Hepatocellular-cholangiocarcinoma with sarcomatous change:Clinicopathological features and outcomes
2022-01-07YeRongQinJingPengLiuXuFengZhngXueMinLiuYiLvJunXiXing
Ye-Rong Qin , b , N Jing , Peng Liu , b , Xu-Feng Zhng , b , Xue-Min Liu , b , Yi Lv , b ,Jun-Xi Xing , b , *
a Department of Hepatobiliary Surgery, First Affiliated Hospital of Xi’an Jiaotong University, No. 277 West Yan-ta Road, Xi’an 710061, China
b National Local Joint Engineering Research Center for Precision Surgery and Regenerative Medicine, First Affiliated Hospital of Xi’an Jiaotong University,Xi’an 710061, China
c Department of Pathology, First Affiliated Hospital of Xi’an Jiaotong University, Xi’an 710061, China
Combined hepatocellular-cholangiocarcinoma (cHCC-CCA) is a rare type of cancer, accounting for 0.4%-14.2% of hepato-carcinoma.Hepatic sarcoma is only less than 2% of primary malignant liver tumors. Thus, sarcomatoid cHCC-CCA is extremely rare. To the best of our knowledge, only twenty-six cases had been reported in the literature [ 1 -19 ]. Consensus cannot be reached due to the difficulty of preoperative pathological diagnosis and disagreement for the selection of the treatment. Therefore, clinicopathological features and outcome data about sarcomatoid cHCC-CCA are essential for diagnosis and treatment. We reported in this article three cases with clinical data and prognostic analysis, and summarized 26 cases of sarcomatoid cHCC-CCA from published literatures.
Case 1: A 46-year-old man with hepatitis B virus infection was referred and admitted to our hospital due to mild, right upper abdominal pain. The enhanced computed tomography (CT) scan showed liver cirrhosis and a tumor in the liver. There was no evidence of tumor metastasis ( Fig. 1 A, B). Details were presented in Table 1 . The patient underwent segmentectomy of liver segments V, VI and IVb. The tumor showed a heterogeneous pattern on microscopic examination ( Fig. 1 G-I). Microscopic analysis revealed that the relative proportions of the tumor consisted of 20%of poorly differentiated HCC, 80% of CCA with massive necrosis and some parts of sarcomatoid component. Immunohistochemistry results were shown in Fig. 1 M-O and Table 2 . Intra- and extrahepatic metastases were presented by CT scan 2 months postoperatively ( Fig. 1 C). The patient was treated with transcatheter hepatic arterial chemoembolization (TACE) to control the disease progression. Unfortunately, the patient eventually died four months after liver surgery because of extensive metastasis.
Case 2: A 60-year-old man had 20-year history of hepatitis B-related cirrhosis. He had mild, upper abdominal pain for a month. Enhanced CT scan detected patchy enhancement and delayed phase washout lesion in the right posterior hepatic section( Fig. 1 D-F). The patient underwent right posterior hepatectomy.Pathologically, the tumor was 4.0 × 3.5 × 2.5 cm with grayishwhite, fish-like specimen plane and complete pseudocapsule. Microscopically, the tumor mainly comprised carcinomatous element,which was poorly differentiated with combined HCC (60%) and foci of CCA (40%, some area presented sarcomatoid tissue) ( Fig. 1 J,K).Bone metastases were found. The patient died 32 months after hepatectomy.
Case 3: An asymptomatic, 50-year-old man had a 16-year history of hepatitis B-related cirrhosis. CT scan revealed the hepatic masses at the left lateral segment and the right inferior segment of the liver. Liver transplantation was performed one month after TACE. The tumor consisted of moderately-poorly differentiated HCC, 30% of CCA and 15% of sarcomatoid component ( Fig. 1 L).The patient was alive at the last follow-up in September 2019, 8 months after liver transplantation.
Combining with the three cases in this article, the clinicopathological features and prognosis of the 29 patients were comprehensively analyzed. Baseline information was presented in Table 1 . Hepatectomy, liver transplantation and vascular interventional therapy were performed in 16 (80.0%), 2 (10.0%) and 6(30.0%) cases, respectively (9 cases not available). The 1-, 2- and 3-year overall survival rates of the entire cohort were 43.3%, 32.5%and 16.2%, respectively ( Fig. 2 A, 9 cases not available), while the 1-, 2- and 3-year overall survival rates of the patients who underwent hepatectomy or transplantation were 51.6%, 38.7% and 19.4%,respectively ( Fig. 2 B).

