Strategies to improve regenerative potential of mesenchymal stem cells
2022-01-07MahmoodChoudhery
Mahmood S Choudhery
Mahmood S Choudhery, Department of Biomedical Sciences, King Edward Medical University, Lahore 54000, Punjab, Pakistan
Mahmood S Choudhery, Department of Genetics and Molecular Biology, University of Health Sciences, Lahore 54600, Punjab, Pakistan
Abstract In the last few decades, stem cell-based therapies have gained attention worldwide for various diseases and disorders.Adult stem cells, particularly mesenchymal stem cells (MSCs), are preferred due to their significant regenerative potential in cellular therapies and are currently involved in hundreds of clinical trials.Although MSCs have high self-renewal as well as differentiation potential, such abilities are compromised with “advanced age” and “disease status” of the donor.Similarly, cell-based therapies require high cell number for clinical applications that often require in vitro expansion of cells.It is pertinent to note that aged individuals are the main segment of population for stem cell-based therapies, however; autologous use of stem cells for such patients (aged and diseased) does not seem to give optimal results due to their compromised potential.In vitro expansion to obtain large numbers of cells also negatively affects the regenerative potential of MSCs.It is therefore essential to improve the regenerative potential of stem cells compromised due to “in vitro expansion”, “donor age” and “donor disease status” for their successful autologous use.The current review has been organized to address the age and disease depleted function of resident adult stem cells, and the strategies to improve their potential.To combat the problem of decline in the regenerative potential of cells, this review focuses on the strategies that manipulate the cell environment such as hypoxia, heat shock, caloric restriction and preconditioning with different factors.
Key Words: Hypoxia; Stem cell aging; Growth factors; Heat shock; Caloric restriction
INTRODUCTION
Stem cell-based therapies hold great promise for neurodegenerative diseases, cardiovascular diseases, immunological disorders, skin diseases and cancers.Mesenchymal stem cells (MSCs) are adult stem cells found in many adult (bone marrow, adipose tissue, dental pulp, peripheral blood, menstrual blood) and neonatal tissues (cord blood, cord tissue, Wharton’s jelly, Chorionic villi), have potential for self-renewal and multi-lineage differentiation as well as the capacity to secrete many therapeutic factors with chemoattractive, immunomodulatory, angiogenic and antiapoptotic functions[1,2].Although MSCs originate from the mesoderm, they can differentiate not only into a variety of mesenchymal tissues (such as bone, cartilage, adipose, and haematopoietic tissue) as well as into non-mesodermal tissues (such as glial cells and neurons).MSCs have low immunogenicity, have immunomodulatory and immunoregulatory properties, are easy to isolate and culture.Due to these properties MSCs are considered ideal for replacing damaged or lost cells and tissues in the body and are currently the focus of scientists in hundreds of clinical trials (www.clinicaltrials.gov).
The regenerative potential of MSCs, however; may be compromised with advanced age and disease conditions of the cell donors.Aging is a normal physiological process in living organisms that affects the cells, tissues, and organs of the body.The age of adult resident stem cells is directly proportional to the age of the donor and therefore the functional properties of stem cells severely deteriorate with increasing age of donors.As the stem cells age, their regenerative potential declines as evidenced by the slow healing of wounds in aged individuals[3].It is also pertinent to note that this decline in regenerative potential of stem cells plays a critical role in initiation of number of age-related diseases in old people.With advance age, the ability of stem cells to properly function is compromised leading to cell apoptosis, senescence and complete loss or at least decline in their regenerative potential[4,5].Studies indicate that the therapeutic potential of stem cells significantly declines with an increase in stem cell agein vitroandin vivo[3,6].Similarly, underlying disease conditions of donors also seem to upset stem cell function[7].In addition, number of adult stem cells is very low in their adult niches while stem cell-based therapies often require large number of cells for a potential positive effect.To obtain a high cell number, cells are usually expandedin vitro.Thein vitroexpansion deteriorates stem cell function and does not often give desired results after transplantation[8].Thus, the regenerative potential of cells is significantly compromised when isolated from “old”, “unhealthy” persons and especially within vitroexpansion.
The main segment of the population who can get benefits from regenerative therapies are the aged individuals with diseases[9].However, the autologous use of unhealthy stem cells derived from aged donors does not seem to give the desired results due to their compromised function.The solution to the problem is either to use cells isolated from young donors or rejuvenate the unhealthy cells before use.Autologous use of stem cells is preferred for cell based regenerative therapies and therefore use of stem cells from young donors for transplantation into aged people is not without problems.Autologous use of stem cells for such patients (aged and diseased) does not seem to give the required results due to their age or disease status.This seems a major roadblock for cellular therapies and therefore it is essential to improve the regenerative potential of “aged” and “diseased” stem cells for their successful autologous use.Studies indicate that compromised stem cell function can be reversed using various strategies before clinical use.Previously, many strategies to improve the regenerative potential of stem cells were proposed and described in different studies.In the current review such strategies have been comprehensively described to address major clinical hurdles faced due to the reduced regenerative potential of compromised cells.The review will open new avenue for the stem cell based regenerative therapies for their autologous use in aged and diseased patients.In the current review, age and disease depleted function of resident adult stem cells, and the strategies to improve their potential have been described.To combat the problem of decline in the regenerative potential of cells, we aim to focus on the strategies that manipulate the cell environment such as heat shock, hypoxia, caloric restriction (CR), preconditioning with different factors.
