Regulating the fate of stem cells for regenerating the intervertebral disc degeneration
2022-01-07SobiaEkramShumailaKhalidAsmatSalimIrfanKhan
Sobia Ekram, Shumaila Khalid, Asmat Salim, Irfan Khan
Sobia Ekram, Shumaila Khalid, Asmat Salim, Irfan Khan, Dr.Panjwani Center for Molecular Medicine and Drug Research, International Center for Chemical and Biological Sciences, University of Karachi, Karachi 75270, Sindh, Pakistan
Abstract Lower back pain is a leading cause of disability and is one of the reasons for the substantial socioeconomic burden.The etiology of intervertebral disc (IVD) degeneration is complicated, and its mechanism is still not completely understood.Factors such as aging, systemic inflammation, biochemical mediators, toxic environmental factors, physical injuries, and genetic factors are involved in the progression of its pathophysiology.Currently, no therapy for restoring degenerated IVD is available except pain management, reduced physical activities, and surgical intervention.Therefore, it is imperative to establish regenerative medicine-based approaches to heal and repair the injured disc, repopulate the cell types to retain water content, synthesize extracellular matrix, and strengthen the disc to restore normal spine flexion.Cellular therapy has gained attention for IVD management as an alternative therapeutic option.In this review, we present an overview of the anatomical and molecular structure and the surrounding pathophysiology of the IVD.Modern therapeutic approaches, including proteins and growth factors, cellular and gene therapy, and cell fate regulators are reviewed.Similarly, small molecules that modulate the fate of stem cells for their differentiation into chondrocytes and notochordal cell types are highlighted.
Key Words: Stem cell; Intervertebral disc; Degeneration; Inflammation; Cell therapy; Gene modification
INTRODUCTION
Intervertebral disc (IVD) degeneration is a progressive, inflammation-driven cascade that leads to structural and mechanical failure, strongly associated with lower back pain (LBP), representing a global health burden.The worst aspect(s) of degenerative disc disease (DDD) is/are pain, discomfort, emotional distress, and functional disability, affecting the quality of life and causing socioeconomic burden[1].Altered cellular microenvironment within the disc, reduced cell viability due to structural failure, and functional inadequacy are the leading causes of the adverse condition in LBP[2,3].IVD degeneration (IVDD) treatments can only mitigate painful symptoms and improve flexibility and body movements[4].
Around 84% of the population experience an event of LBP sooner or later in their life span; 50% of them are younger age group (18 to 44 years), otherwise adulthood (45 to 64-years), and generate almost 80% of health care expenditure[5].Even though the correct etiology of LBP remains obscure[6], IVDD results due to the loss of nucleus pulposus (NP) and/or annulus fibrosus (AF), which leads to the reduction in water content, diminished glycosaminoglycans (GAGs), and extracellular matrix (ECM), and collagen II deterioration in the NP region[7].This remodeling results in reduced IVD height, osteophyte development, facet joint arthritis, and bending of vertebral bodies, which are reflected through magnetic resonance imaging (MRI)[8].Spine fusion is the only available option, but it greatly reduces the flexion of the body.With the disease advancement, pharmaceutical or otherwise postoperative intervention is needed to reduce symptomatic pain and reserve the flexion of the spine[9].Despite the innovations in IVD surgery, patients with the progressive disorder cannot receive the benefits of surgical intervention because of the associated morbidities.
Perinatal stem cells and their derivatives can offer an improved therapeutic approach for the treatment of disc degenerated diseases.Mesenchymal stem cells (MSCs) are being utilized to rectify the pathogenesis of DDD[10].This review presents an overview of IVD biology and how cellular signaling plays a role in IVD homeostasis.We also review the opportunities and challenges for the utilization of cell-based therapy for IVD regeneration.
CELLULAR SIGNAL IN IVD
The development of IVD in embryogenesis relies on the coordinated network of molecular signals arising in the notochord and neural tube plate[11].Following signaling pathways are involved in the IVD.
Sonic hedgehog
Sonic hedgehog (Shh) signaling plays a vital role in tissue morphogenesis, regulation, presenting information about embryonic patterning, and degree of cell fate differentiation and proliferation[12,13].Somite stalks evolve in response to Shh and Wnt (wingless-related integration site) dependent regulatory pathways, while a sclerotome tissue generates only under the activating impact of the Shh pathway[14].A specific attribute of the Shh intracellular signaling cascade works through synergistic interaction with Noggin-cascade, a direct antagonist of the bone morphogenetic proteins (BMPs) pathway in the induction of sclerotome growth[14,15].Noggin molecules are primitively expressed by the notochord cells blocking BMP signaling from developing vertebral bodies till the formation of the AF[16,17].
Paired box genes
Paired box (Pax) genes encode transcription regulators for proliferation, differentiation, apoptosis, and migration of pluripotent cells during embryogenesis.Expression of Pax genes plays an essential role in subsequent cell differentiation of distinct populations of IVD[18-20].It is proved thatPax1andPax9genes are entirely involved in the IVD formation.When these genes are obliterated, IVD and vertebral bodies do not develop, forming an irregular cartilaginous core[21].Pax1gene expression in all sclerotome tissues is intervened by the activity of Shh and Noggin regulatory pathways in the notochord cells[22,23].After IVD development, expression of thePax1gene arises exclusively in the tissue of IVD primordium (precursor of the AF) enclosing the notochord.Hence, thePax1gene impacts the notochord advancement by activating cell expansion which turns into the NP.
SRY-box genes
The SRY-box (Sox) family is involved in developing the vertebral column[24,25].Sox5,Sox6, andSox9genes are of significant importance for IVD development and growth.Sox5 and Sox6 are present in the cells of the notochord and the sclerotome[26].In the mice deprived ofSox5andSox6genes, the development of the notochordal membrane was weakened.This is associated with the evidence that these genes are key players in genesis IVD and intercellular proteins, including collagen II and aggrecan[26,27].Lack of notochordal membrane prompts apoptosis of the notochordal cells (NCs) and disrupts the development of IVD segments.In the cells with knockout Sox9, notochord development starts, which is degraded due to the deprivation of the notochordal membrane matrix and inhibits the formation of sclerotome[28].
Transforming growth factor-β genes
Transforming growth factor-β (TGF-β) signaling pathways are effectively involved in advancing IVD and vertebral bodies.TGF-β intercellular signaling cascade stimulates cellular migration, proliferation, differentiation, and IVD matrix synthesis[29].TGF-β3 is actively synthesized in the perichordal membrane during the condensation stage of embryogenesis and promotes the development of the AF and vertebral bodies.Blockage of the TGF-β2 receptors inhibits the synthesis of type II collagen leading to defective NP, the exterior part of the AF, and inadequate IVD mineralization.TGF-β2 receptors participate in the differentiation of IVD tissue and vertebral bodies, producing spine[30].
IVDD
DDD is a complex, multifactorial process, the etiology of which is not well known.Thus, there are no particular criteria to differentiate the IVDD from the physiological retardation of development, maturation, or adaptive tissue remodeling[31].IVDD has perhaps been best defined as an “aberrant cell-mediated response to progressive structural failure”[32].Heredities, ecological causes, mechanical factors, aging, systemic and toxic mediators are identified as risk factors[33].This mechanism begins with alterations to the cellular IVD microenvironment leading to structural and functional failure[34].Interestingly, evidence showed that the early disappearance of NC density in NP is crucial for IVD stability and induces impairment in the ECM anabolic/catabolic proportion, resulting in the change of the IVD mechanical properties[25,35].IVDD is related to expanded ECM disruption[36], abnormal matrix formation[37], cellular apoptosis[38], inflammation[39], and regulation of sensory nerve and blood vessel in-growth into a normal avascular and neural tissue[40].