Fig. 1. Abdominal computed tomography and microscopic findings of the resected specimen. A-C: Arterial phase, suggestive of poor blood supply liver cancer or cholangiocarcinoma ( A ), delayed phase ( B ) and 2 months after the operation ( C ) of Case 1, intrahepatic metastasis was considered. D-F: Baseline scan ( D ), arterial phase ( E ) and portal venous phase ( F ) of Case 2. In Case 1, hepatocellular carcinoma cells coexisted with cholangiocarcinoma cells (hollow star) and carcinosarcoma cells (triangle) (HE staining, original magnification × 40) ( G ), better differentiated hepatocellular carcinoma showed polymorphic cells with oval or round nuclei and eosinophilic cytoplasm(HE staining, original magnification × 200) ( H ) and the majority of the tumor was composed of poorly differentiated cholangiocarcinoma cells (solid star) (80%) with carcinosarcoma cells (triangle) (HE staining, original magnification × 100) ( I ). In Case 2, cholangiocarcinoma cells (solid star) coexisted with carcinosarcoma cells (triangle) (HE staining, original magnification × 100) ( J ), and the majority of the tumor was composed of poorly differentiated hepatocellular carcinoma cells (60%) (HE staining, original magnification × 200) ( K ). The sarcomatoid was composed of spindle-shaped cells in Case 3 (HE staining, original magnification × 100) ( L ). Immunohistochemical expression from the first case (original magnification × 100) showed that cholangiocarcinoma cells were positive for CK7 ( M ) and CK19 ( N ), and that the sarcomatoid component was diffuse and strongly stained with vimentin ( O ). HE staining: hematoxylin-eosin staining.
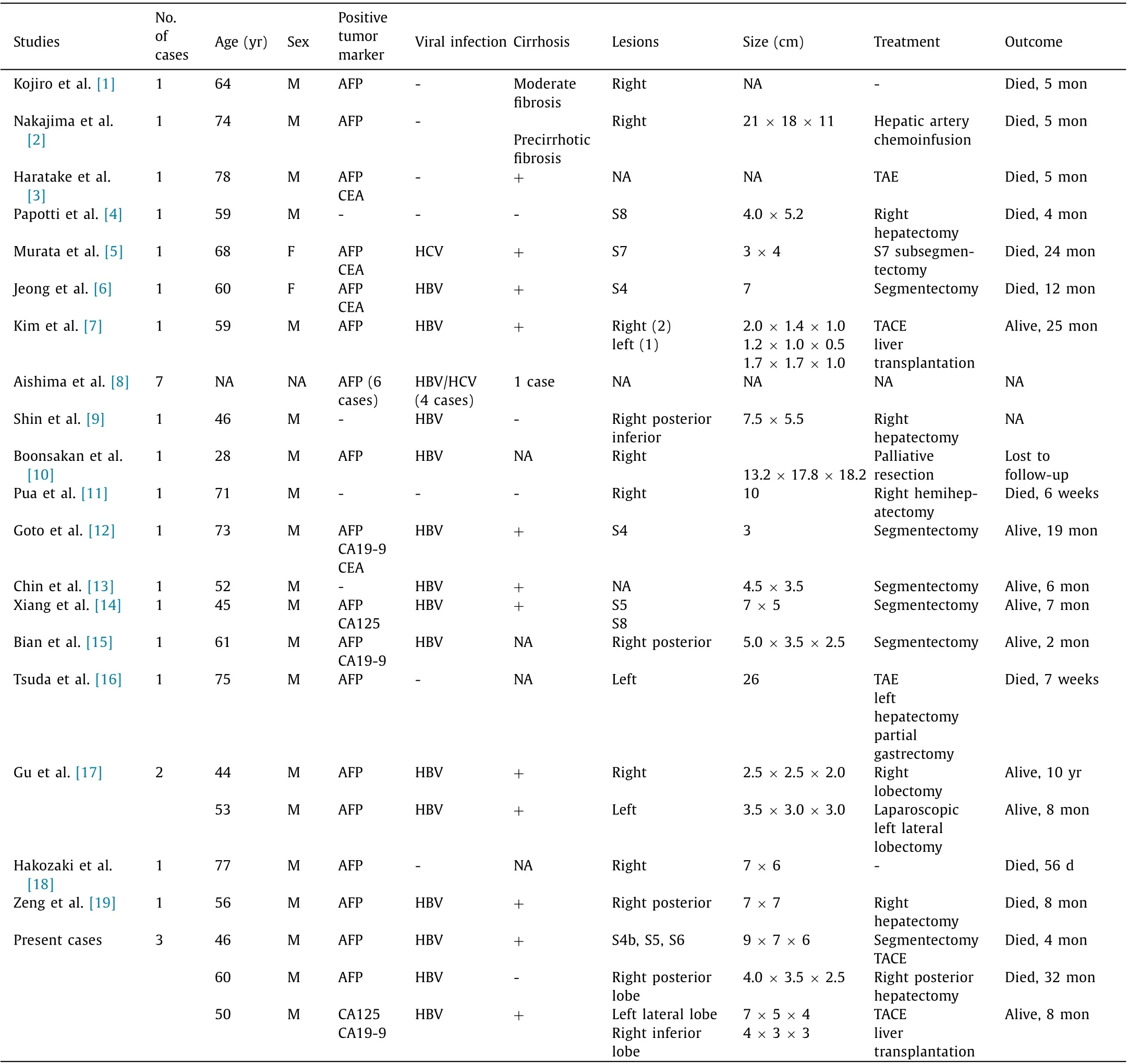
Table 1 Summary of reported cases of liver carcinosarcoma with combined hepatocellular cholangiocarcinoma.
