the capability to convey EVs and soluble proteins through non-invasive pathways of administration are required.These challenges will be groundbreaking, and will provide impressive clinical outcomes in the treatment of acute and chronic lung diseases.
ACKNOWLEDGEMENTS
The authors are thankful to Tabriz University of Medical Sciences, Tabriz, Iran.
杂志排行
World Journal of Stem Cells的其它文章
- Application of mesenchymal stem cells derived from human pluripotent stem cells in regenerative medicine
- Strategies to improve regenerative potential of mesenchymal stem cells
- Dental mesenchymal stromal/stem cells in different microenvironments— implications in regenerative therapy
- Regulating the fate of stem cells for regenerating the intervertebral disc degeneration
- Bone marrow mesenchymal stem cell therapy regulates gut microbiota to improve post-stroke neurological function recovery in rats
- SmartFlareTM is a reliable method for assessing mRNA expression in single neural stem cells