The onset of the IVDD is believed to be mainly in the NP[41].The decline of the key essential proteoglycan, aggrecan[42], reduces additional ECM production in the NP, and causes decreased hydration[43], a deficit of IVD height, and general failure to resist compressive burden[44].Compression pressures are hence dispensed through the NP to the adjacent AF, which leads to altered biomechanical function of AF and structural failure with radial and circumferential tears in the AF[45].These fissures and tears facilitate the in-growth of nociceptive nerves and blood vessels, resulting in the secretion of inflammatory pain-related mediators, thus leads to radial disc bulges or herniation of the NP into the contiguous spine, causing LBP[34].
Although the IVDs degenerate with aging and can be asymptomatic, a pathological process of IVDD is followed by pain.It has been revealed that a large number of people with no pain show degenerative disc changes that further complicate the differentiation of typical age-related degeneration from pathological conditions[46].An increase in catabolic action of matrix-degrading proteases, pro-inflammatory cytokines, and contemporary immune cell infiltration is proposed to define disc degeneration factors[39].Furthermore, lower disc pH, reduced nutrition, and calcified cartilaginous endplate (CEP) create an unfavorable environment for restoring the disc[47].Presently, there are symptomatic cures for advanced phases of DDD but no effective disease-modifying therapies[48].
Inflammation in degenerated IVD
Degenerated IVD cells produce higher concentrations of pro-inflammatory mediators, which suggest their role in the pathogenesis of IVD.A variety of cytokines, chemokines, and enzymes have been associated with IVDD, including interleukins (IL), interferons, tumor necrosis factor-alpha (TNF-α), matrix metalloproteinases (MMPs), prostaglandin E2 (PGE2), nitric oxide (NO), and aggrecanase.Among these, TNF-α and cytokines of the IL-1 family have been most widely investigated.Both TNFα and IL-1β are produced by IVD cells, and they acquire strong association in the pathogenesis of IVDD[49,50].Degenerated and herniated discs exhibit upregulated expression of both pro-inflammatory chemokines, TNF-α and IL-1β[51].Both have been found to activate ECM degrading enzymes and reduce ECM constituent synthesisin vitro[49,52].Recent studies showed that both TNF-α and IL-1β molecules induce increased MMP expression, particularly MMP-1, -2, -3, -7, -8, and -13.These MMPs are well recognized for their proteolytic activity towards collagen and proteoglycans (PGs)[53].Also, IL-1β, as a pro-inflammatory cytokine, upregulates the vascular endothelial growth factor (VEGF), brain-derived neurotrophic factor, and nerve growth factor expressions to stimulate the neovascularization and neoinnervation of IVD that eventually lead to inflammation and discogenic pain[24].Another study concluded that IL-1β is a master regulator in the disc cells that influence other cytokines and chemokines[54].IL-1β and TNF-α in NP cells contribute to the secretion of chemoattractant molecules such as C-C motif ligand 5/regulated 5 (CCL5/CCR5), regulated upon activation, normal T cell expressed and presumably secreted (CCL5/RANTES) or chemokine C-X-C motif ligand 6 (CXCL6)[55], and are involved in the migration of MSCs.
Another pro-inflammatory cytokine that has been involved in the pathogenesis of IVDD is IL-6, which is also secreted by NP cells[56].Indeed, degenerated IVD tissue samples contain a significantly higher expression of IL-6[57].Notably, numerous genetic variations in cytokine genes have been correlated with IVD degeneration.Traditionally, inflammation has mainly been considered as a primary reaction to infection at the site of tissue injury; however, it is not sure if it is a cause or outcome of IVD degeneration and herniation[58].During degeneration, increased aggrecan and collagen breakdown occur within the disc tissue with significant changes in IVD cell phenotype and increased levels of inflammatory cytokines[47].With an advanced degeneration phase, clefts and tears are developed in the AF and NP, which leak into the external environment.This allows immune cell activation and the invading blood vessels to pervade the IVD through the clefts and tears of the AF[59].
THERAPEUTICS FOR DEGENERATIVE INTERVERTEBRAL DISCS
Modern treatments for IVDD remain a subject of debate.Despite the known consequences of the IVD pathological cascade, the treatment options for IVDD are limited.The traditional conservative therapy for chronic LBP involves a wide range of treatment modalities, including bed rest, physiotherapy, analgesic and anti-inflammatory medications, acupuncture, and chiropractic[60].Approximately, 75%-90% of chronic LBP patients obtain satisfactory results with conservative treatment[34,61].The pain symptoms can be overcome by administering anti-inflammatory mediators, for example, opioids, steroids, non-steroidal anti-inflammatory drugs, and muscle relaxants[39].These anti-inflammatory drugs have effective short-term alleviation for back pain, but they cannot reverse the progression of IVDD[62].If conservative management does not have the desired effect, the constant pain sensation progresses because of the nerve compression[63].
Interventional procedures for IVDD include spinal surgical interventions, such as discectomy, spine fusion, and total disc replacement to manage the degenerated disc.The main surgical treatment alternatives for IVDD are spinal fusion and the replacement of the whole disc.Spinal fusion surgery, fusing two vertebrae, provides stability to the spine, which can be attained by a range of surgical interventions, such as posterolateral fusion, anterior and posterior lumbar interbody fusion.The minimally invasive methods to the lumbar spine for interbody fusion include lateral lumbar interbody fusion[64].Spinal fusion is considered as a gold standard treatment option for LBP[65].The results of three randomized controlled trials, which compared spinal fusion with conservative treatment, showed substantial clinical improvement in only a limited number of patients[45].
Moreover, spinal fusion could accelerate the degenerative process in adjacent vertebrae[66], and it mitigates painful symptoms, irrespective of repairing disc structure and mechanics; therefore, its efficacy remains controversial.Disc arthroplasty has the advantage of removing the degenerated IVD and restoring it with a prosthesis that can permit flexibility between the discs[67].Moreover, disc arthroplasty does not restore the mechanical movement of the native joint[61].The additional motionpreserving surgical procedure includes posterior dynamic stabilization.These surgical procedures contain the installation of pedicle screws over a motion segment associated with a flexible graft.These devices intend to limit motion over the interspace to control discogenic pain[68].The disadvantages of the surgical therapies can be extreme invasiveness, the increased possibility of recurrences, and failure of mechanical properties with contiguous segment degeneration.In most cases, some surgical intrusions and conservative treatments have low efficiency with lack of sustainable long-term effects.Instead of targeting the pathophysiology of the degenerative progression, they target the clinical symptoms[69].
Recent surgical treatment options for symptomatic degenerated IVD are still far from optimal outcomes.Hence, there is a substantial necessity for new therapies that focus on relieving painful symptoms and reestablishing IVD structure and mechanical loading capacity by explicitly addressing the underlying biological causes of DDD.
NOVEL THERAPEUTIC APPROACHES
The advancements in research and development have encouraged scientists to search for innovative pharmacological therapies in the regeneration of the IVD that mitigate painful symptoms by restoring and maintaining mechanical function.Depending on the stage of degeneration, different biological treatment options are used that alter the cascaded events at the molecular level.Figure 1 summarizes various therapeutic options for disc degeneration diseases.The three major groups of biological approaches for disc regeneration are divided as follows: (1) In the early stage of IVDD (grade II-III), growth factor injections may be effective; (2) In the intermediate stage of degeneration (grade IV), gene therapy or cell therapy may be required; and (3) In the advanced stage of IVDD (grade V), tissue engineering approaches are needed[70].
