Recent advances in immunotherapy for hepatocellular carcinoma
2022-01-07AbidAliKhanZhiKunLiuXiaoXu
Abid Ali Khan a , b , Zhi-Kun Liu a , b , Xiao Xu a , b , c , *
a Division of Hepatobiliary and Pancreatic Surgery, Department of Surgery, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310 0 03, China
b Key Lab of Combined Multi-Organ Transplantation, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, Ministry of Public Health, Hangzhou 310 0 03, China
c Department of Hepatobiliary and Pancreatic Surgery, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou 310 0 06,China
Keywords:Hepatocellular carcinoma Immunotherapy Immune checkpoint inhibitor Adoptive cellular therapy Immune evasion Combination therapy Predictive biomarkers
ABSTRACT
Introduction
Liver cancer is globally responsible for 6% of cancer-related deaths every year, with hepatocellular carcinoma (HCC) accounting for about 90% of primary liver cancer cases [1] . Surgical resection and liver transplantation are the only curative treatments for HCC. The 5-year survival rate after successful liver transplantation is 75%. However, due to different factors such as organ shortage, treatment cost, strict selection criteria and disease staging, the number of patients who can benefit from liver transplantation is limited. Treatment for advanced HCC is more challenging because of the limited benefits from radical surgery, leaving systemic treatment as a last resort [2] .
Conventional systemic chemotherapy has failed to improve survival in patients with HCC, yet was associated with severe toxicities [3] . Combination chemotherapy has also ended up with unsatisfactory outcomes in clinical studies [4] . The FDA-approved molecular targeting drugs such as sorafenib and lenvatinib have shown survival benefit of up to three months. However, their clinical benefits are limited by toxicity, low overall survival advantage, and drug resistance [ 5 , 6 ]. Recently, a relatively novel immune-based treatment approach via enhancing the body’s natural anticancer immune response has revolutionized the treatment of advanced HCC. Nivolumab was the first immune-based therapy approved in September 2017 for advanced HCC followed by the approval of pembrolizumab in 2018. Both nivolumab and pembrolizumab work by inhibiting the so-called PD-1/PD-L1 immune checkpoint, which is one of the main strategies exploited by tumor cells to evade the anticancer immune response [7-9] .
Nivolumab has shown a clinically significant advantage over the current standard treatment, sorafenib, in terms of median overall survival (OS), overall response rate (ORR), and safety and tolerability [10] . According to the data reported from a recent phase III randomized study of 743 patients with advanced HCC, the median OS was 16.4 mon in the nivolumab group (n= 371) and 14.7 mon in the sorafenib group (n= 372), and ORR was 15% in the nivolumab group and 7% in the sorafenib group. Furthermore,nivolumab showed a better safety and tolerability profile compared to sorafenib [11] . With the recent success of immune checkpoint inhibitor (ICI) based treatment for advanced HCC, challenges such as low response rate and acquired resistance in previously respondent patients still exist. Researchers have recently identified several predictive biomarkers that could help in clinical decision making when using anti-PD-1/PD-L1 therapy for HCC.
Besides significant success in ICIs, other immunotherapies such as cancer vaccine, chimeric antigen receptor T-cells (CAR-T), natural killer cells (NK cells), cytokines, and combination immunotherapies, have also shown promising outcomes in clinical trials. In this review, we summarized recent advances in the most promising immune-based approaches for HCC, currently approved therapies,with those going through clinical trials. Furthermore, this review discusses the mechanism of immune escape and the role of immunotherapy in enhancing the immune response to cancer.
Escaping the immune surveillance
The basic molecular mechanism in HCC initiation and progression has been broadly researched during the last decade. HCC has a high level of immunogenicity, which is thought to elicit a potential anticancer immune response, but the underlying chronic inflammatory process and the immunosuppressive tumor microenvironment (TME) help HCC evade the immune response [12] . Burnet and Thomas in 1957 originally hypothesized that the immune system worked as a surveillance system that was programmed to recognize and destroy the emerging cancer cells [13] . Tumor cells must be antigenic, i.e., able to be recognized by the immune system as different from normal cells, and immunogenic, i.e., capable of inducing an immune response, to be eliminated successfully[14] . Loss of antigenicity and immunogenicity can make the tumor cells escape from the immune surveillance. “Cancer immune editing” is another important concept which describes the process of the interaction between tumor cells and the immune system,which consists of three phases, including elimination, equilibrium and escape. In the elimination phase, tumor cells are recognized and eliminated by the immune system. In the equilibrium phase,certain tumor cells may go unrecognized by the immune system and avoid elimination, but there exists a balance between new cell proliferation and apoptosis, so the tumor does not grow. In the escape phase, an immunosuppressive microenvironment is created that helps tumor cells escape from cytotoxic T-cells [15] .