STEM CELL FUNCTION DETERIORATES WITH ADVANCED AGE, DISEASE AND EXTENSIVE IN-VITRO EXPANSION
It has long been known that advanced age is linked with reduced reparative and regenerative potential (Figure 1).With increasing age, the body becomes unable to maintain tissue turnover and homeostasis.It is believed that reduced repair of organs and tissues at the organismal level is due to diminished functional capabilities of tissue resident stem cells[10].Stem cells in the body reside in a special microenvironment called stem cell niches.Stem cells respond to the niche signals either by proliferating, differentiating or by remaining in quiescent state.Such a response ensures that tissues and organs needs are accurately met[9].In aged individuals, this response is significantly delayed taking longer to repair and heal the damaged tissues and organs[11].Stem cells residing in the elderly are affected by the age related changes and thus are not as affective for tissue rejuvenation as are the cells from young donors.In a past study, it was found that the function of stem cells isolated from aged mice was adversely affected[3].Interestingly, this decreased function of stem cells from aged mice was corrected by exposing the old mice to factors present in the serum of healthy young mice.This parabiotic pairing (shared circulatory system) of old and young mice restored the diminished proliferation and differentiation potential of aged cells[10].The general properties of stem cellsi.e., self-renewal and differentiation are significantly decreased with donor age making the aged stem cells less efficient to respond to signals from niches and growth factors.The yield, number of colonies, proliferation as well as differentiation potential of cells isolated from different animal and human tissues was negatively affected by donor age[12-15].In addition, aged stem cells exhibited more senescent (p16, p21, SA-β-gal) and apoptotic (p53, annexin V, caspases) features as well as reduced SOD level, telomeres shortening, high ROS levels and diminished functional ability (wound healing, angiogenesis, migrationetc.)[12-15].These findings of different reports indicate that donor age has negative impact on basic stem cells characteristics and thus adversely affect the regenerative potential of stem cells.
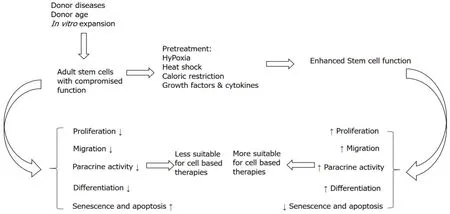
Figure 1 Increased donor age, disease conditions and in vitro expansion of cells reduce stem cell potential, making the cells less suitable for cell-based therapies.
Similar to donor age, various diseases of donors particularly the age-related diseases such as diabetes and heart failure also make the cells unhealthy and therefore limit their therapeutic potential.In healthy individuals the stem cell niche is tightly regulated by the combined action of local and systemic factors.In diseased conditions, however; an altered microenvironment changes stem cell properties that result in compromised quality of their use for regenerative therapies.It has been shown that disease conditions of cell donors negatively impact the function of endogenous progenitor cells[16].Diabetes (type I & II) has been shown to lower the number of CD34+KDR+EPCs[17,18].Pérezet al[19] (2018) has comprehensively discussed the diseases that potentially affect stem cell behavior[19].Diseases such as osteoporosis, cardiovascular diseases, diabetes, obesity, hypercholesterolemia, glucocorticoid imbalance, arthritis, cancer and aplastic anemia have been shown to negatively impact a variety of stem cell types[19].Generation of oxidative stress with certain diseases and the resultant compromised stem cell proliferation, differentiation and mobilization are well documented in literature[18-20].Diabetes, for example, negatively regulates stem cell proliferation, differentiation, paracrine activity, SOD activity, chemotactic ability, angiogenesis and heart repair[21].Similarly, stem cells isolated from adipose tissue of obese persons show low yield, impaired migration and angiogenesis[22-24].It has been shown that the effects of disease conditions are similar to those that are portrayed by aged donors.For example, the production of ROS, telomere shortening, reduced expression of telomerase, high expression of apoptotic and senescent markers and resultant reduced repair and regenerative capability are manifested with advanced age and also with onset of certain diseases[25].It is pertinent to mention here that the onset of diseases in aged individuals affects the regenerative potential of stem cells more adversely as compared to diseases in young donors.
High number of stem cells are needed for cell-based therapies to fully appreciate their therapeutic potential for repair of damaged tissues.However, stem cells are found in low numbers in most adult tissues and thereforein vitroexpansion is required to obtain large number of cells.MSCs have high regenerative potential but they are also vulnerable to replicative senescence[26].In prolongedin vitrocultures, stem cells become senescent and undergo deleterious changes such as reduced proliferation and multi-lineage differentiation capability, shortening of telomere length and morphological changes.Studies indicate that passaging of the cells for prolonged times negatively affected their potential applications for tissue engineering and regenerative medicine[27].The passaging of stem cells from “old” and “unhealthy” donors is particularly risky to obtain desired results as these cells already have compromised characteristics as mentioned above.
As stem cells are the basis of tissue engineering and regenerative medicine applications, a reduced regeneration potential of stem cells due to increased donor age, disease condition orin vitroexpansion may compromise the efficacy of autologous cell therapies.Due to medical advancements, life expectancy has been significantly increased that resulted in a substantial increase in the aged population.Similarly, due to unhealthy lifestyles the frequency of occurrence of diseases has also been increased.As a result, stem cell based therapies are becoming more and more popular in recent years.It is therefore important to use different strategies to improve the stem cell function before use in patients to obtain the desired medical improvements.
ENHANCEMENT OF COMPROMISED STEM CELL FUNCTION
With time researchers have adopted different methodologies and protocols in an attempt to enhance compromised stem cell function.These modifications include best source of stem cells, type of serum for culture, cell plating density, glucose concentration, cell delivery method, transplant method, timing and dosages, which have improved some aspects of cell therapy but not up to the optimal level.The limited improvement is due to low numbers or poor survival of the cells after transplantation due to a harsh ischemic environment at the host site[3,28,29].To compensate for the reduced functions of stem cells, researchers were encouraged to investigate novel strategies to improve the compromised stem cell function to maximize the therapeutic effect of stem cells.In this regard significant attention has been given to strategies that manipulate the culture conditions such as hypoxia, heat shock, CR, and preconditioning with different factors.
Hypoxic preconditioning
Oxygen concentration can be adjusted during cell culturing to optimize cell function for cell based regenerative therapies[30].Naturally stem cells reside in niches inside the body where oxygen concentrations are significantly lower as compared to normal oxygen concentrations.Studies indicate that oxygen concentration in different tissues and organs depends on the distance from the capillaries.Oxygen tension in the lungs for example is 20% which lowers to 2% to 9% when entering other organs and tissues.Oxygen concentration in tissues that are important stem cell sources (such as adipose tissue, bone marrow, placenta, cord tissueetc.) is variable and is low as compared to normoxic conditions (Table 1).For example, it is 2%-10% in adipose tissue[31] and 1%-6% in bone marrow[32,33].So, although stem cells reside in anatomical sites that are relatively oxygen deficient, conventionally they are culturedin vitrounder normoxic conditions (20%-21%) in CO2incubators regardless of their source and oxygen concentration in the tissues from where they are isolated.So hypoxic physiological niches in which most type of stem cells normally reside are largely ignored which may make the cells unhappy and unhealthy.