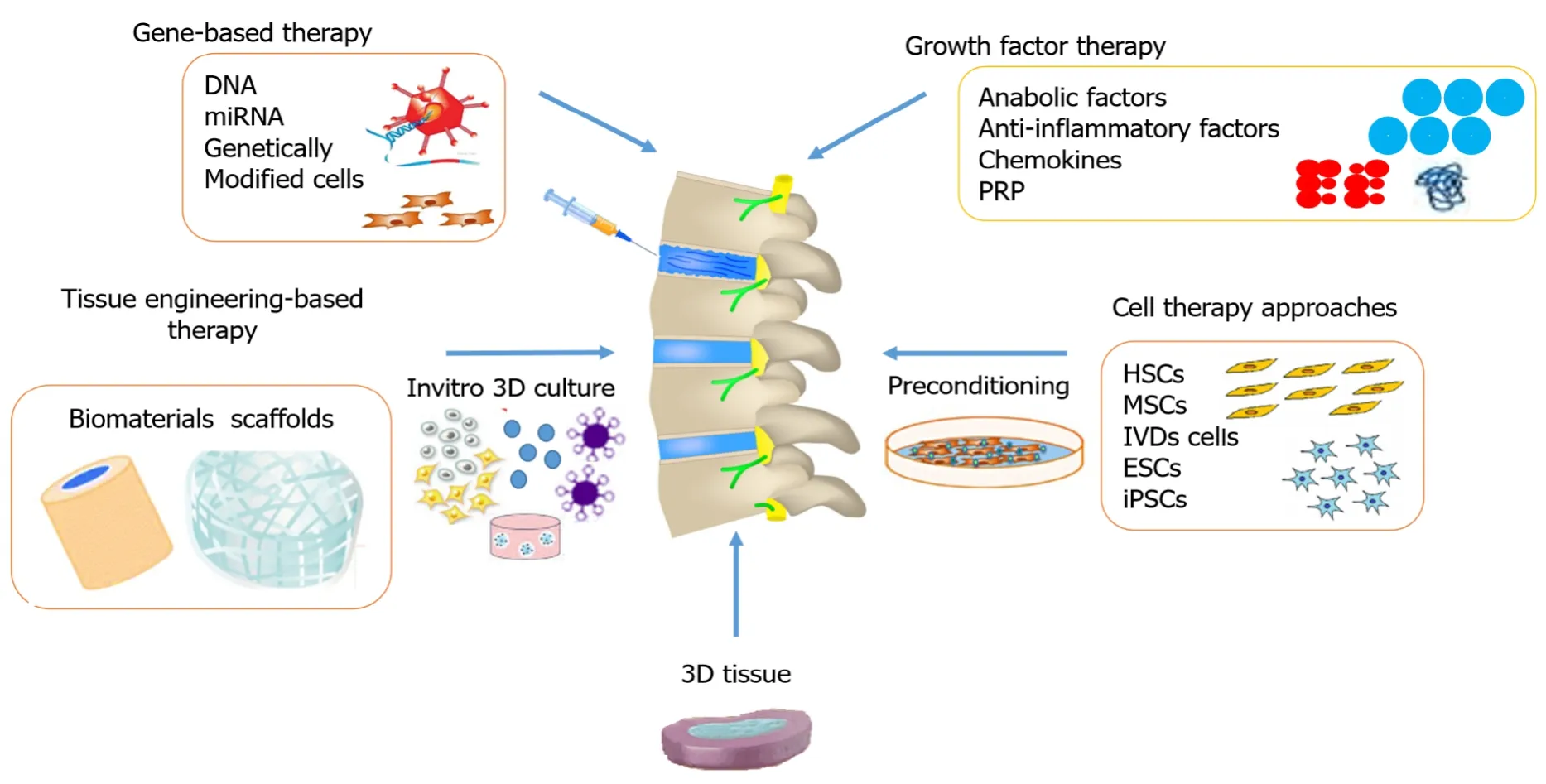
Figure 1 Different approaches used for restoring a degenerated disc.
Growth factor therapy
The therapeutic use of growth factors enhances the matrix synthesis and delay degeneration by reducing inflammation[71,72].Growth factors are the peptides or polypeptides that target specific receptors present on the surface of the cell, thereby influencing cell proliferation, differentiation and increasing their ability to synthesize the ECM[73,74].Specific growth factors that include BMPs and TGF-β family members are used to stimulate osteogenic and chondrogenic differentiation[75,76].
The first successful exogenous administration of TGF-β1 in animal models showed the enhanced synthesis of PGs in the NP.Severalin vitroandin vivoanalyses on BMP-2 and -7, TGF-β, epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), growth and differentiate factor 5 (GDF-5), and insulin-like growth factor 1 (IGF-1) revealed that they stimulate the synthesis of ECM[77-82].
In chronic conditions of IVDD, cocktails of growth factors may be needed because the growth factors have a short half-life and are unstable that limits their use as direct injection into the IVD.The administration of multiple injections of growth factors could enhance gradual release at target site or gene-based delivery system to obtain the desired effect.Currently, the primary focus is on platelet-rich plasma (PRP) that is used as a possible therapeutic option to promote IVD regeneration[83-86].Some limitations like the absence of standardization of the dosage, the process of preparation, and identification of mode of action need to be settled[87].
Gene-based therapy
In the last few decades, gene-based therapy has achieved wide research applications to focus on the regeneration of the IVD structures.The introduction of genes encoding the chondrocyte-specific proteins is directly transferred into the effectual host tissues[88].The gene-dose impact needs to be characterized for a safe and effective treatment.In contrast, certain findings have revealed inadequate outcomes of direct gene approach into the host cells[89].Nonetheless, there are limited investigations that support the efficacy of this approach[90-93].Recently, lentiviruses are believed to be competent vectors for gene transfer because they can deliver a substantial quantity of genetic material into the host cell's genome.The most frequently studied factors are TGF-β3, Sox-9, GDF-5, BMP family including 2, 7, and 12, connective tissue growth factor (CTGF), Wnt, IL-1, tissue inhibitor of metalloproteinases (TIMP-1), and LIM mineralization protein 1 (LMP-1), that are reported to enhance the synthesis of collagen type II and aggrecan in NP cells[94-106].Genes involved in the development of IVD are summarized in Table 1.
Cell therapy approaches
Regardless of the development of various treatment alternatives, the conservative and surgical therapeutic approaches are not exceptionally valuable for treating degenerated disc disease.These are usually incapable of delivering any solution to reestablish the structural and mechanical function of degenerated IVD.This situation has prompted the advancement of a regenerative medicine-based approach that substitutes the apoptotic and necrotic cells and limits cell death in IVD by targeting different cellular and molecular events[107].Out of several approved cellular and molecular approaches, the utilization of stem cell therapies has shown superior outcomes, and stem cell transplantation is being used to restore the degenerated IVDs[70].Stem cells are undifferentiated cells that can differentiate into particular cell types and are broadly utilized as a cell therapy approach.Stem cells exist in a quiescent condition, and they self-renew in the propagation process.Stem cells are being researchedin vitroandin vivoaccording to the need for the desired effect.Stem cell research has reformed the eventual fate of regenerative medicine because of its capability to recover impaired and damaged organs from treating various debilitating syndromes.The sources of stem cells and their properties are summarized in Table 2.Investigations are being made to comprehend the mechanism of regeneration at the molecular level to address the possible solutions for degenerative diseases and understand the basic pathogenesis and progression of different disorders.