Cancer cells express tumor antigens which are recognized by the immune system. However, circulating cytotoxic T-lymphocytes do not directly recognize tumor antigens unless processed and presented by major histocompatibility complex class 1 (MHC-1) and MHC-2 molecules on the tumor cells and the antigen-presenting cells. This process is known as “antigen presentation”. Alteration in tumor antigens recognition and presentation may lead to cancer cell proliferation. Tumor evolves different strategies such as lowering the expression of MHC-1 molecule to avoid detection by T-cells,stopping the expression of essential co-stimulatory molecules such as CD80 or CD86 for T-cell activation, recruiting regulatory T cells(Tregs) which produce immunosuppressive cytokines such as IL-10 and TGF-βto create an immunosuppressive microenvironment,and upregulating the immune checkpoints [ 12 , 16 ]. Immunotherapy works by enhancing tumor antigens recognition and presentation,and by blocking the immunosuppressive forces such as immune checkpoints.
Immunotherapy targets in HCC
Recently, the success in PD-1/PD-L1 inhibitors has revolutionized the treatment of advanced HCC, while other potential immunotherapies such as adoptive cellular therapy, therapeutic cancer vaccine, cytokine therapy and combination immunotherapy, have shown promising results in preclinical and clinical trials. Apart from PD-1/PD-L1 inhibitors, other ICIs, such as anticytotoxic T-lymphocyte-associated protein 4 ( CTLA-4) and anti-LAG-3, have also shown exciting results in recent clinical trials( Table 1 ) [ 9 , 17-25 ].
ICIs
James P. Alison and Tasuku Honjo were awarded the 2018 Nobel Prize in Physiology or Medicine for their discovery of immune checkpoints, the negative immune regulators [26] . The role of immune checkpoints in tumor immune escape is well established, and several mainstream anticancer immunotherapy strategies have focused on manipulating the immune checkpoints such as PD-1/PD-L1, LAG-3, and CTLA-4, using monoclonal antibodies( Fig. 1 ) [27] .
PD-1/PD-L1 blockers
Physiologically, PD-1/PD-L1 interaction is critical in immune regulation, which protects against autoimmunity by promoting apoptosis of antigen-specific T-cells and preventing apoptosis of Treg [28] . HCC cells may exploit PD-L1 expression, prevent apoptosis and escape the immune response. Blocking PD-1/PD-L1 interaction is thus a potential immunotherapy strategy against HCC [29] .PD-1 inhibitors, nivolumab 3 mg/kg/week IV and pembrolizumab 200 mg/3 weeks IV are previously approved monotherapies for advanced HCC. In a recent phase III clinical trial, nivolumab challenged sorafenib as a first-line treatment modality for HCC by showing a clinical advantage over sorafenib in terms of safety and tolerability, and median OS (16.4 mon vs. 14.7 mon). The study results showed that grade 3/4 treatment-related adverse events (AEs)were reported in 81 patients (22%) in the nivolumab group versus 179 (49%) in the sorafenib group. AEs related treatment discontinuation in sorafenib group was double (8%) compared to that of the nivolumab group (4%) [11] . Pembrolizumab has also shown clinical advantages in patients who progressed on sorafenib. Results from the phase III keynote-240 trial showed that median OS in HCC patients who received pembrolizumab as second-line therapy was 13.9 mon (95% CI: 11.6 to 16.0 mon) as compared to that of the placebo group (median 10.6 mon; 95% CI: 8.3 to 13.5 mon) [30] .
The recent approval of combination ICIs therapies such as nivolumab plus ipilimumab (anti-CTLA-4) and atezolizumab (anti-PD-L1) plus bevacizumab [vascular endothelial growth factor(VEGF) inhibitor] has further broadened the therapeutic options for advanced HCC [ 19 , 31 ]. Early data from the IMbrave150 study(n= 501) showed that ORR was 65% and the disease control rate(DCR) was 70% with atezolizumab (1200 mg IV every three weeks)plus bevacizumab (15 mg/kg IV every three weeks). Progressionfree survival (PFS) in the atezolizumab plus bevacizumab group was 6.8 mon compared to that of sorafenib (4.3 mon). It is reported that 57% of patients in the combination group and 55% of patients in the sorafenib group experienced grade 3/4 AEs [32] .Another combination of nivolumab at dose 1 mg/kg plus ipilimumab at dose 3 mg/kg was approved on March 11, 2020 for patients previously treated with sorafenib based on results from the Checkmate-040 (n= 140) clinical trial, in which ICIs showed ORR of 35% (including complete response in 12%), overall DCR of 54% (95% CI: 39.3%-68.2%). Twenty-nine percent of patients discontinued treatment while 65% delayed treatment due to adverse reactions. Common treatment-related AEs were rash (53%), pruritus (53%) and musculoskeletal pain (41%), cough, and diarrhea [10] .Overall, recent clinical statistics support anti-PD-1/PD-1 therapy for advanced HCC because of its safety and tolerability, as well as clinically significant results in terms of survival advantage, ORR, and DCR, etc.

Table 1 Summary of clinical status of potential immunotherapies for HCC.

Fig. 1. Monoclonal antibodies targeting the immune checkpoints.
In the VEGFLiver100 trial, combination of avelumab (anti-PDL1) at dose 10 mg/kg IV Q2W + axitinib (VEGF inhibitor) at dose 5 mg orally BID, showed median PFS of 5 mon, confirmed OS 13%and 6 mon and PFS rate of 35% in HCC [33] . Another combination of durvalumab (anti-PD-L1) with tremelimumab (anti-CTLA-4) has recently been given orphan drug status by the FDA for the treatment of HCC. In an early phase I/II study of the ongoing HIMALAYA trial (n= 40), the combination of durvalumab and tremelimumab showed confirmed ORR in 17.5%, ORR of 25.0% and the median time to response of 8 weeks (range 7.6-24.0 weeks) among 40 advanced HCC patients, and the combination was generally well tolerated with grade 3/4 treatment-related AEs or serious AEs in 25% patients [34] .