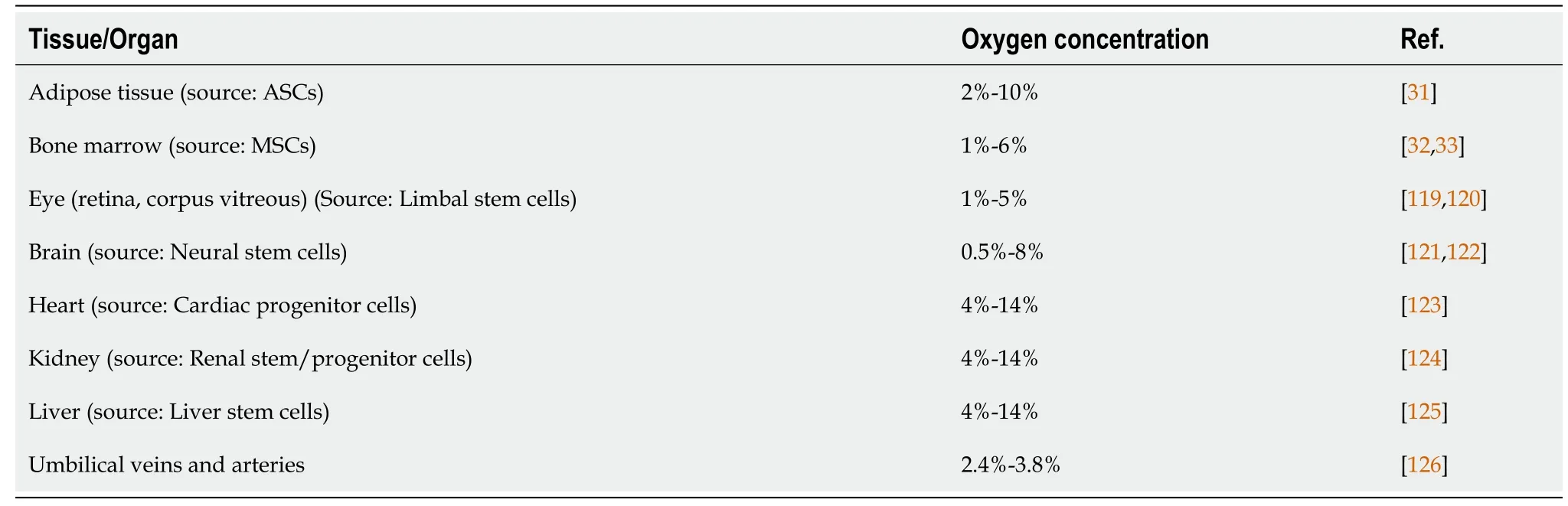
Table 1 Oxygen concentrations in various stem cells niches
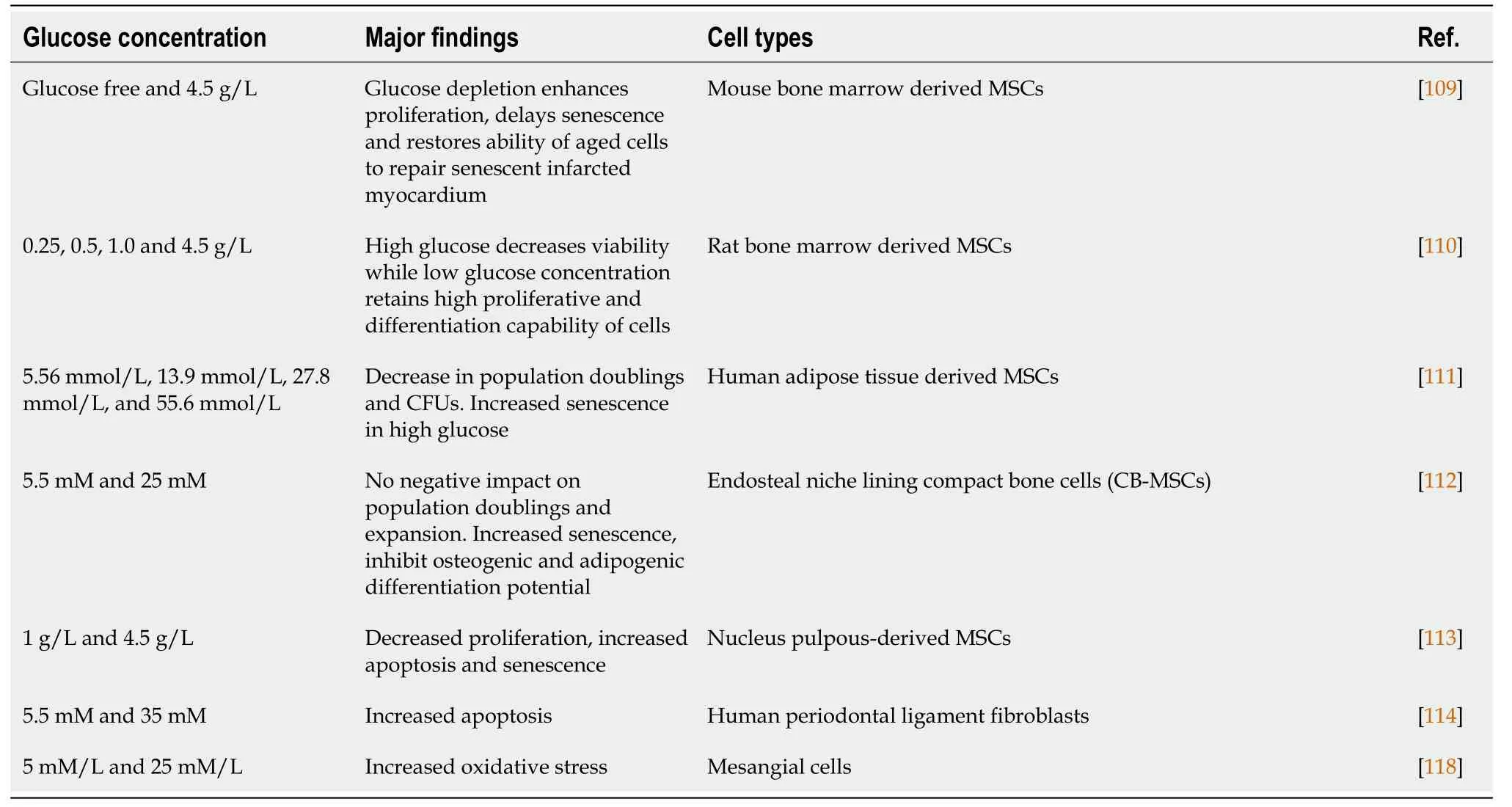
Table 2 Effect of glucose concentrations on cells
Being an important component of the stem cell microenvironment, oxygen tension provides signals for maintenance of stem cell properties[34].Studies indicate that the cells may grow better if the samein vivooxygen concentrations are provided to them forin vitroculturing.Stem cell culturing under hypoxia is physiologically more relevant to their niche and thus can affect the regenerative potential of cells.Culturing the cells under hypoxic conditions may improve their regenerative potential in terms of their improved proliferation, differentiation, adhesion, angiogenesis and growth factor secretion.