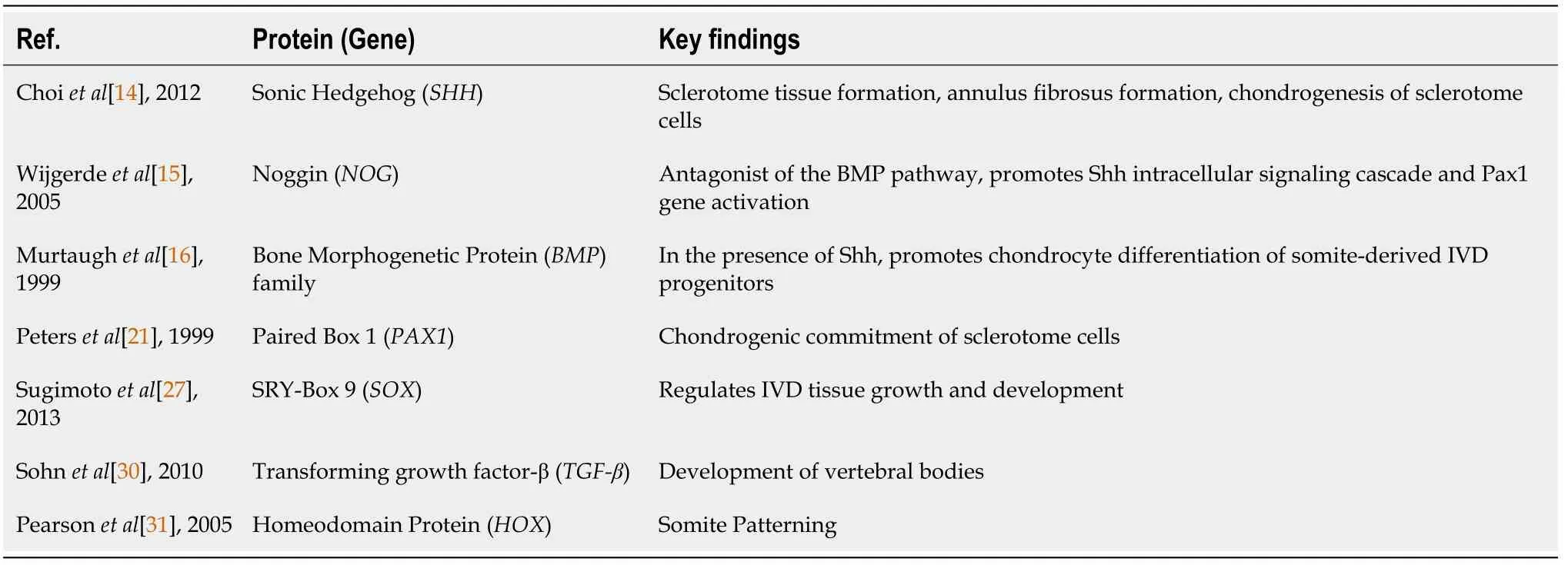
Table 1 Modifying genes essential for the development of intervertebral disc
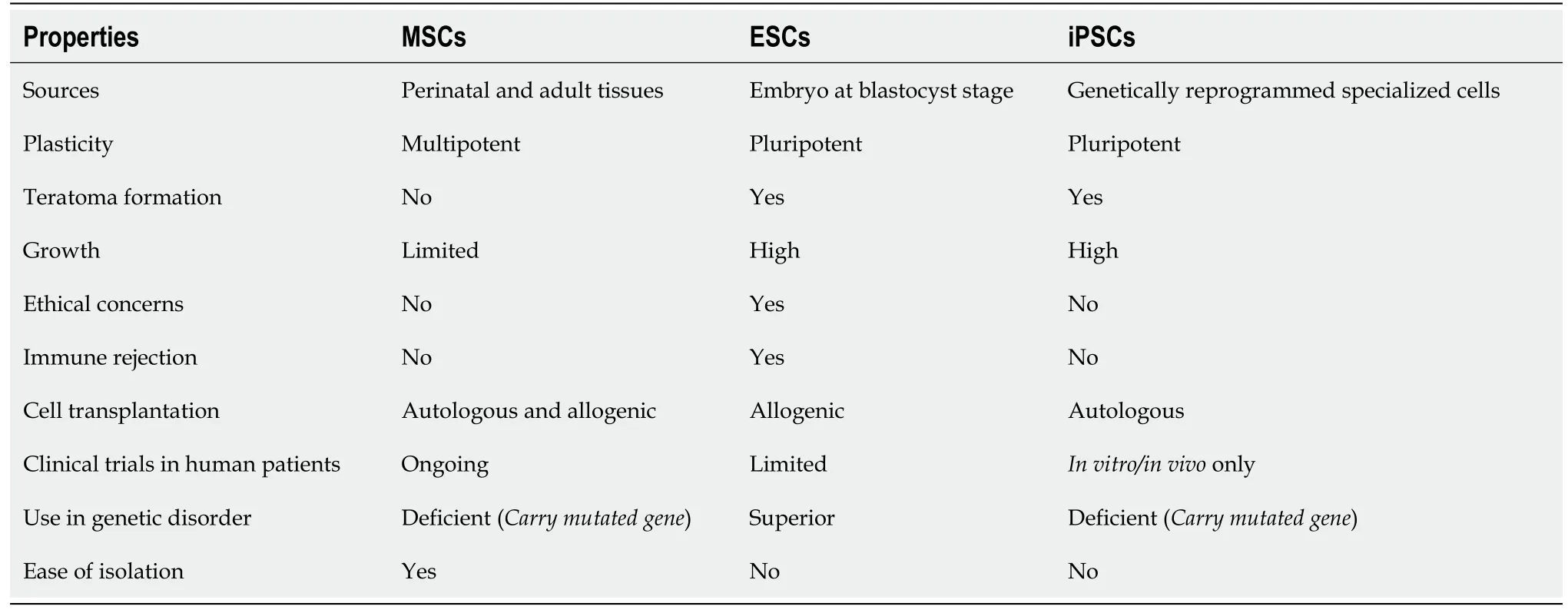
Table 2 Variation in properties of different sources of stem cell types
STEM CELLS FOR IVD REGENERATION
Stem cells from different sources are involved in the regeneration of disc diseases.A comparison of MSCs and other cell types is presented in Table 3.Different cellular approaches used for the regeneration of IVDs are highlighted in Table 4.