These accelerated approvals have generated great excitement and opened the door to new systemic treatments for advanced liver cancer. However, low ORR and acquired resistance in previously respondent patients are still posing a challenge to anti-PD-1/PD-L1 therapy. In a recent clinical study by Lee et al., anti-PD-1 dual therapy of nivolumab and pembrolizumab has shown a significantly higher response rate in HCC patients as compared to PD-1 monotherapy (46.2% vs. 20.8%,P= 0.049) [35] . Lee MS et al. reported that atezolizumab (PD-L1 inhibitor) combined with bevacizumab (anti-VEGF) showed improved median PFS in HCC patients compared to atezolizumab monotherapy (5.6 mon vs. 4.3 mon)[36] . All these results mentioned suggest that combined ICIs may be a potential therapeutic strategy to improve clinical outcomes in resistant patients.
Predictive biomarkers for PD-1/PD-L1 drugs
An upward trend in the number of cancer patients eligible for ICIs treatment has been observed over the years. A recent retrospective analysis showed that the estimated percentage of eligible cancer patients for the FDA-approved six ICIs was 1.54% in 2011 and increased to 43.63% in 2018 [37] . Currently available immunebased monotherapy or combination therapy for HCC typically has an ORR of 14%-35%. Therefore, predictive biomarkers are critical for selecting eligible patients for immunotherapy and for avoiding unnecessary costs and treatment-related toxicity in ineligible patients.
Several genetic and protein biomarkers have been discovered recently that could predict the clinical efficacy of anti-PD-1/PD-L1 therapy. Some important biomarkers include PD-L1 expression, tumor mutation burden (TMB) and circulating PD-L1, etc. Higher expression of PD-L1 by tumor cells has been associated with poor OS in HCC and better response to anti-PD-1/PD-L1 therapy [38] .In a phase II dose expansion cohort study, the response rate with nivolumab was 17.2% versus 32% in HCC patients with PD-L1<1%and PD-L1 ≥1%, respectively [39] .
TMB is another biomarker to predict response to ICIs. In non-small lung cancer (NSCLC) patients, higher TMB indicates worse prognosis, poor OS, and better response to PD-1/PD-L1 inhibitors [40] . Response to PD-1/PD-L1 inhibitors in patients with different types of tumors may be three times higher in patients with high TMB than in patients with low TMB. However, in the previous study, good responses to PD-1/PD-L1 inhibitors in cancer patients have been reported despite low TMB [41] .
De-glycosylated PD-L1 is another potential predictive biomarker to guide anti-PD-L1 immunotherapy in HCC. Glycosylation of PDL1 has been associated with its stability and resistance to anti-PDL1 antibody. Enzymatic de-glycosylation of PD-L1 can significantly improve the affinity of anti-PD-L1 antibody and signal intensity,which may be used to quantify PD-L1 molecules and to improve the efficacy of PD-1/PD-L1 inhibitors in HCC [42] . Soluble PD-1/PDL1 has recently been identified as a potential predictive biomarker of response to immunotherapy in different cancers. Similar to the membrane bonded PD-1/PD-L1 molecules, a high serum concentration of PD-L1 has also been associated with cancer aggressiveness and with better response to PD-1/PD-L1 inhibitors [43] .
The role of tumor-infiltrating lymphocytes (TILs) as a predictive biomarker for anti-PD-1/PD-L1 therapy was recently revealed in different cancers, such as melanoma, breast cancer, and NSCLC [ 44 , 45 ]. Uryvaev et al. [45] showed that CD8 + /CD4 + TILs ratio was positively correlated with response to anti-PD-1 therapy in patients with melanoma (n= 30) and NSCLC (n= 26). In patients with melanoma, a CD8 + /CD4 + TILs ratio less than 2 predicted a low response to anti-PD-1 immunotherapy response rate(30%;P= 0.006), while a CD8 + /CD4 + TILs ratio greater than 2.7 predicted a relatively better response (response rate 80%). Furthermore, their study reported that response rate to PD-1/PD-L1 inhibitors was 90% in tumors with CD8 + TILs count above 1900/mm2(P= 0.0 0 01), while tumors with TILs count less than 866/mm2showed a response rate of only 30%. Similar conclusion was obtained in NSCLC patients, where tumors with CD8 + TILs count less than 886/mm2showed a response rate of 16.7% (P= 0.046), while TILs CD8 + count of 886-1899/mm2showed a response rate of 60%(P= 0.017) [ 44 , 45 ]. The previous study has also demonstrated that PD-1 high CD8 + tumor infiltrate was associated with better response to anti-PD-1/PD-L1 immunotherapy in HCC [46] .
The specificity and sensitivity of the above predictive biomarkers are low. However, genetic pre-testing may still be considered before starting immunotherapy for advanced HCC patients to select the most suitable patients for the therapy and to prevent the treatment-related side effects and unnecessary financial losses.