There is a clear consensus on the fact that hypoxia promotes the proliferative potential of cells.It has been shown that hypoxic insult significantly improves survival, stemness and proliferation of MSCs derived from adipose tissue[35] and bone marrow derived stem cells[36,37].Proliferative potential of MSCs was significantly higher in hypoxic culture condition as compared to normoxic conditions[38] in long term cultures.Oxygen concentrations of 1%-5% has been demonstrated to significantly increase the proliferation of MSCs while maintaining their normal morphology[36,37].Similarly, the proliferative potential of BM-MSCs was significantly enhanced under hypoxia[39].In this study, 1% hypoxia significantly enhanced the proliferative potential of BM-MSCs.Collectively, these studies indicate that hypoxic insult increases the self-renewal potential of stem cells.Some studies however indicate that initially hypoxia has a negative affect on cell viability and proliferation, however, reoxygenation following hypoxia promotes these processes[40].
Low oxygen concentrations also help maintenance of stemness characteristics of cells.In periodontal ligament cells[41], adipose tissue MSCs[42] and dental pulp cells[41], 2% hypoxia maintained the cell stemness for prolonged periods of time.Under 24-h hypoxic conditions mRNA expression of pluripotency markers Oct-4, Sox-2 and c-Myc upregulated significantly concomitant with increased protein expression of these markers[41].
The effect of hypoxia on differentiation of stem cells has also been investigated by number of researchers with conflicting reports and therefore the role of hypoxia in the differentiation of stem cells remain controversial.Regarding differentiation of stem cells into adipocytes, culturing the cells under hypoxic conditions seems to inhibit it[39].Carrièreet al[43] (2004), reported decreased adipocyte differentiation of 3T3-F442A preadipocytes in 1% hypoxia[43].Similarly, Hunget al[39], 2012 observed compromised adipogenic potential of bone marrow derived MSCs when hypoxia was applied for 4 wk[39].In another study, it has been demonstrated that hypoxia negatively regulates the differentiation of ASCs.The authors demonstrated that hypoxia reversibly arrested ASCs in an undifferentiated state and maintains the expression of pre-adipocyte factor 1 (Pref-1) that has been shown to negatively regulate adipogenic differentiation[44].Contrary to these findings an extreme hypoxia (0.2%) induced more adipogenic differentiation that resulted in more lipid droplets accumulation and upregulation of adipocyte specific genes such as LPL, CFD, PGAR and HIG2[45].Under severe hypoxia, significantly lower adipogenic differentiation was observed as compared to differentiation of BM-MSCs in normoxic conditions[46].However, as indicated in another report, hypoxic preconditioning (2% oxygen) of adipose tissue derived MSCs induces more adipocyte differentiation[47].
Hypoxia however favors differentiation of MSCs into osteocytes.Studies indicate that hypoxia promotes osteogenic differentiation of MSCs[39].In another report hypoxia positively regulated osteogenesis of MSCs derived from rat bone marrow.In this study, hypoxic preconditioned rat derived MSCs produced more bone when implanted into rats[48].Moreover, Tsaiet al[49], (2011) demonstrated that culturing of cells under hypoxic conditions significantly promoted their osteogenesis and chondrogenesisin vitroand their bone repair abilityin vivo[49].Similarly, in a number of studies 1% to 5% oxygen enhanced the chondrogenic differentiation of ASCs[50-53].Interestingly, Jurgenset al[53], 2012 found that hypoxia can promote differentiation of cells into chondrocytes to the same extent as transforming growth factor-b1[53] and enhance the expression of hypoxia inducible transcription factor-2a, SOX5, SOX6, and SOX9, and that of aggrecan, versican, and collagens II, IX, X, and XI[54].Contrary to these results D’Ippolitoet al[55] (2006) reported reduced osteogenic commitment of human bone marrow derived MSCs when cultured and differentiated under hypoxic conditions[55].These interesting findings indicate that hypoxic effect may be cell source and species specific.Chenet al[56], 2015 set the hypoxic conditions at 0.2% and found that this extreme hypoxia can impair the osteogenic differentiation as indicated by the attenuation of alkaline phosphatase (ALP) activity and the reduced expression of osteogenic markers osteocalcin and osteopontin[56].