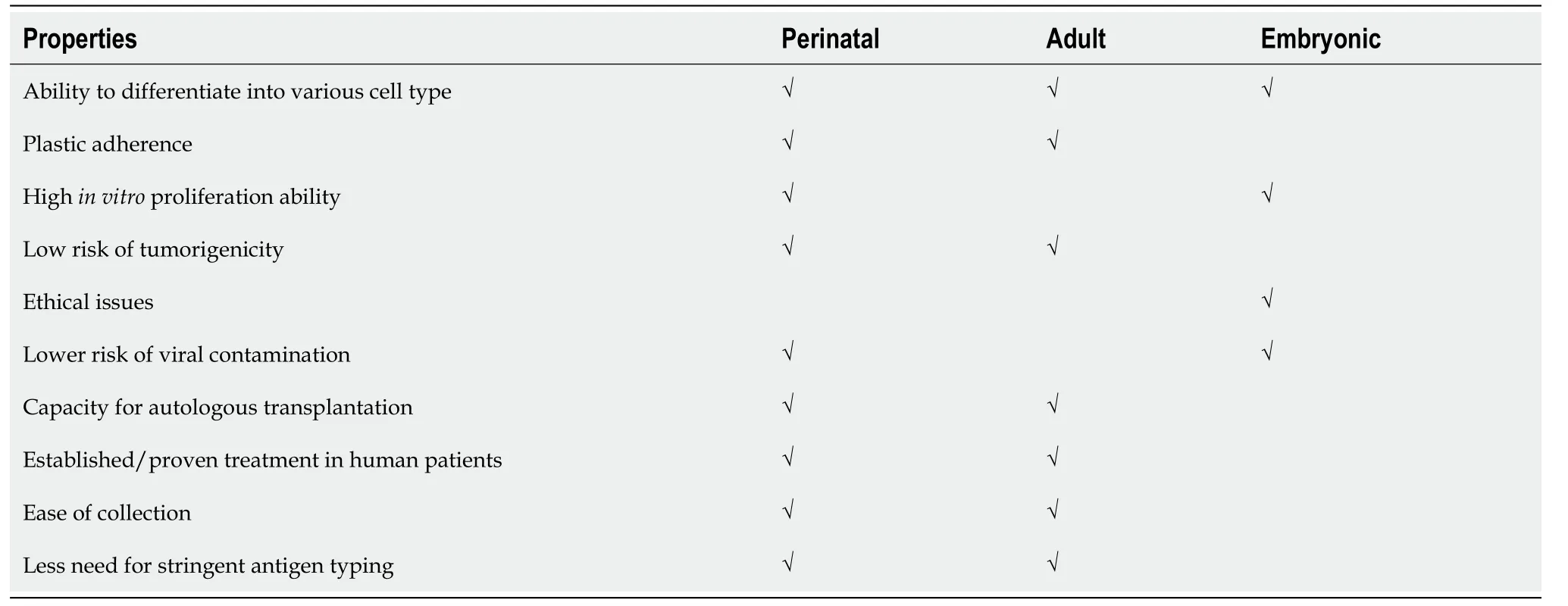
Table 3 Human umbilical cord-derived mesenchymal stem cells compared with other stem cells sources
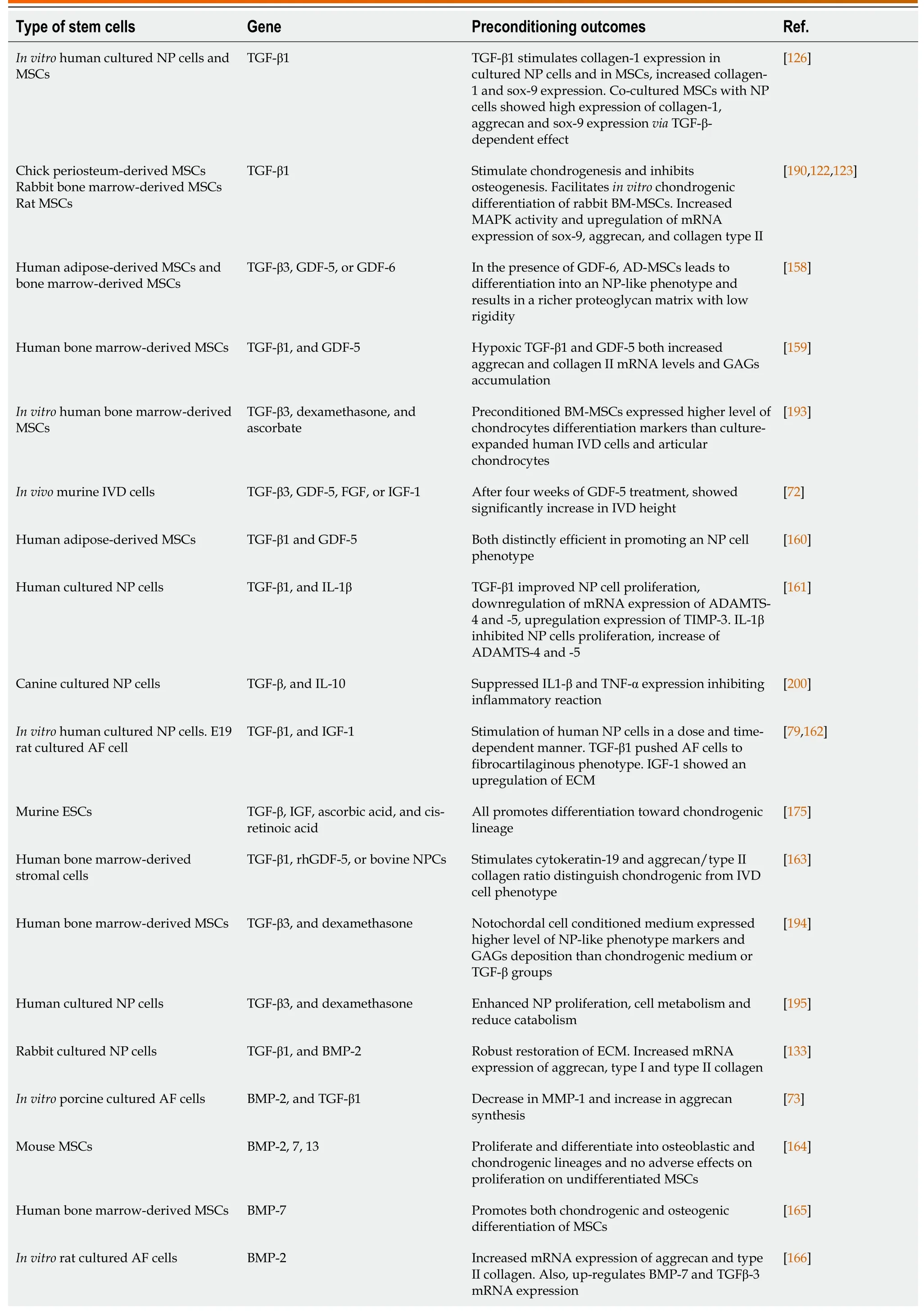
Table 4 Summary of studies on cellular therapeutic approaches for regenerative potential of the degenerated disc
Hematopoietic stem cells
Hematopoietic stem cells (HSCs) possess the capability to differentiate into blood cells.HSCs express CD34 molecules, while non-hematopoietic stem cells, including MSCs, do not show CD34 expression.These cells were injected into the rat IVDD model to investigate which population of cells might acquire disc-identical cells for treating IVDD.It is reported that HSCs can survive in the NP of host IVDs up to 42 d, while non-HSCs were detected up to 21 d only[108].However, this was nullified by further confirmation that HSCs cannot cure DDD.Although HSCs can only induce blood cells and cannot differentiate into chondrocyte-like cells and repair disintegrated NP, this has begun a novel era of scientific investigation for tissue regeneration.It is demonstrated that HSC transplantation of autologous pelvic bone marrow (BM) cells for the degenerated disc in clinical trials yielded no efficient recovery[109].
MSCs
The therapeutic use of MSCs is based on their two basic characteristics,i.e., they can be used to treat different diseases and can be isolated from the autologous source.MSCs are considered as a treatment choice for several diseases like DDD, stroke, myocardial ischemia, diabetes, and neurodegenerative diseases[110-113].MSCs can be readily isolated due to their adherent property.MSCs possess the excellent capability to differentiate into three mature lineages, namely bone, adipose, and cartilage, as well as into endothelial, myogenic[114-116], epithelial[117], and neural cell types[118] under specific conditions when guided by appropriate growth factors or pharmacological inducers.They possess the remarkable proliferative capability in cell culture with excellent stability in their phenotype and differentiation potential[119].