Anti-CTLA-4
CTLA-4 is a protein receptor expressed on the surface of activated T-cells. Its higher expression has been associated with poor survival in cancer patients [47] . CTLA-4 plays a key role in developing peripheral tolerance to self-proteins by neutralizing the function of CD28 [48] . It binds to the B7 co-stimulatory receptor on the surface of antigen-presenting cell (APC) with affinity ten times higher and outcompetes its homolog of the immune costimulatory protein CD28 ( Fig. 1 ) [49] . Blocking CTLA-4 reverses the inhibitory signal to T-cell activation, making it a potential target for immunotherapy.
Anti-CTLA-4 monoclonal antibody, ipilimumab, has previously been approved for the treatment of metastatic melanoma [50] .Although there is no anti-CTLA-4 monotherapy for HCC available yet, the recent approval of combined ipilimumab and nivolumab in 2020 has been a significant development in CTLA-4 based immunotherapy. In the Checkmate-040 trial, nivolumab + ipilimumab showed a significant ORR of 33% (16/49; 95% CI: 20%-48%) [19] .
Another CTLA-4 inhibitor, tremelimumab, has shown significant clinical benefits when combined with radiofrequency ablation (RFA) or chemoablation. In a clinical trial (n= 32), patients with advanced HCC receiving tremelimumab plus RFA or chemoablation showed significant clinical benefits of median OS 12.3 mon, the 6- and 12-mon PFS in 57.1% and 33.1% of patients respectively, while no dose-limiting toxicities were encountered [26] .These results suggest that combination immunotherapy approach may be a potential modality for advanced HCC in the future. Several clinical trials of combination immunotherapies are currently underway, with highly anticipated results ( Table 2 ).
Lymphocyte-activation gene-3 (LAG3)
LAG3 is another immune checkpoint that can down-regulate the immune system similar to PD-L1 and CTLA-4. LAG-3 is a protein expressed both on CD4 + and CD8 + T lymphocytes, NK cells,dendrites, and other cells. The role of LAG-3 in CD8 + T cells exhaustion has previously been established in the murine study [51] .Preclinical studies have shown that blocking the LAG-3 checkpoint was associated with enhanced CD8 + T and CD4 + cell proliferation, production of pro-inflammatory cytokines such as IL-2,IFN-γand IL-4, and inhibition of the immunosuppressive activity of induced Treg cells [ 52 , 53 ]. LAG-3 expression is upregulated by tumor-infiltrating CD8 + T-cells in HCC which provides a clue for its role in the immune escape [51] . Blocking LAG-3 can augment T-cells activity, making it a potential immunotherapy target.
Early results from a phase I/II study in 2017 showed that 8 out of 68 melanoma patients who were initially resistant to other immunotherapy showed response to combined relatlimab (anti-LAG-3) plus nivolumab, and the combination was also well tolerated [54] . Anti-LAG-3 as a monotherapy or in combination with PD-1/PD-L1 inhibitors is undergoing phase I/II clinical trials for the treatment of solid tumors, including HCC (NCT01968109) [55] .LAG-3 may be a more effective target for immunotherapy thanPD-1/PD-L1 or CTLA-4, as LAG-inhibition not only activates the CD8 + cytotoxic T-cells but also down-regulates the immunosuppressive Treg cells. Furthermore, since PD-1/PD-L1 and LAG-3 are both highly expressed in HCC, combining anti-LAG-3 with anti-PD-1/PD-L1 may significantly enhance the efficacy of ICIs.
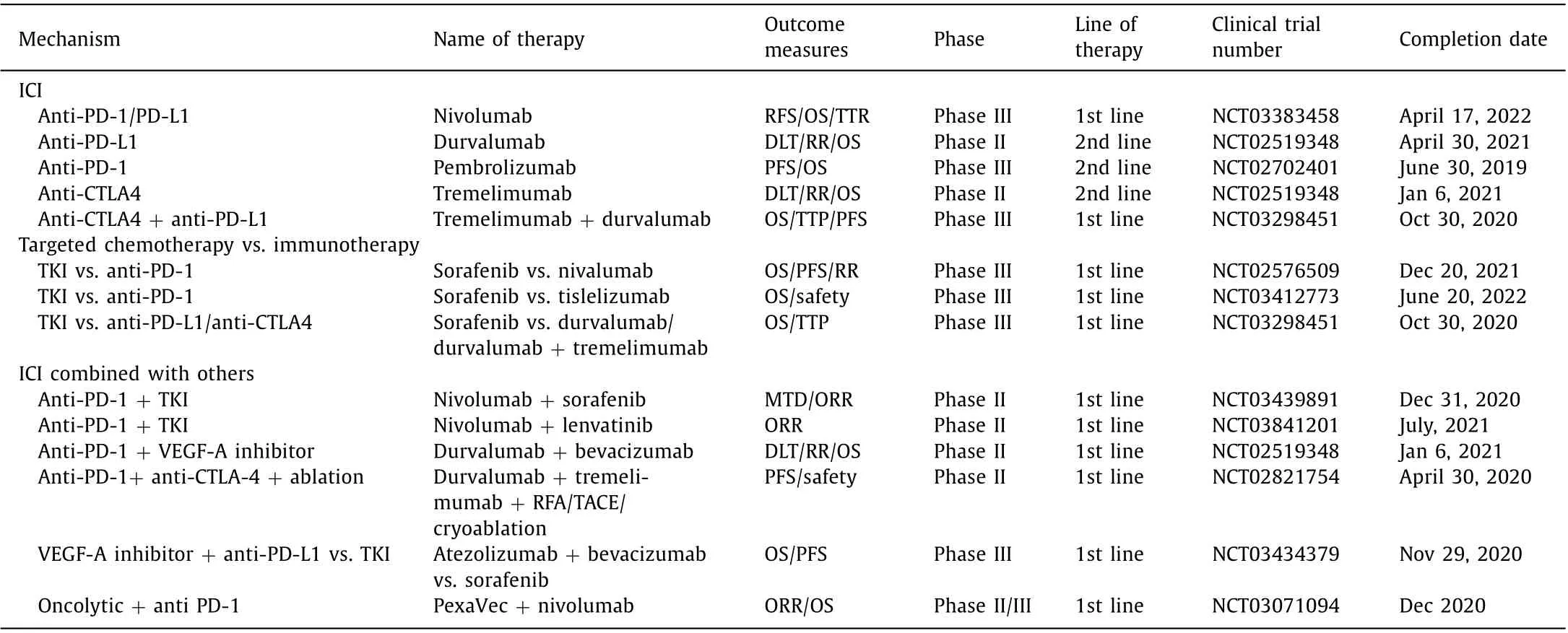
Table 2 Summary of important ongoing immunotherapy clinical trials for HCC.