The key regulators that alter the cellular and molecular functions of stem cells during hypoxia are reactive oxygen species, HIF-1a and micro RNAs.The electrontransport chain within the mitochondria is the major source of ROS production in the cells.Although accumulation of high ROS levels in the cells may cause adverse effects in terms of genetic and physiological dysfunction, and induction of senescence and apoptosis[57-59], low ROS levels function as signaling molecule and positively affect cell characteristics by serving as second messengers, triggering the phosphorylation of signaling molecules[60,61] such as tyrosine kinase.Activation of tyrosine kinases leads to the activation of the PI3K/Akt and MAPK signaling pathways that also can alter stem cells characteristics.Different microRNAs such as miR-210 have been found to consistently induced during hypoxia.miRNA-210 is regulated by HIF-1a and ROSrelated pathways during hypoxia[62].HIF-1a is a master transcription factor that regulates many genes involved in the differentiation of cells.It becomes activated during hypoxia and directly binds with the HIF-responsive element (HRE) to alter stem cell functions.
In conclusion, hypoxia has a profound impact on the biological and functional properties of stem cells and could be used as a strategy to improve their regenerative potential before clinical use (Figure 1).Hypoxia not only enhances the self-renewal potential of cells but also their differentiation into multiple cell types.However, it must be noted that the inconsistent or controversial reports in the literature are probably due to the use of different hypoxia levels, variable durations of exposures and a variety of cell types.The question is not if hypoxia alters stem cell function but rather the use of the correct hypoxic preconditioning for different cell types for an accurate period of time that is most important.In addition, it is important to note that previous studies have often been performed using H2O2for short time periods.However, development of sophisticated trigas CO2incubators now provide a more refined way of culturing the cells under hypoxia for long periods of time (Figure 2).
Heat shock
Hormesis is a phenomenon in which low doses of a harmful stressor produce a cascade of beneficial biological effects.Temperature is one such stressor that has recently been used to manipulate the cell functionality.The hormetic effect of high and low temperature for a short period of time has been shown to effectin vivo-as well asin vitro-age-related dysfunction in cells.Temperatures below and above the standard culture temperature (32 ℃ and 41 ℃) have been shown to prevent or reverse aging and age related impairment, and significantly impact the regenerative potential of cells.Adult stem cells exhibit therapeutic potential for regenerative medicine and tissue engineering applications.However, age related changes may make these cells less effective for medical use to treat various diseases and disorders (Figure 1).Similarly,in vitroexpansion of adult cells negatively affects the regenerative potential of cells as indicated by a decline in adipogenic, osteogenic, chondrogenic and myogenic differentiation potential of MSCs within vitropassaging (Figure 1).Adult stem cells are found in low numbers in their niche but are required in large number for clinical use and therefore many promising tissue engineering and regenerative medicine applications require expansion to obtain large numbers of cells.The expansion of cells results in increased senescence and apoptosis, and reduced regenerative potential representing a severe limitation for their use.Expansion of cell at high or low temperatures can significantly enhance the regenerative potential of stem cells and thus could be used as a strategy to enhance their potential.
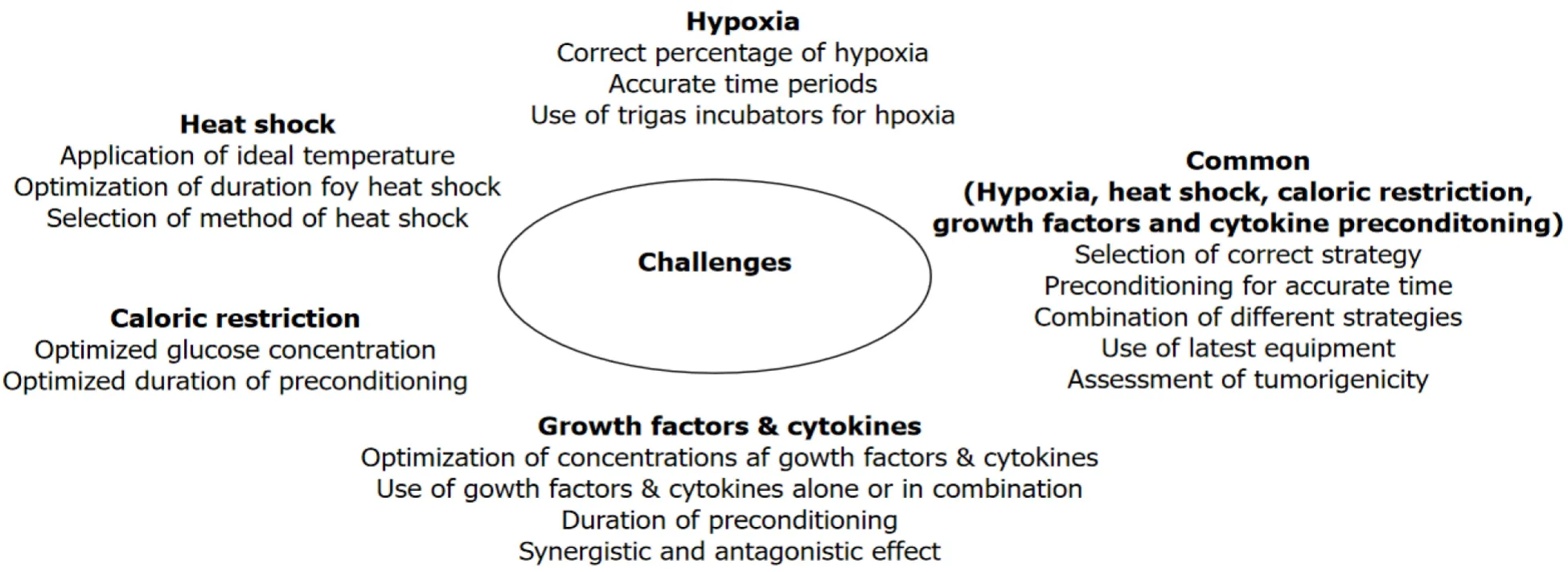
Figure 2 Challenges and limitations in using preconditioning strategies such as hypoxia, heat shock, caloric restriction and growth factor & cytokine.
