Hepatic adenomatosis in glycogen storage disease: Radio-pathological correlation
2022-01-07JoLouroAnAlvesJosBrndMnuelFrn
João M Louro , , An M Alves , José R Brndão , Mnuel Frnç
a Department of Radiology, Centro Hospitalar Universitário do Porto EPE, Porto 4099-001, Portugal
b Department of Anatomical Pathology, Centro Hospitalar Universitário do Porto EPE, Porto 4099-001, Portugal
Glycogen storage disease type I (GSD type I), also known as von Gierke disease, is an autosomal recessive disorder of the glycogen metabolism pathway, caused by a deficiency of glucose-6-phosphatase (GSD type Ia) or glucose-6-phosphate translocase(GSD type Ib) [1] . These enzymes are crucial in the last step of both glycogenolysis and gluconeogenesis, and their deficiency results in excessive glycogen and fat accumulation in the liver, kidney and intestinal mucosa [1] . GSD type I has an overall incidence of 1/10 0,0 0 0 live births [1] , and 80% of patients are type Ia [2] . Patients commonly present between 3 and 6 months of age with severe hypoglycemia, hepatomegaly, hypertriglyceridemia and growth retardation. They often develop renal disease, hyperuricemia and, in those with GSD type Ib, recurrent infections due to neutropenia. Diagnosis is usually straightforward; nevertheless,genetic testing is necessary for confirmation [ 1 , 2 ].
A diet based on uncooked starch, a low glycemic index carbohydrate, and frequent meals is the mainstay of treatment, with the goal of preventing hypoglycemia and other metabolic abnormalities [2] . Nonetheless, GSD type I is a multisystemic disorder requiring multidisciplinary management [1] .
Hepatocellular adenomas (HCAs) are a common complication of GSD type I, often growing in number and size from the second decade of life and requiring careful follow-up due to the risk of malignant degeneration [1] . Here, we report a case of a 22-yearold woman with GSD type Ia submitted to liver transplant due to multiple HCA unresponsive to dietary therapy.
Pre-transplant magnetic resonance imaging (MRI) revealed 16 lesions suggestive of inflammatory-HCA (I-HCA), the largest one with 11 cm, which had gradually grown in number and size. These lesions were heterogeneously hyperintense on T2-WI, some with internal cystic degeneration ( Fig. 1 A). After contrast injection these lesions were hypervascular on arterial phase ( Fig. 1 B), showing progressive enhancement on the portal venous ( Fig. 1 C) and delayed venous phases. On MRI performed with hepatobiliary contrast agent (Gd-EOB-DTPA), these lesions exhibited hypointensity on hepatobiliary phase due to decreased contrast uptake ( Fig. 1 D).None of the lesions demonstrated signal loss on chemical-shift T1-WI. Furthermore, none of the lesions showed restriction on diffusion-weighted imaging or other imaging signs of malignancy.
Histopathological evaluation of the explanted liver showed multiple and large yellow-tanned nodules at macroscopy, some with cystic degeneration and internal hemorrhage. Microscopically,these lesions were confirmed to be I-HCA, demonstrating architectural distortion, disorganized cords of hepatocytes and aberrant portal spaces with no bile ductules or portal venules ( Fig. 2 B).These nodules also presented multiple areas of peliosis and sinusoidal dilatation ( Fig. 2 C).
HCAs are the most common liver lesions to develop in patients with GSD type Ia, with prevalence ranging from 16 to 75%. They have a mean age of presentation of 14.8 years and progress in size and/or number in 50% of patients [1] . Compared to the general population, HCAs in GSDs are greater in number, more likely to have bilobar distribution and have no sex predisposition. As in the general population, the most common HCA pathologic subtype is I-HCA [3] . Pathogenesis is likely multifactorial, although good metabolic control has been associated with decreased formation and progression of HCA [4] .
Macroscopically, HCAs are 1 to 20 cm nodules with a yellow-tanned color due to their fat content, have large peritumoral/intratumoral vessels and can show central necrosis or hemorrhage. A pseudocapsule may sometimes be seen due to adjacent compression of the liver parenchyma [5] . Microscopically, HCAs are monoclonal neoplasms with sheet or cord-like arrangements of benign hepatocytes containing a varying amount of intracellular fat.HCAs rich in intracellular fat show diffuse signal loss on chemicalshift T1-WI, a finding most commonly seen on hepatocyte nuclear factor-1 alpha inactivated HCA [5] .