Adoptive cellular therapies
In adoptive cellular therapy, a patient’s autologous or allogeneic immune cells are taken, sensitized, and activated against specific tumor antigenexvivoand then re-infused back to the patient [56] . TILs, cytokine-induced killer cell (CIK), CAR-T, cytotoxic T-lymphocytes, and NK cells may be used for this purpose.
CAR-T therapy
T-cells are genetically engineered to express artificially produced receptor (CAR) which gives them the ability to target tumor cells expressing a specific antigen ( Fig. 2 ) [28] . CAR-T therapy has been previously approved for acute lymphoblastic leukemia (ALL)and diffuse large B-cell lymphoma in which CAR-T cells target the CD19 expressing cells [57] . The majority of CAR-T therapy clinical trials over the last 30 years were carried out for hematological cancers, thus very little is known about its mechanism, safety, and efficacy in solid tumors including HCC [58] .
Recent preclinical studies have identified a number of potential targets for CAR-T therapy in HCC. In animal models, HCC cells positive for glypican-3 (GPC-3), MUC1, EGFR, and CEA can be potentially targeted and eliminated by specific CAR-T cells [59] . CAR-T immunotherapy in HCC has proven to be effective in animal studies, however, potential toxicities to normal cells expressing the target antigen may occur. In a recent study, CAR-T cells showed effi-cacy against liver cancer cell line, SMMC7721. The study reported that adoptive transfer of EGFRvIII CAR-T cells demonstrated antitumor activity bothinvitroand in animal models [60] . Results from a phase I clinical study (n= 13) suggest that genetically modified CAR-T cells targeted against GPC-3 may be a safe and potentially effective treatment for recurrent HCC. Shi et al. reported that most of the total thirteen patients with advanced GPC-3 + HCC who received CAR-GPC-3 + T cell therapy showed clinically manageable grade 1/2 treatment-related toxicities, while only one patient experienced a grade 5 cytokine release syndrome. They further reported that the OS rate at 6 mon was 50.3%, 1-year and 3-year OS rates were 42% and 10.5%, respectively, with a median OS duration of 279 days (95% CI: 48-615 d), and objective responses in 2 patients [61] .
Results from these preliminary studies of CAR-T immunotherapy are promising and it is now crucial to identify antigen targets that are expressed only by malignant cells but not by the normal cells to avoid potential toxicities. Further research is needed to identify tumor-specific antigens to guide the future CAR-T immunotherapy for HCC.
CIK cell therapy
CIK cell therapy is an attractive novel immunotherapy approach.Immune effector cells including CTLs and NK cells are generated byexvivoexposing of autologous human peripheral blood mononuclear cells (PBMC) to recombinant cytokines (IL-2, IL-1, IFN-γ) and anti-CD3 antibody. Cytokines provide a strong stimulation for proliferation and maturation of the effector immune cells (CIK cells)[62] . Heterogeneous CD3 + CD56 + cells can be amplified 10-100 times in a cytokine cocktailinvitro, giving CIK cells, like NK cells(CD56 + ) and cytotoxic T lymphocytes (CD3 + ), the ability to destroy cancer cells i.e., the MHC-dependent cytotoxicity of T cells and the MHC-independent cytotoxicity of NK cells [63] .