Furthermore, they can be smoothly transformed with the ability to home at the transplantation site.MSCs are immunologically inactive, which makes them ideal candidates for transplantation[120].MSCs have great capability to differentiate into chondrocyte-like cells that phenotypically resemble NP cells in chondrogenic induction conditions[121-123].MSCs promote the regeneration of endogenous tissue by secreting cell survival factors[124].
Tissue-specific stem cells
CEP, AF, and NP-derived stem cells are isolated from the adult IVD, namely cartilage endplate stem cells, AF stem cells, and nucleus pulposus stem cells (NPSCs), respectively.These cells are effective candidates for IVD recovery.Trials with disc stem cells revealed remarkable advantages in homing and retention in the IVD niche, differentiation capability, and functional competency.However, limitations in harvesting, separation, and proliferation of disc stem cells and low potency hinder researchers from using them for therapy[125].Studies to overcome IVD injury using disc derived stem cells showed their ability to replace affected tissue by producing disc-specific collagen type II and proteoglycan, and restoring disc hydration to physiological state[126,127].
Embryonic stem cells
Embryonic stem cells (ESCs) originate from the inner cell mass of blastula and possess an excellent tendency to differentiate into different cell types.They proved themselves as stable and relatively better source for disc regeneration involvingin vitroproduction of NCs.These NCs are the first to form NP during the embryogenesis of the disc.Researchers have successfully differentiated ESCs into chondrocyte-like cells[128].However, ESCs display tumorigenic properties, can cause teratoma formation, and also pose ethical concerns because of their embryonic origin, which limit their application for IVDD therapy[69].
Induced pluripotent stem cells
Induced pluripotent stem cells (iPSCs) are derived from genetically reprogrammed somatic cells to an embryonic-like state.The introduction of pluripotency genes and factors in adult terminally differentiated cells is a major discovery of this era.In 2006, mouse iPSCs were first reported by Shinya Yamanaka together with his co-investigators who revealed that fibroblasts might be reprogrammed to an ESC-like cells by four pluripotent gene-induced expressionsi.e.Sox2, octamer-binding transcription factor 3/4 (Oct3/4), Kruppel-like factor 4 (Klf4) and Myelocytomatosis (c-myc).These iPSCs were identical to the mouse ESCs because they express pluripotent markers and can differentiate into any cell lineage[119,129].In subsequent years, they performed
several experiments using human fibroblasts and successfully reprogrammed them to iPSCs by applying the same factors.A different team of researchers attained a similar achievement with minor alterations of Lin-28 and Nanog rather than c-myc and Klf4[130].iPSCs possess a great tendency to differentiate into each of the three germ layer cells containing NCs[131,132].Despite their ability to induce chondrogenesis, iPSCs might be susceptible to tumorigenesis because of their extreme pluripotent nature.
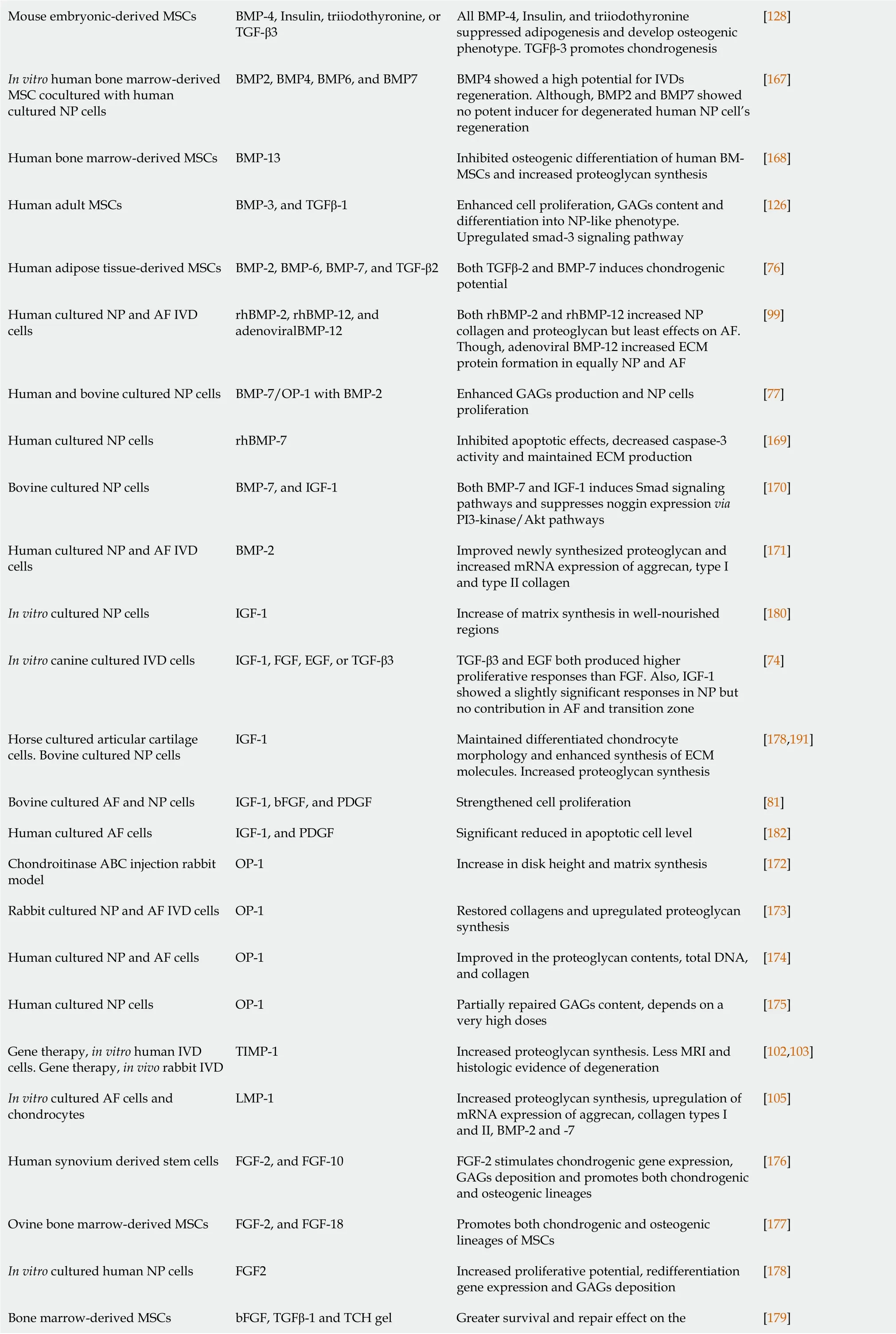
Mouse embryonic-derived MSCs BMP-4, Insulin, triiodothyronine, or TGF-β3 All BMP-4, Insulin, and triiodothyronine suppressed adipogenesis and develop osteogenic phenotype.TGFβ-3 promotes chondrogenesis[128]In vitro human bone marrow-derived MSC cocultured with human cultured NP cells BMP2, BMP4, BMP6, and BMP7 BMP4 showed a high potential for IVDs regeneration.Although, BMP2 and BMP7 showed no potent inducer for degenerated human NP cell’s regeneration[167]Human bone marrow-derived MSCs BMP-13 Inhibited osteogenic differentiation of human BMMSCs and increased proteoglycan synthesis[168]Human adult MSCs BMP-3, and TGFβ-1 Enhanced cell proliferation, GAGs content and differentiation into NP-like phenotype.Upregulated smad-3 signaling pathway[126]Human adipose tissue-derived MSCs BMP-2, BMP-6, BMP-7, and TGF-β2 Both TGFβ-2 and BMP-7 induces chondrogenic potential[76]Human cultured NP and AF IVD cells rhBMP-2, rhBMP-12, and adenoviralBMP-12 Both rhBMP-2 and rhBMP-12 increased NP collagen and proteoglycan but least effects on AF.Though, adenoviral BMP-12 increased ECM protein formation in equally NP and AF[99]Human and bovine cultured NP cells BMP-7/OP-1 with BMP-2 Enhanced GAGs production and NP cells proliferation[77]Human cultured NP cells rhBMP-7 Inhibited apoptotic effects, decreased caspase-3 activity and maintained ECM production[169]Bovine cultured NP cells BMP-7, and IGF-1 Both BMP-7 and IGF-1 induces Smad signaling pathways and suppresses noggin expression via PI3-kinase/Akt pathways[170]Human cultured NP and AF IVD cells BMP-2 Improved newly synthesized proteoglycan and increased mRNA expression of aggrecan, type I and type II collagen[171]In vitro cultured NP cells IGF-1 Increase of matrix synthesis in well-nourished regions[180]In vitro canine cultured IVD cells IGF-1, FGF, EGF, or TGF-β3 TGF-β3 and EGF both produced higher proliferative responses than FGF.Also, IGF-1 showed a slightly significant responses in NP but no contribution in AF and transition zone[74]Horse cultured articular cartilage cells.Bovine cultured NP cells IGF-1 Maintained differentiated chondrocyte morphology and enhanced synthesis of ECM molecules.Increased proteoglycan synthesis[178,191]Bovine cultured AF and NP cells IGF-1, bFGF, and PDGF Strengthened cell proliferation [81]Human cultured AF cells IGF-1, and PDGF Significant reduced in