Hepatocytes are separated by thin-walled sinusoids solely supplied by arteries, resulting in its hypervascularity on arterial-phase imaging [5] . Additionally, I-HCAs show sinusoidal dilatation and areas of peliosis, histological features that correlate with portalvenous enhancement and T2 signal hyperintensity on MRI [6] .
HCAs are also susceptible to hemorrhage, due to their extensive arterial supply and poor connective tissue support. As there is no true capsule, bleeding may spread into the liver or abdominal cavity. On MRI, acute hemorrhage is hyperintense on T1-WI due to methemoglobin, while chronic hemorrhage shows hypointensity on T1-WI and T2-WI due to hemosiderin [5] .
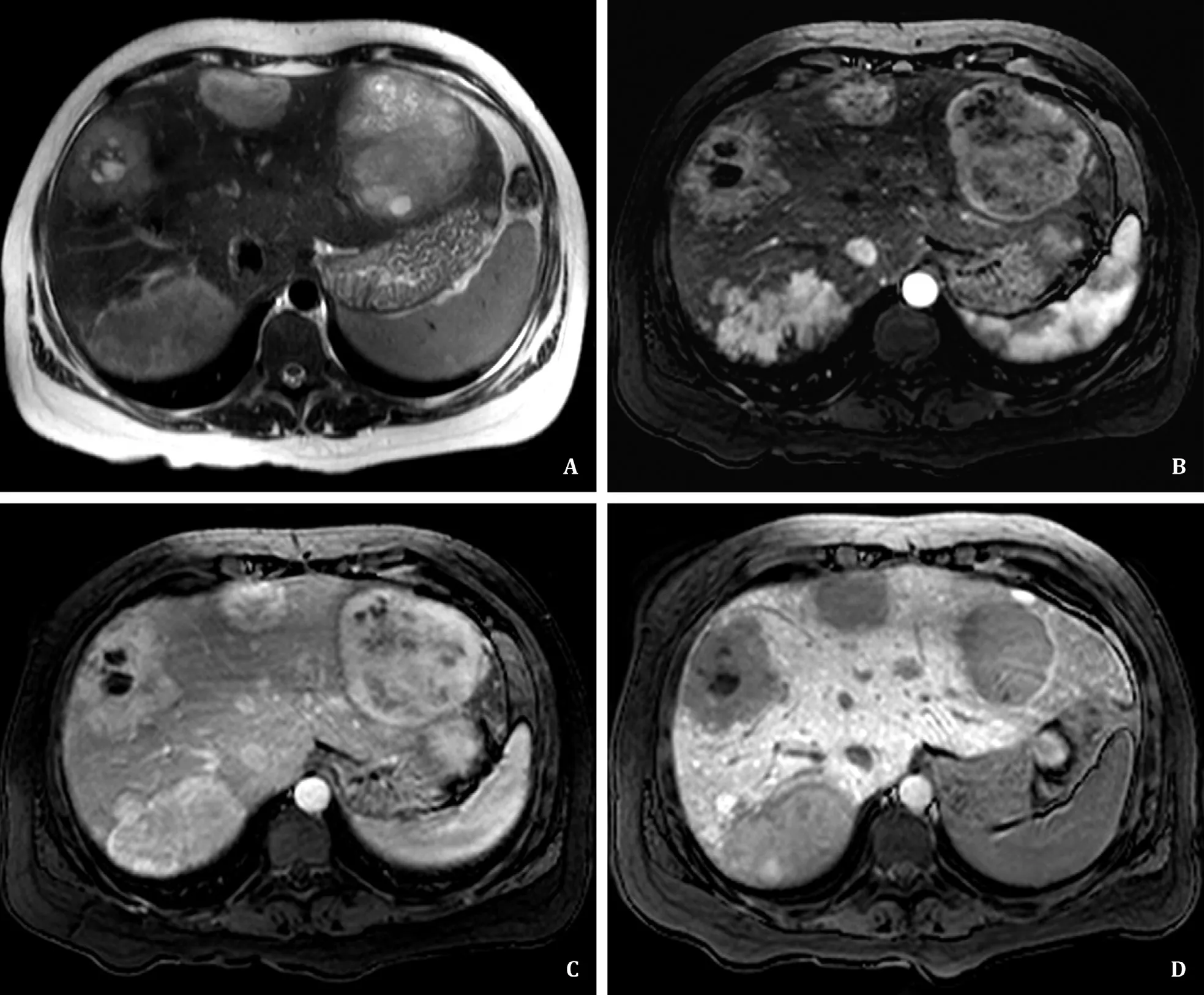
Fig. 1. A: Axial T2-WI image showing multiple histologically confirmed inflammatory-hepatocellular adenoma (I-HCA). They are heterogeneously hyperintense and some present areas of cystic degeneration. B and C: Axial T1FS-WI images post-gadolinium in arterial ( B ) and portal venous phases ( C ), depicting the lesions’ arterial and delayed hyperenhancement, respectively. D: Axial T1FS-WI image at hepatobiliary phase, 20 min after contrast injection (Gd-EOB-DTPA), demonstrates the hypointense signal of the I-HCA compared to that of the liver parenchyma.