Results from a clinical phase I trial of HBV positive advanced HCC patients (n= 13) showed that infusion ofexvivogenerated CIK-cells could significantly increase CD3 + CD8 + , CD3 + CD56 + , and CD25 + cells from 33.5% ± 10.1%, 7.7% ± 2.8%, and 12.3% ± 4.5%to 36.6% ± 9.0% (P<0.05), 18.9% ± 6.9% (P<0.01), and 16.4% ± 5.9% (P<0.05), respectively. Furthermore, the average viral load was significantly reduced from 1.85 × 106copies of DNA/mL to 1.41 × 105copies of DNA/mL in 3 months and the treatment was also well tolerated [62] . Adjunct CIK cell therapy has shown clinical benefits in terms of PFS rate and OS in patients with HCC who had received curative surgical therapy, RFA, or transarterial chemoembolization (TACE). In one randomized control trial of 127 patients grouped into CIK1 (n= 41), CIK2 (n= 43),and control (n= 43), the 1-year, 2-year, and 5-year PFS rates were 83.1%, 31.7%, and 23.3% in the CIK1 group; 84.7%, 30.5%, and 19.4%in the CIK2 group; and 82.6%, 20.9% and 11.2% in the control group,respectively. However, no statistically significant difference in OS was observed among the three groups [64] .

Fig. 2. Key steps involved in CAR-T cells cancer therapy.
In another randomized control trial in which post-operation RFA or ethanol injection treated HCC patients were enrolled(n= 230), the CIK cell therapy group received adjunct CIK-cell treatment 16 times during 60 weeks. A significant difference in PFS was observed between the treatment group (44 mon) and the control group (30 mon) [65] . Data from these studies highly favor CIK cell immunotherapy as an attractive approach for HCC in the future.
NK cell immunotherapy
NK cells unlike T-cells do not require MHC molecules for antigen presentation or preceding sensitization to antigen to elicit an immune response [66] . Mature NK cells are CD3-, CD16 + and CD56 + , and express a number of activating and inhibitory receptors. The activating natural cytotoxicity receptors (NCRs) recognize viral and bacterial or proliferating cell nuclear antigen (PCNA) and release cytotoxic granzymes or perforin to induce apoptosis of the target cells, while CD 16 plays its role in antibody-dependent cellmediated cytotoxicity [67] .
NK cells preferentially target cells with low or lacking MHC-1 molecules, thus are particularly important against malignant cells with low MHC-1 expression [68] . NK cell therapy requires adequate cell number and purity for clinical use.Exvivoexpansion of peripheral blood NK cells, umbilical cord blood, and human embryonic stem cells is carried out to generate bulk of NK cells [69] . Different cell culturing protocols for NK cell expansion are currently available, from expansion capacity of 60-180 fold over two weeks up to 10 × 1010fold over three weeks [70] .
Combination allogeneic NK cell therapy has shown significant clinical efficacy in advanced HCC. In a clinical study of 61 patients with advanced HCC, the PFS in the cryoablation (cryo) plus NK cell group (n= 35) was 9.1 mon with response rate of 60%, compared to PFS of 7.6 mon and response rate of 46.1% in the cryoablation or NK cell alone group (n= 26). The main treatment-related side effect was fever, while only a few patients experienced manageable side effects such as fatigue, chill, pain, and pleural effusion [71] . Allogeneic NK cells combined with irreversible electroporation (IRE)have been reported to significantly improve median OS in HCC patients. Results of a clinical study (n= 40) showed that OS of the combination group (NK cell + IRE,n= 20) was 10 mon compared to 8.9 mon in the IRE alone group (n= 20,P= 0.007) [72] .
Genetic modification of NK cells may improve its efficacy and specificity. Yu et al. showed that GPC-3 targeted CAR-NK-cells have enhanced tumor infiltration capacity and more potent anticancer activity in HCC xenograft. GPC-3 targeted CAR-NK cells infiltrate GPC-3 + HCC xenograft leading to decreased tumor proliferation and increased apoptosis [23] . NK cells transfer either alone or in combination with ablation therapy may be a potential treatment approach for advanced HCC. Therefore, further clinical trials are needed to investigate its efficacy and safety in solid tumors.
Therapeutic cancer vaccine
Therapeutic cancer vaccines are designed to recognize a specific antigen and generate a targeted anticancer immune response [73] .The first cancer vaccine was experimented by Dr. William Coley in 1891. He found that stimulation of the immune system by injecting streptococcus organism in sarcoma patients resulted in shrinkage of cancer [74] .
Immune cells are educated to mount anticancer immune response byexvivopulsing of tumor associated antigen (TAA), such as prostatic acid phosphatase (PAP) and alpha-fetoprotein (AFP), to dendrites cells of a patient in the presence of immune-stimulating factors such as granulocyte macrophage colony-stimulating factor(GM-CSF) and cytokines, and then the activated dendrites cells were re-infused back to the patient [75] . Therapeutic vaccines have shown clinical benefits in different cancers such as sarcoma,NSCLC, prostate cancer, and non-Hodgkin’s lymphoma [76] . HCC vaccines include autologous or allogeneic HCC cells, antigen peptides (i.e, AFP, GPC-3), and dendrites cells.
AFP vaccine based clinical trial in the early 20 0 0s and a phase I/II trial (n= 10) of AFP pulsed dendrites cells vaccine based clinical trial in 2006, did not report any significant clinical outcome in terms of survival in advanced HCC patients. However, the results showed that AFP peptide plus autologous dendrites cells vaccine could increase IFN-γproduction, and promote AFP specificT-cells immune response [77] . Furthermore, autologous dendrites cells vaccination was reported to be safe and well-tolerated by HCC patients in a clinical phase I/II trial (n= 30) [78] . In a recent murine model study, Teng et al. showed that dendrites cells vaccine alone or in combination with PD-L1 inhibitor could potentially improve survival in patients with HCC [79] .