The anti-aging effect of heat shock treatment has been well documented in a series of studies with interesting results.Heat shock treatment has been found to maintain the long, spindle shaped morphology of MSCs by preventing or reducing age-related alterations such as the irregularly enlarged and flattened shape of cells[63,64].Similar results were obtained by Choudheryet al[65], (2015) in a study in which the stressed cells (HS at 41 ℃) exhibited more thin, long and spindle shaped morphology of MSCs as compared to control cells that had more flattened morphology (a typical age-related alteration)[65].Heat shock also enhanced viability of cells at different passages during expansion of cells.There were significantly more viable cells at passage 5 and passage 8 when a mild heat shock was applied as compared to non-treated cells[65].In this study, the percentage viability as determined by the trypan blue exclusion assay as well as flow cytometry using 7-AAD/Annexin V was significantly higher at different passages[65].
A significant increase in the proliferative potential of cells was observed when cells were treated with mild heat shock.The number of cumulative population doublings were increased 10% to 15% as a result of heat shock treatment for a short period of time[64].In another study, the maximum population doublings were higher for cells that underwent heat shock at 41 ℃ for 60 min once in a week.The cells that were treated with heat shock achieved 36.0 ± 3.4 doublings while the cells in control group achieved only 26.2 ± 1.1 doublings.The doubling time was also shorter for heat shocked MSCs (2.1 ± 0.2 d) as compared to those that were not treated with heat shock (3.2 ± 0.2 d)[65].Self-renewal is a complex regulatory process under the control of various transcription factors such as Nanog, Oct4, Sox2, STAT3 and others[66].These transcription factors work in collaboration to regulate self-renewal of cells.Interestingly, the heat-shock proteins expressed as a result of stress (e.g.heat shock stress) interact with these transcription factors to regulate normal cell development and functioning[67].HSP90, HSP70 and HSP27 are also particularly involved in cell self-renewal[68].
The anti-aging effect of repeated mild heat stress on cell growth and other cellular and biochemical characteristics has been well documented[63].In another study, heat shock alleviated apoptosis in BMSCs and improved survival[69].The protective effects of heat shock in this study were attributed to elevated levels of heat shock proteins HSP70 and HSP90 along with attenuation of autophagy.Heat shock has been shown to enhance the survival of transplanted cells concomitant with reduced apoptosis and senescence[65,70].After heat shock treatment, the expression of senescent associated markers such as β-galactosidase, P16 and P21 were significantly downregulated in cultures of cells that were subjected to heat shock[65].Fenget al[71], (2010) explored the cytoprotective effects of HSP90 on rat MSCs.In this study apoptosis was induced with hypoxia and serum deprivation, and heat shock improved viability, paracrine effect and elevated Bcl-2/Bax and Bcl-xL/Bax expression in MSCs[71].
It is pertinent to note that differentiation of MSCs into various lineages was also elevated after heat shock treatment.MSCs, under exposure to heat shock produced more extracellular matrix (that stained black with von Kossa staining) as compared to non-heat-shocked MSCs.The expression of lineage-specific osteogenic genes such as ALP, osterix, ostepontin, bone morphogenetic protein 2 (BMP2) and osteocalcin as assessed with RT-PCR was also upregulated in heat-shocked MSCs[56,65].Adipogenic induced MSCs cultures that were exposed to repeat heat shock showed more oil red O uptake and expression of markers of adipogenesis such as peroxisome proliferatoractivated-receptor-g (PPAR-g) and lipoprotein lipase (LPL)[65].Similarly, in pellet culture a periodic heat shock enhanced the chondrogenic differentiation of human MSCs as depicted by increased sulfated glycosaminoglycan and increased expression of collagen type II and aggrecan in heat-shocked pellets than non-heat-shocked cell pellets[57].Besides the above-mentioned effects, the novel effects of heat shock have been explored onin vitrowound healing[72], angiogenesis[73], neuroprotection and neurodegeneration[74].Furthermore, heat shock treatment seems to be an effective way to protect the cells even after transplantation.Recently it has been shown that mild heat stress significantly enhanced the viability concomitant with reduced apoptosis and senescence of transplanted cells[65,70].Chenet al[75], (2018) demonstrated that heat stressed bone marrow derived MSCs inhibited apoptosis of ovarian granulosa cells and enhanced their repair effect when transplanted in a chemotherapy induced rat model.In this study, the chemotherapy-induced rat model was established by intraperitoneal injection of cyclophosphamide by giving an initial dose of 50 mg/kg followed by a dose of 8 mg/kg for 14 d[75].
Heat shock response is an evolutionary conserved genetic response to various physiological, pathological, chemical and environmental stresses[76].This response of heat shock (and other stressors) leads to the induction of special type of proteins in cells called heat shock proteins (HSPs).HSPs may function as molecular chaperones and can help in stabilization of intracellular proteins, repairing damaged proteins, and assisting in protein translocation[68,77-80].Studies indicate that HSPs can interact with various transcription factors and thus are involved in various cell signaling pathways.Therefore, alterations in the expression of HSPs directly affect stem cell characteristics such as their proliferation capacity as well as differentiation and aging.
In conclusion, it is clear that hormetic effects of mild heat shock can affect the regenerative potential of adult stem cellsin vitroand these effects help in better performance of these cells after transplantation (Figure 1).However, applying the correct hormetic conditions for stem cells from different sources is challenging.The temperature as well as the duration of heat shock treatment is important for optimal results.In addition, it is also important to select a method of application of heat shock in cell cultures.Instead of incubators, water baths may be more useful for this purpose for quick heat transfer.
GROWTH FACTORS AND CYTOKINES
The use of growth factors and cytokine preconditioning (Figure 1) can also influence the therapeutic potential of stem cells by improving self-renewal, cell survival, paracrine activity and differentiation potential concomitant with reduced senescence and apoptosis[81,82].The growth factors interact with the receptors present on the cells and activate various downstream signaling pathways to influence numerous cell characteristics.Stem cells particularly MSCs release a number of growth factors and cytokines that influence the cells and tissues in an autocrine or paracrine manner.The half-life of these growth factors, however; is very short and therefore their stable therapeutic effects are limited.