apoptotic cell level [182]Chondroitinase ABC injection rabbit model OP-1 Increase in disk height and matrix synthesis [172]Rabbit cultured NP and AF IVD cells OP-1 Restored collagens and upregulated proteoglycan synthesis[173]Human cultured NP and AF cells OP-1 Improved in the proteoglycan contents, total DNA, and collagen[174]Human cultured NP cells OP-1 Partially repaired GAGs content, depends on a very high doses[175]Gene therapy, in vitro human IVD cells.Gene therapy, in vivo rabbit IVD TIMP-1 Increased proteoglycan synthesis.Less MRI and histologic evidence of degeneration[102,103]In vitro cultured AF cells and chondrocytes LMP-1 Increased proteoglycan synthesis, upregulation of mRNA expression of aggrecan, collagen types I and II, BMP-2 and -7[105]Human synovium derived stem cells FGF-2, and FGF-10 FGF-2 stimulates chondrogenic gene expression, GAGs deposition and promotes both chondrogenic and osteogenic lineages[176]Ovine bone marrow-derived MSCs FGF-2, and FGF-18 Promotes both chondrogenic and osteogenic lineages of MSCs[177]In vitro cultured human NP cells FGF2 Increased proliferative potential, redifferentiation gene expression and GAGs deposition[178]Greater survival and repair effect on the Bone marrow-derived MSCs bFGF, TGFβ-1 and TCH gel [179]
IVD: Intervertebral disc; BMP: Bone morphogenetic protein; EGF: Epidermal growth factor; FGF: Fibroblast growth factor; IGF-1: Insulin-like growth factor-1; OP-1: Osteogenic protein-1; PDGF: Platelet-derived growth factor; TGF-β1: Transforming growth factor-β1; ADAMTS: A disintegrin and metalloproteinase with thrombospondin motifs; TIMP: Tissue inhibitor of metalloproteinases; TNF-α: Tumor necrosis factor-α; MMP: Matrix metalloproteinase; IL-1β: Interleukin-1 beta; IL-1Ra: IL-1 receptor antagonist; SOX-9: SRY-box transcription factor-9; rhGDF-5: Recombinant human growth and differentiation factor-5; LMP-1: LIM mineralization protein-1; WNTs: Wingless-related integration site; VEGFR: Vascular endothelial growth factor receptor; LacZ: β-galactosidase; CTGF: Connective tissue growth factor; GCSF: Granulocyte colony-stimulating factor; PRP: Platelet-rich plasma; AF: Annulus fibrosus; GAGs: Glycosaminoglycans; NP: Nucleus pulposus; ECM: Extracellular matrix; IVDD: Intervertebral disc degeneration; MSCs: Mesenchymal stem cells; BM: Bone marrow; AD: Adipose tissue; ESCs: Embryonic stem cells; NPCs: Nucleus pulposus cells; MRI: Magnetic resonance imaging; DNA: Deoxyribonucleic acid; mRNA: Messenger ribonucleic acid; TCH: Temperature-responsive chitosan hydrogel; MAPK: Mitogen-activated protein kinase; PI3: Phosphatidylinositol 3; Akt: Protein kinase B.
Tissue engineering-based therapy
MSCs face challenges like survival following transplantation, inadequate paracrine secretion, and limitations in cell homing.These hindrances in the effectiveness of MSCs can be overcome by improving their potential of migration, homing, propagation, and differentiation into the preferred cell type.Thus, selecting an appropriate scaffold for stem cells can better serve for the re-development of the lost tissue.Injectable bio-materials or micro and nanoscale scaffolds are preferable for biocompatibility, cell infiltration, and remodeling of the transplanted cells.Upon preconditioning, the fully biocompatible material can also target cell attachment, proliferation, normal morphology, and elevated expression of desired factors.Thus, the strategy has the advantage of inducing differentiationin vitroand transplanting cellsin vivo[133,134].
CURRENT ISSUES RELATED TO TREATING DEGENERATIVE INTERVERTEBRAL DISC
IVD is the largest avascular structure in the human body that has limited efficiency for regeneration.Due to a vascular nature of IVD, tendency to develop strategy for their treatment and regeneration is low[135].Rehabilitation, surgical interventions, posttrial treatment, and standardized procedures for the subjects should be deemed mandatory.In the case of the local treatment, a small incision should be made[136].Therefore, surgeries for injecting therapeutic cells should be minimally invasive.In addition, safety concerns such as high intensity of neuropathic pain and secondary infections and genuine diagnosis of complications are significant.One of the critical aspects of designing clinical trials with lower back injuries is the level of injuryinduced cases[137].In selecting subjects with an exclusively specific level of damage, the distance of the injured spinal segment, route of administration, and phenomenal interaction of cell or drug action should be considered[138].Therefore, long term patient follow-up with standardized measurement scales, such as the American Spinal Injury Association Scale for neurological levels, Normal Rating Scale (pain and spinal cord independence level), Modified Ashworth Scale (for spasticity), and International Association of Neurorestoratology Spinal Cord Injury Functional Rating Scale (for the report of functionality) are essential[139].Current IVDD animal models are of limited significance as most are different from human disc degeneration[140].Factual information can be obtained from animal models; however, the limitations are that the studies were generally applied on young rodents with the recently damaged disc in which normal tissue repair mechanisms are still active to heal the degeneration.It is also difficult to quantify the amount of pain.Therefore, researchers use alternate methods to examine disc regeneration or repair success by performing biochemical, molecular, and histological assessments.