GPC-3 peptide vaccine has been tried as an adjuvant therapy after surgery in a clinical phase II trial. Results showed that 1-year recurrence rate in the vaccinated group (n= 350) was reduced by approximately 15%, and the 5-year and 8-year survival rates were improved by approximately 10% and 30% respectively, as compared to the unvaccinated group (n= 33) [80] . Although HCC vaccine is a promising therapeutic option, data from previous studies are still limited. Therefore, further exploration is needed to evaluate the efficacy and safety of HCC vaccines.
Cytokine therapy
Cytokines make a large and diverse category of small proteins(~5-20 kDa) secreted by the immune cells in response to antigens and are necessary for cellular signaling, immune regulation,and hematogenesis [81] . Cytokines such as interleukine-2 (IL-2)and interferon-α(INF-α) have successfully been used for the treatment of advanced melanoma, renal cell carcinoma, and hairy cell leukemia. IL-7, IL-12, IL-15, IL-21, and GM-CSF also have shown efficacy in pre-clinical studies [82] . IL-2 is predominantly produced by CD4 + T cells (Th1), NK cells, and dendrites cells. The anticancer role of IL-2 is concentration-dependent, which means that high serum concentration of IL-2 saturates the receptors for activation of cytotoxic T-cells and enhances anticancer response, while low concentration favors the activation of Treg cells that produces inhibitory cytokines such as IL-10, thus down-regulating the anticancer immune response [83] .
In a phase II trial of 255 patients with stage 4 renal cancer, patients treated with a high dose of recombinant IL-2 (600 000 to 720 0 0 0 IU/kg) showed an objective response rate of 15% (37/255),complete response in 7% patients, partial response in 8% patients,and median OS of 16 mon. However, the response rate with low dose recombinant IL-2 was clinically negligible [84] . Also, severe toxicities, high cost, and global unavailability pose a challenge to its use as a standard treatment.
Genetically engineered IL-2-expressing adenovirus (RAD-IL-2)may also be a promising immunotherapy approach for HCC. In a recent animal model study, HCC mice treated with RAD-IL-2 showed reduced tumor growth and improved OS compared to placebo. RAD-IL-2 could significantly enhance the anti-tumor cytotoxic T-cells response, increase interferon-γproduction, and enhance CD4 + and CD8 + T-cells recruitment into the tumor microenvironment [85] .
Another immune-stimulating cytokine, serum IL-7, is recently being investigated for the treatment of HCC. Previous studies have found that the concentration of IL-7 was down-regulated in HCC patients, and its stimulation could enhance CD8 + T cell activity and reduce PD-1 expression by cytotoxic T cells [86] . A combination of anti-PD-1/PD-L1 antibody and IL-7 may produce synergic anticancer activity. Treatment of murine HCC models with IL-2 expressing recombinant adenovirus (RAD-IL-2) can significantly enhance CD8 + T lymphocyte activity, reduce tumor volume, and improve survival [85] . The potential of cytokine-based immunotherapy in kidney cancer and advanced melanoma has been demonstrated. However, further clinical trials are needed to determine its clinical efficacy and safety in advanced HCC.
Others
ICI combined with ablation therapy
The inflammatory molecules released by direct killing of tumor cells with ablation therapy activate the immune system, thus combining ablation therapy with immune-enhancing drugs like tremelimumab may enhance the anti-tumor immune response in a synergic fashion and may enhance the efficacy of ICIs in HCC [25] .CTLA-4 inhibitor, tremelimumab, in combination with ablation can significantly enhance the anti-tumor immune response in HCC and improve clinical outcome in terms of patient’s response, median OS, and PFS. Results from a clinical phase I/II trial of 32 advanced HCC patients who received tremelimumab for 3 mon at 2 dose level (3.5 mg/kg and 10 mg/kg IV) every 4 weeks for 6 doses and ablation therapy on day 36, showed that confirmed partial responses in five out of nineteen evaluable patients (26.3%; 95%CI: 9.1%-51.2%), significant HCV viral load reduction in 12 of 14 HCV + patients, 6 and 12 mon probabilities of tumor progression of 57.1% and 33.1%, respectively, median time-to-tumor progression of 7.4 mon (95% CI: 4.7-19.4 mon) and OS of 12.3 mon (95% CI:9.3-15.4 mon). The treatment was well tolerated with only few patients reported pruritus [25] . Preclinical animal model experiments have demonstrated an enhanced effect of ICIs in mice previously treated with RFA plus oncolytic virus (HSV-1). A phase II clinical trial (n= 90) for dual anti-CTLA-4 and anti-PD-1/PD-L1 combined with TACE, RFA and cryoablation is currently ongoing for the treatment of HCC and biliary tract diseases (NCT02821754)( Table 2 ) [87] .
Data from previous clinical trials and murine studies have shown the potential of combination of ablation with immuneactivating agents in HCC patients. This suggests that ICIs in combination with ablation are a promising treatment modality for HCC and should be validated in further clinical trials.