BM-MSCs when preconditioned with stromal derived factor 1 showed enhanced survival, proliferation, migration, secretion of pro-survival genes (AKT-1, BCL-2, Erk) and pro-angiogenic factors (bFGF, VEGF) concomitant with reduced apoptosis and senescence[83].In another study, BM-MSCs were treated with 0.05 μg/mL of SDF-1 that enhanced cell survival, engraftment and vascular density and suppressed apoptosis.Further, injection of the SDF-1 preconditioned MSCs in a rat model of left anterior descending artery ligation also improved myocardial function by increasing cell proliferation and reducing infarct size and fibrosisviaSDF/CXCR4 signaling[84].Preconditioning of BM-MSCs with 10 ng/mL to 100 ng/mL of SDF-1 also reduced hypoxia induced apoptosis[85].TGF-Beta inhibits differentiation of BM-MSCs into adipocytes and osteocytes.Interestingly, however, the same growth factor promotes osteogenesis in the presence of IBMX (usually present in adipogenic differentiation medium).TGF-β1 is a potent stimulator of tissue regeneration[86] and it can switch adipogenic differentiation into osteogenic differentiation.Pretreatment of MSCs with TGF-β1 improves wound healing in a murine wound model by adhesion and migration to the wound site[87].Further, TGF-β1 enhanced fibronectin production as well as survival of human umbilical cord-derived MSCs in a rat model of lipopolysaccharide-induced acute lung injury[88].However, a previous study demonstrated that TGF-β1 induces senescence through production of ROS in periodontal ligament stem cells[89].A 3 d preconditioning of AT-MSCs with tumor necrosis factor-alpha (TNF-α) significantly promoted proliferation, mobilization and differentiation into osteocytesviaactivation of ERK1/2 and MAPK signaling pathways.These results were confirmed by gene silencing with siRNA that partially inhibited ERK1/2 signaling and osteogenic differentiation of MSCs[90].TNF-α preconditioning has been shown to improvein vitrobone regeneration by up-regulating BMP2.Further, it stimulated the cell proliferation and differentiation[91].IFN-γ pretreatment improved the therapeutic efficacy of MSCs by enhancing the secretion of immunomodulatory molecules such as PGE2, HGF, TGF-β, and MCP-1[92].MSCs pretreated with IFN-γ inhibited natural killer cell activation and NK mediated cytotoxicity by upregulating the synthesis of indoleamine 2,3-dioxygenase (IDO) and prostaglandin E2[93].In another study MSCs were pre-stimulated with IFN-γ to enhance their immunosuppressive and therapeutic propertiesin vitroandin vivo[94].A combination of different growth factors may produce contrary results.For example, a combination of interleukin (IL)-1 and TNF-α inin vitrocultures of MSCs inhibited the osteogenesis and adipocyteviaactivating the canonical nuclear factor-kappa B (NF-kB) signaling[95].Similarly, when cells were treated with a combination of bFGF and steroid hormones an enhanced neural differentiation was observed as indicated by upregulation of beta III-tubulin (β-III tubulin) and microtubule-associated proteins-2 (MAP-2) during 4 d of treatment[96].
Certain cytokines have also been shown to influence the regenerative potential of stem cells.IL-1β preconditioning of MSCs activated several biological processes such as cell survival, cell migration, cell adhesion, chemokine production, angiogenesis and modulation of the immune response[96].More specifically MSC preconditioning with IL-1β significantly upregulated the expression of certain cytokines (TNF-α, IL-6, IL-8 and IL-23A), chemokines (CCL5, CCL20, CXCL1, CXCL3, CXCL5, CXCL6, CXCL10 and CXCL11) and adhesion molecules [vascular cell adhesion molecule (VCAM)-1, intercellular adhesion molecule (ICAM)-1 and ICAM-4][96].In another study, synovial MSCs when pretreated with IL-1β, showed significantly higher proliferation as well as chondrogenic potential[97].To induce these results, TGF-β seemed to activate Akt, extracellular signal-regulated kinase 1/2 (ERK1/2), focal adhesion kinase (FAK), and p38,viaTGF-β type I receptor in MSCs[97].Xinariset al[98], preconditioned MSCs with insulin-like growth factor-1 (IGF-1) before administration and found it effective in terms of migration and homing of cells which was required for the restoration of renal function following acute kidney injury[98].Interestingly when the diabetic MSCs were preconditioned with a combination of IGF-1 (50 ng/mL) and fibroblast growth factor-2 (FGF-2) (50 ng/mL), upregulation of IGF-1, FGF-2, Akt, GATA-4, Nkx 2.5 and downregulation of p16INK4a, p66shc, p53, Bax and Bak occurred[99].
In conclusion, preconditioning of cells with different growth factors and cytokines may enhance regenerative potential of stem cells (Figure 1).Although preconditioning of MSCs with different growth factors and cytokines can influence significantly the biological properties of MSCs, there are number of challenges to use this strategy successfully for optimum benefits.For example, will the same dose or concentration of cytokines and growth factors influence MSCs isolated from different sources? Some growth factors and cytokines may influence MSC function synergistically and antagonistically when used in combination[40].Therefore, optimization of amalgamation of growth factors and cytokines as well as their concentrations is required for better results.Similarly, MSCs behave differently in culture conditions such as in 3D cultures and hypoxic conditions and therefore preconditioning in such conditions should be optimized (Figure 2).
CR
CR refers to consuming significantly reduced calories as compared to calories taken ad libitum.At the organismal level, it was first reported in 1935 that reduced caloric intake can extend the mean and maximum life span in rodents[100].Since then beneficial effects of CR were observed in animals of other species such as rats, mice, dogs, fish, flies, worms, yeast and humans[101-103].CR is now an established antiaging strategy for prolonging lifespan and has also been applied on stem cells to rejuvenate them.CR as a non-genetic dietary intervention reduces the energy metabolism in cells and can positively affect regenerative potential of cells by extending their life span and making the cells healthy.