Few ethical concerns should be considered while performing pre-clinical studies to translate into clinical trials.Using scientific validity, fair subject selection, favorable distribution of risks-benefits ratio, and informed consent is necessary to make clinical research ethical, which is considered challenging in disc diseases[141].Typical successful measurements comprise proportions of morphology (e.g., IVDs height, AF delamination, and IVD degeneration grade through MRI and histology), cellularity, ECM quality and quantity, cytokine levels, and biomechanics (e.g.pressure/volume testing, compressive strength, and range of motion)[142].Further, leakage of the delivering cells or drugs is a concern because small escape is possible while injecting.Cell therapy may upregulate the production of some growth factors, which may not be suitable for disc repair, as the cells intrinsically express a high level of growth factors, for example, TGF-β1 and bFGF, that can mediate blood vessel formation, trigger inflammatory mechanism and regulate abnormal disc cell differentiation.Therefore, extensive studies related to the toxicity of biochemical factors in the intervertebral disc are necessary before they are applied in clinical trials.Furthermore, safety with any type of gene therapy is a major consideration.These limitations make direct application of biological approaches difficult to treat disc injuries from animals to humans[143,144].
ENHANCING THE IVD REGENERATION POTENTIAL BY HUMAN PERINATAL MSCs
The implantation of MSCs is considered a promising therapeutic approach for IVD regeneration.MSCs are primarily found in adipose tissue, dental pulp, BM, and peripheral blood.Recent advances with MSCs have shown that they can be isolated from a variety of postnatal organs such as skin, bone, cartilage, periodontium, pancreatic islets, skeletal muscle, periosteum, and synovial membrane/fluid as well as from perinatal tissues like umbilical cord tissue, umbilical cord blood (UCB), AF, and placenta[107,145,146].The human perinatal umbilical cord is an optimistic source of MSCs.Like BM stem cells, human umbilical cord-derived MSCs (hUC-MSCs) are the noncontroversial source.The cells have rapid self-renewal properties and possess various advantages, making them promising therapeutic candidates[147].Some of the advantages are as follows: (1) They are accessible in massive amounts, considering plenty of umbilical cord (UC) with around 135 million births globally every year; (2) They can be effectively collected and manipulated without any adverse effect on the infant or mother; (3) There are no predetermined ethical issues that need to be managed in contrast with ESCs; (4) They show more significant proliferative potential compared to BM-MSCs[148]; (5) They possess minimal immunogenicity[149]; (6) There is minimal possibility of viral contamination[150]; (7) They possess a relatively large harvest size as compared to MSCs from BM[151]; and (8) They need less stringent antigenic typing, and there may be less rejection[152].
Studies have shown that MSC isolation and characterization from Wharton’s jelly (WJ) tissue can be easily performed[153,154].In addition, several current clinical trials explain the utilization of UC matrix-derived MSCs.It is early to relatein vivoresearch of tissue regeneration utilizing MSCs derived from UCB compared to other sources to understand better the capability of hUC-MSCs to regenerate degenerative discs.Clinical trials showed that hUC-MSC transplantation could be a promising substitute for the treatment of prolonged discogenic LBP[155] due to better survival in the avascular niche of the IVD[156] with differently manipulating transplanting cells[157].
DIFFERENTIATION of MSCs TOWARDS CHONDROGENESIS
Stem cells have been treated with small molecules to improve their renewing capability.Numerous proteins and small molecules have been examined in this perspective such as TGF-β[158-163], BMPs[164-171], osteogenic protein (OP)[172-175], bFGF[176-179], IGF[180-182], GDF-5[183,184], granulocyte colony-stimulating factor (GCSF)[185], Wnt[186], CTGF[187], decalpenic acid, β-glycerophosphate, isobutyl methylxanthine, purmorphamine, ascorbic acid, and heparin-binding growthassociated molecule (HB-GAM)[188,189].TGF-β has been found to lead periosteumderived stem cells towards chondrogenic lineage and inhibit osteogenic differentiation in extreme density culture[190].High concentrations of IGF-1 can impose the expression of chondrogenic proteins in BM-derived MSCs[191].Ascorbic acid, nonorganic phosphates, and dexamethasone increase the differentiation potential of BMderived stem cells towards osteoblasts in CEPs[192-195].Similarly, pleiotrophin (PTN) has also been reported to differentiate stem cells derived from human BM into chondrocytes[196].Dexamethasone, insulin, and soluble factors have also been shown to stimulate chondrogenic differentiation of MSCsin vitro[197].
Chemical treatment to improve cell survival
Cell survival at the transplantation site is the most critical challenge.Numerous cells die soon after implantation at the site of injury[156].Direct stimulation of stem cells into specific lineage by using growth factors and small molecules to increase their survival in host tissue is the most practical approach.Investigations showed that the expression of particular cell survival factors could enhance cell feasibility and survival in diseased tissue[198,199].TGF-β is a growth factor associated with several cellular processes including cell proliferation and differentiation[200].The rabbit model of IVDD induced through nucleus aspiration and infused with a combination of TGF-β1, fibrin glue, and rabbit MSCs, produced improved results[201].Similarly,in vitrotransdifferentiation phenomenon of MSCs into different cell types showed that transplanted cells could combine with native cells to give better performance in the damaged tissue[202].
Chemical treatment to improve stem cell homing
For enhanced regeneration, proficient cell homing is essential because the curative impact primarily depends on the effective cell engraftment following transplantation.Various investigators have utilized chemokine/cytokines receptors associated with MSC homing to enhance cell attachment at degenerated tissues[203], including CCR1, 2, 4, 7, 9, and in addition, CXC chemokine receptor-5, -6[204].CCL5/RANTES has been identified as a chemoattractant secreted by degenerative IVD in organ culture[55].Moreover, the possibility of different cytokines associated with the pathogenesis of IVD degeneration, specifically TNF-α and IL-1β, play an important role in controlling MSC recruitment to the IVD[101,205-207].In vitroandin vivoresearch studies showed that molecular pre-requisite of MSCs with growth factors like TNF-α and stromal-derived-factor-1 (SDF-1) represent primary signaling cues to elevate VEGF production[208].MSC conditioned medium improved neuronal survival in several neurological disorders such as neurodegenerative diseases, stroke, and spinal injuries[209].Moreover, the conditioned medium acquired from articular cartilage stimulated the chondrogenic potential of MSCs and ECM development.The paracrine influence of prominin-1 or CD133+ endothelial progenitor cells from cord blood releases biologically active molecules in the conditioned medium along with microvesicles, which stimulate cell growth and homing.CD133+ cell derivatives with microvesicles possess messenger RNAs for various pro-angiopoietins and antiapoptotic factors, containing bFGF, receptor tyrosine kinase (c-kit) ligand, IGF-1, VEGF, and IL-8, contributing to withstand harsh microenvironment of the disc[210].
CONCLUSION
In conclusion, this review highlights regenerative medicine-based approaches for the regeneration of IVDD.Numerous potential therapeutic options were identified for the development of cellular therapies.The harsh microenvironment of the degenerative disc poses challenge to the survival of implanted cells.Therefore, possible strategies are needed to enhance the ability of the transplanted cells by preconditioning, chemical modification, genetic manipulation, and augmentation of growth and survival factors to help cells withstand the harsh disc microenvironment.The ultimate goal is to ensure that the transplanted cells survive, integrate and differentiate into desired cell types to regenerate and restore the normal physiological function of the IVD.
杂志排行
World Journal of Stem Cells的其它文章
- Stem cell-derived biofactors fight against coronavirus infection
- Application of mesenchymal stem cells derived from human pluripotent stem cells in regenerative medicine
- Strategies to improve regenerative potential of mesenchymal stem cells
- Dental mesenchymal stromal/stem cells in different microenvironments— implications in regenerative therapy
- Bone marrow mesenchymal stem cell therapy regulates gut microbiota to improve post-stroke neurological function recovery in rats
- SmartFlareTM is a reliable method for assessing mRNA expression in single neural stem cells