Oncolytic virus plus ICI
Attenuated viruses have been in use for vaccination for decades.An attenuated virus’s disease causing ability is weakened but retains immunogenicity. Oncolytic viruses destroy tumor by replicating virally inside the tumor cells and by activating anti-tumor immune response [88] . Oncolytic viruses may be genetically engineered to replicate preferentially within the tumor cells. The exact mechanism of how oncolytic virus such as talimogene laherparepvec (T-VEC) produces the anti-tumor response is not clear.However in theory, the dying viral infected cells produce GM-CSF which attracts dendrites cells, the APCs then present the tumor antigens to T-cells for activating the immune response [89] . Some oncolytic virus therapies are approved for advanced melanoma in a few countries, and for head and neck cancer in China [ 90 , 91 ].
Pexa-Vec, a genetically engineered virus armed withGM-CSFgene, was previously tried as a first-line treatment in the phase III PHOCUS trial (n= 600) and as a second-line treatment after sorafenib in the phase II TRANSVERS trial (n= 129) [92] . Unfortunately, these trials failed to reach primary endpoints. However,Pexa-Vec immunotherapy was generally well tolerated. Previous data from a phase II trial showed statistically significant improvement in OS of HCC patients receiving high dose Pexa-Vec (1 × 109PFU) compared to the low dose (1 × 108PFU), OS 14 mon versus 6.7 mon. Pexa-Vec is also being tested in combination with nivolumab (Opdivo) in a phase II trial by “Transgene” company(n= 36) [93] .
Currently, there is no approved oncolytic virus therapy for HCC.However, results from previous trials are promising. Oncolytic virus immunotherapy in combination with ICI, targeted chemotherapies,or loco-regional therapy is a promising strategy for future trials.Combining oncolytic virus with ICIs may boost anticancer immune response synergistically as ICI enhances cytotoxic T-cells activation and infiltration, while genetically engineered immunogenic oncolytic virus may direct the immune response against the infected tumor cells.
Combined ICI (anti-PD-1/PD-L1 plus anti-CTLA-4)
The 5-year OS rate in HCC is less than 20% [94] . Response rate using single ICI for HCC is less than 20%. Studies have shown combination immunotherapy enhanced the anti-tumor effect with dual PD-1/CTLA-4 blockade [95-97] . Dual ICI therapy has shown considerable survival benefit in advanced melanoma, while phase III study to assess the efficacy and safety of durvalumab (anti-PD-L1)plus tremelimumab (anti-CTLA-4) combination therapy for patients with advanced HCC who have not received prior systemic therapy is currently underway ( Table 2 ) [98] .
Conclusions
The global burden of mortality due to HCC is one of the highest. With the failure of conventional chemotherapy and unsatisfactory outcome of molecular targeted drugs, immune-based treatment approaches have become a new focus of research in HCC treatment. First FDA-approved immunotherapy for HCC, nivolumab,has shown clinical superiority in terms of OS and safety over the current standard of treatment, sorafenib, in a multi-center phase III CheckMate-459 study [11] . Recently approved ICIs, such as nivolumab and pembrolizumab, have revolutionized HCC treatment, while the potentials of other approaches, particularly combination immunotherapy, cellular therapy, cytokines, and cancer vaccines have also been demonstrated in preclinical and clinical trials.
Diagnostic and prognostic biomarkers are important in clinical decision making when considering immunotherapy for cancer patients. PD-L1 expression level and TMB have been identified as potential biomarkers to predict the clinical outcome when using ICIs [99] . De-glycosylated PD-L1 may be used to quantify PD-L1 molecule, and to improve the efficacy of PD-1/PD-L1 inhibitors in HCC [100] . Genetic pre-testing is advisable for patients in whom treatment with ICI is planned. However, high cost and low specificity of genetic pre-testing limit its routine clinical use. More predictive biomarkers are thus needed to be identified which could not only predict treatment outcome but also are cost-effective.
Our current knowledge of immune-based approaches for HCC is just like the tip of an iceberg. Therefore, further research is needed to identify new biomarkers and uncover the unique immune escaping mechanism of HCC. Hopefully, once the ongoing trials are completed, more effective and safe immune therapeutic drugs will become available in the near future.
Acknowledgments
None.
CRediT authorship contribution statement
Abid Ali Khan: Conceptualization, Data curation, Writing - original draft. Zhi-Kun Liu: Data curation, Writing - original draft. Xiao Xu: Funding acquisition, Supervision, Writing - review & editing.
Funding
This study was supported by grants from the National S&T Major Project (2017ZX10203205) and Key Research & Development Plan of Zhejiang Province (2019C03050).
Ethical approval
Not needed.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Hepatocellular-cholangiocarcinoma with sarcomatous change:Clinicopathological features and outcomes
- Toll-like receptors and hepatitis C virus infection
- Involvement of the circular RNA/microRNA/glucose-6-phosphate dehydrogenase axis in the pathological mechanism of hepatocellular carcinoma
- Progress in hepatitis B virus-related acute-on-chronic liver failure treatment in China: A large, multicenter, retrospective cohort study using a propensity score matching analysis ✩
- From conventional two-stage hepatectomy to ALPPS: Fifteen years of experience in a hepatobiliary surgery unit
- Melatonin attenuates hepatic ischemia-reperfusion injury in rats by inhibiting NF- κB signaling pathway