Glucose is an essential source of energy for all types of cells in the body although elevated levels of glucose have been shown to be associated with reduced mobilization, proliferation, homing and repair potential[104,105].Similarly, stem cells isolated from diabetic patients and animals exhibited reduced yield, viability, proliferation, angiogenesis, differentiation and wound healing ability[106,107].Cells are cultured in stem cell media that contain various components including glucose to ensure proper functioning and maintenance of cell characteristics.However, cells culturedin vitroin media with high glucose concentration show impaired regenerative potential of cells[108].High glucose concentration in stem cell culture media was found to negatively impact a cell’s viability, differentiation and self-renewal potential[109,110].Based on the findings it was found that the conventional media used to expand cells was not appropriate for long term expansion of cells as it adversely impacted the biological properties of cells[110,111].Thus induction of CR in cells by culturing in low glucose concentration is another area of interest for the enhancement of stem cell function before transplantation.Different protocols ranging from glucose depletion[109] to varying glucose levels[110] were adopted in this regard.
Al-Qarakhliet al[112] comprehensively studied the effect of glucose concentration on expansion as well as differentiation of mesenchymal stromal cells.They found that hyperglycemia negatively impact the proliferation, and osteogenic and adipogenic differentiation of cells with more senescence features in culture[112].To investigate the effect of CR, Stolzinget al[110] (2006) used media with different glucose concentrations for MSC culturing.In this study MSCs cultured in medium with low glucose concentrations were functionally more active as evidenced by enhanced viability, proliferation and differentiation of cells when cultured in caloric restricted media[110].When the biological characteristics of cells cultured in low glucose and` high glucose concentrations were compared, there was significantly more proliferation, colonyforming ability, homing and wound healing potential of cells in low glucose concentrations as compared to high glucose concentration.In addition, high glucose decreased expression of stemness genes (SOX-2, Nanog, Oct-4), survival genes (Sirt-1, Sirt-6, HIF-1α), glucose transporter 1 (Glut-1) concomitant with increases apoptosis and senescence in cells[113].Choudheryet al[109], (2012) cultured the BM-MSCs in glucose free conditioned and optimized the time to perform furtherin vitroand inin vivostudies[109].In this study aged MSCs were pre-conditioned with glucose depletion for 60 min to enhance the age depleted function of stem cells.Preconditioning of aged MSCs with glucose depletion resulted in upregulation of IGF-1, AKT and SIRT-1 concomitant with enhanced viability, proliferation and delayed senescence.Interestingly, the preconditioned aged MSCs after transplantation into heart showed increased expression of paracrine factors (IGF-1, FGF-2, VEGF and SDF-1a) that was associated with significantly improved cardiac performance in mouse model of myocardial infarction[109].High glucose concentrations can impair cell function and induce apoptosis and represent a potential limitation for therapeutic strategies based onex vivoexpansion of stem cells[114].In parallel to these findings some studies suggested a significantly increased apoptosis of β-cells in diabetic patients that resulted in β-cell dysfunction and reduced β-cell mass[115,116].
There are a number of cellular responses to high glucose (Table 2) that ultimately result in functional impairment and cell death[117].High glucose results in generation of reactive oxygen and nitrogen species such as superoxide, nitric oxide and peroxynitrite and their derivatives[117,118].This high glucose induced ROS species results in high glucose-mediated apoptosis and necrosis and ultimately cell death.ROS species produced by high glucose may increase the activity of NF-kB in various cell types and leads to cell apoptosis and death in a process that involves Bax and caspase activation[117].In addition, high glucose concentration in the cell microenvironment activate those proteins that are related to apoptotic cell death including members of the caspase and Bcl-2 families[117].
In conclusion, the biological properties of cells are influenced by the glucose concentration in the culture medium (Figure 1).Previous studies indicate that low glucose concentration in the culture medium enhances cell proliferation, viability and differentiation potential of cells concurrent with reduced senescence and apoptosis.However, not only the glucose concentration but the duration of preconditioning of cells are important parameters to consider.For example, although 1 h preconditioning of MSCs with glucose depletion (0g/L) produced beneficial effects in Choudheryet al[109]’s study[109], culturing of cells without glucose for longer time will definitely produce deleterious effects in cell.Therefore evaluation of the effects of glucose concentrations with respect to time must the carefully considered for preconditioning of different types of cells (Figure 2).
CONCLUSION
Conclusion and future perspectives
Stem cell-based therapies are gaining interest of patients and doctors for their potential to treat diseases that cannot be cured with conventional medicines.Aged patients are the major candidates for stem cell-based therapies.However, studies clearly indicate that stem cell potential for autologous use deteriorates with donor age.The number of regenerative cells in aged and unhealthy individuals is very low, however, for the success of stem cell based regenerative therapies large numbers of cells are required.Cells are usually expandedin vitroto obtain high numbers, however, this expansion further decreases stem cell function and does not give desired results after transplantation.Overall, with increasing donor age, disease condition of donors andin vitroexpansion of cells, regenerative potential of stem cell decreases and it represents a major limitation for the success of cell therapies.To combat the problem of decline in regenerative potential of cells different strategies such as heat shock, hypoxia, caloric restriction and preconditioning with different factors can be appliedin vitrobefore transplantation of cells.The correct application of these strategies have a profound effect on stem cell characteristics to enhance their therapeutic functions.These strategies may be used to enhance the self-renewal, repair and differentiation potential of cells and to keep the cells healthy.Use of these strategies also enhances cell survival and engraftment in hostile microenvironment of the target tissue.The inconsistent reports are due to the use of different levels of factors (hypoxia, glucose, temperature, growth factors & cytokine), variable durations and variety of cell types used in studies.The question is not if these strategies alters stem cell function but rather the use of the correct strategy and condition for an accurate period of time that is most important.
ACKNOWLEDGEMENTS
The author appreciate the critical and helpful comments and suggestions of his colleagues.
杂志排行
World Journal of Stem Cells的其它文章
- Stem cell-derived biofactors fight against coronavirus infection
- Application of mesenchymal stem cells derived from human pluripotent stem cells in regenerative medicine
- Dental mesenchymal stromal/stem cells in different microenvironments— implications in regenerative therapy
- Regulating the fate of stem cells for regenerating the intervertebral disc degeneration
- Bone marrow mesenchymal stem cell therapy regulates gut microbiota to improve post-stroke neurological function recovery in rats
- SmartFlareTM is a reliable method for assessing mRNA expression in single neural stem cells