Fig. 2. A: Photomicrograph of hematoxylin and eosin (HE) stained of normal liver parenchyma in a different patient, demonstrating a normal portal space, composed of a portal vein ( *), biliary ducts (dashed circles) and hepatic artery (arrowhead) (original magnification × 40). B and C: Photomicrographs of an HE stained inflammatoryhepatocellular adenoma (I-HCA) in the reported patient showing distorted hepatocellular architecture, with areas of sinusoidal dilatation and peliosis, most with proteinaceous content (arrows) and others with internal hemorrhage (yellow arrows), surrounded by cords of hepatocytes (*). The portal spaces have an aberrant morphology, with absent portal venules and biliary ducts ( B , original magnification × 40; C , original magnification × 12).
HCA expression of hepatocytes transporters such as OATPB1/B3 and MRP3 is variable, with reduced expression associated with decreased uptake of hepatobiliary contrast and thus hypointensity on hepatobiliary phase [7] . Nevertheless, hepatobiliary contrast uptake and signal hyperintensity on the hepatobiliary phase may be observed, a finding that has been associated with a higher risk of malignant degeneration [8] . Lastly, HCA characteristically lack biliary ductless, a key histologic feature that helps differentiate them from focal nodular hyperplasia [5] .
Accompanying the rise of life expectancy seen in GSD type I patients, hepatocellular carcinoma is increasingly being recognized as a complication, probably arising from prior HCA. Patient surveillance and screening for hepatocellular carcinoma is difficult, as tumor markers are often normal. Furthermore, histological characterization is not usually feasible due to the great number of lesions and imaging does not allow confident differentiation between benign HCA and those with dysplasia or malignant degeneration [1] .Despite this, some imaging signs have been reported to be associated with malignancy on MRI, such as hyperintensity on hepatobiliary phase, as mentioned previously [8] . Also, lower apparent diffusion coefficient values on diffusion-weighted imaging and portal venous or delayed contrast washout, have been related toβ-catenin mutated HCA, the subtype with the highest risk of malignancy [9] . Thus, routine liver imaging is advised, usually by ultrasound every 18-24 months in those up to 18 years of age, and by CT or MRI afterwards [1] .
In GSD type I patients, HCA management ranges from conservative measures such as dietary therapy and imaging surveillance,to surgical resection, partial hepatectomy or liver transplantation.The latter is usually reserved for those with poor metabolic control, liver failure or multifocal refractory lesions without distant metastatic disease [ 1 , 10 ].
In conclusion, GSD type Ia patients commonly develop multiple HCA, which may be unresponsive to conservative treatment,requiring surgical resection or liver transplantation. Awareness of the typical imaging features of these focal liver lesions is crucial due to their increasingly known risk of malignant degeneration.
Acknowledgments
None.
CRediT authorship contribution statement
João M Louro: Conceptualization, Investigation, Writing - original draft. Ana M Alves: Investigation, Writing - original draft. José R Brandão: Investigation, Writing - review & editing. Manuela França: Supervision, Writing - review & editing.
Funding
None.
Ethical approval
This study was approved by the Medical Ethics Committee at our institution. Written informed consent was obtained from the patient.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Hepatocellular-cholangiocarcinoma with sarcomatous change:Clinicopathological features and outcomes
- Recent advances in immunotherapy for hepatocellular carcinoma
- Toll-like receptors and hepatitis C virus infection
- Involvement of the circular RNA/microRNA/glucose-6-phosphate dehydrogenase axis in the pathological mechanism of hepatocellular carcinoma
- Progress in hepatitis B virus-related acute-on-chronic liver failure treatment in China: A large, multicenter, retrospective cohort study using a propensity score matching analysis ✩
- From conventional two-stage hepatectomy to ALPPS: Fifteen years of experience in a hepatobiliary surgery unit