According to World Health Organization classification, the coexistence of hepatocellular and cholangiocellular cells as well as cells intermediate between HCC and CCA suggests a common stem cell origin [ 20 , 21 ]. Morphologically, the two cell types can exist separately, contiguously but independently, or intermingled within a mass. In histopathology, sarcomatoid cHCC-CCA is defined in histopathology as the proliferation of spindle-shaped cells and pleomorphic malignant cells with bizarre nuclei and abundant mitoses [ 5 , 8 , 10 ]. However, the histogenesis of sarcomatoid cHCC-CCA is still unclear. The stem cell theory holds that both carcinomatous and sarcomatous neoplasms arise from differentiated multipotent stem cells [ 9 , 11 , 12 ], and cancer stem cells are thought to be the “root” of a tumor, which possess the ability of indefinite self-renewal to drive tumorigenesis. When it comes to the “conversion” theory, the sarcomatous element is transformed or dedifferentiated from pre-existing carcinoma based on the observation of a transitional zone [ 3 , 10 ]. Xiang et al. [14] proposed a new hypothesis combining stem cell theory and “conversion” theory. They suggested that liver progenitor cells are not determined as stem cells, and that liver progenitor cells are probably the cell origin of liver tumors containing carcinomatous and sarcomatous elements.Besides diffusely expressing mesenchymal markers, most sarcoma cells in the present cases also stained positive for the epithelial membrane antigen suggesting an obvious bi -directionally differentiated feature of the sarcoma, which could have originated from transformed epithelial cells.
Primary sarcomatoid cHCC-CCA is difficult to diagnose precisely and preoperatively. It needs comprehensive diagnosis, including atypical symptoms, hepatitis history, imaging evidence, raised level of tumor markers, etc. Pua et al. [11] suggested that preoperative diagnosis of cHCC-CCA by CT scan based on the identification of the enhancement characteristics of the individual tumor components is possible. The area of nonspecific hypovascular contrast enhancement may be an implication, but it is not specific on the pattern of enhancement for sarcomatous change. Therefore, sarcomatoid changes within cHCC-CCA are still rarer and more reportedcases are needed to establish diagnostic radiological features. Final diagnosis of sarcomatoid cHCC-CCA depends on histology. In our three cases, foci of sarcomatous elements were typically differentiated. For instance, the spindle cells diffusely expressed mesenchymal markers and CK19.
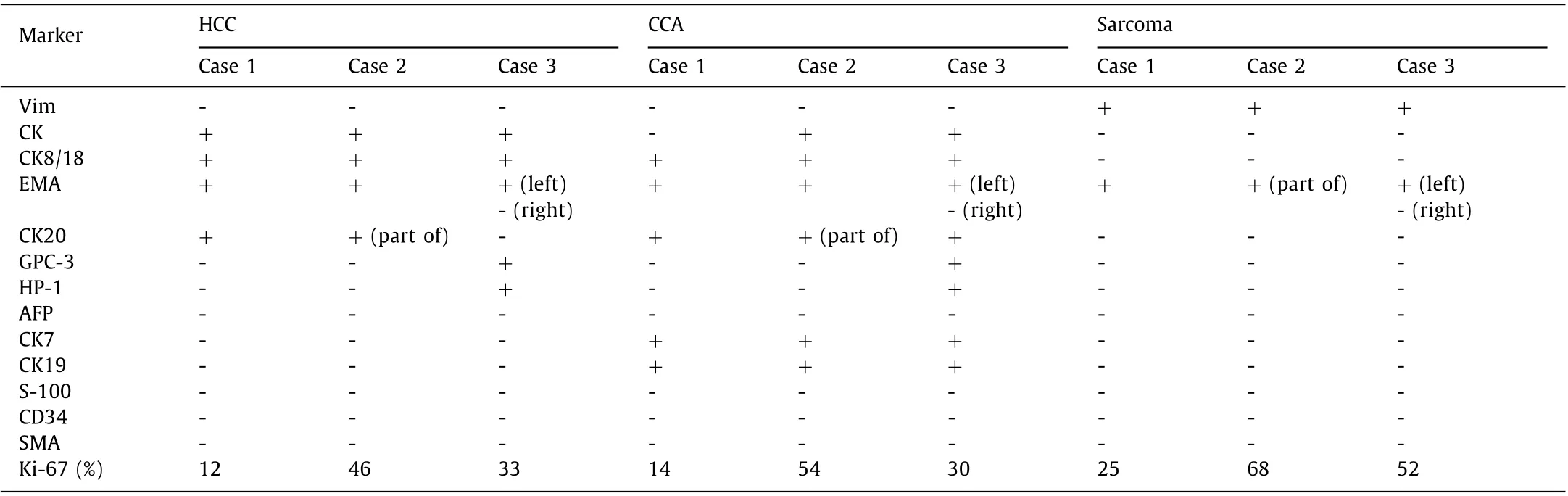
Table 2 Immunohistochemistry stain of three cases.
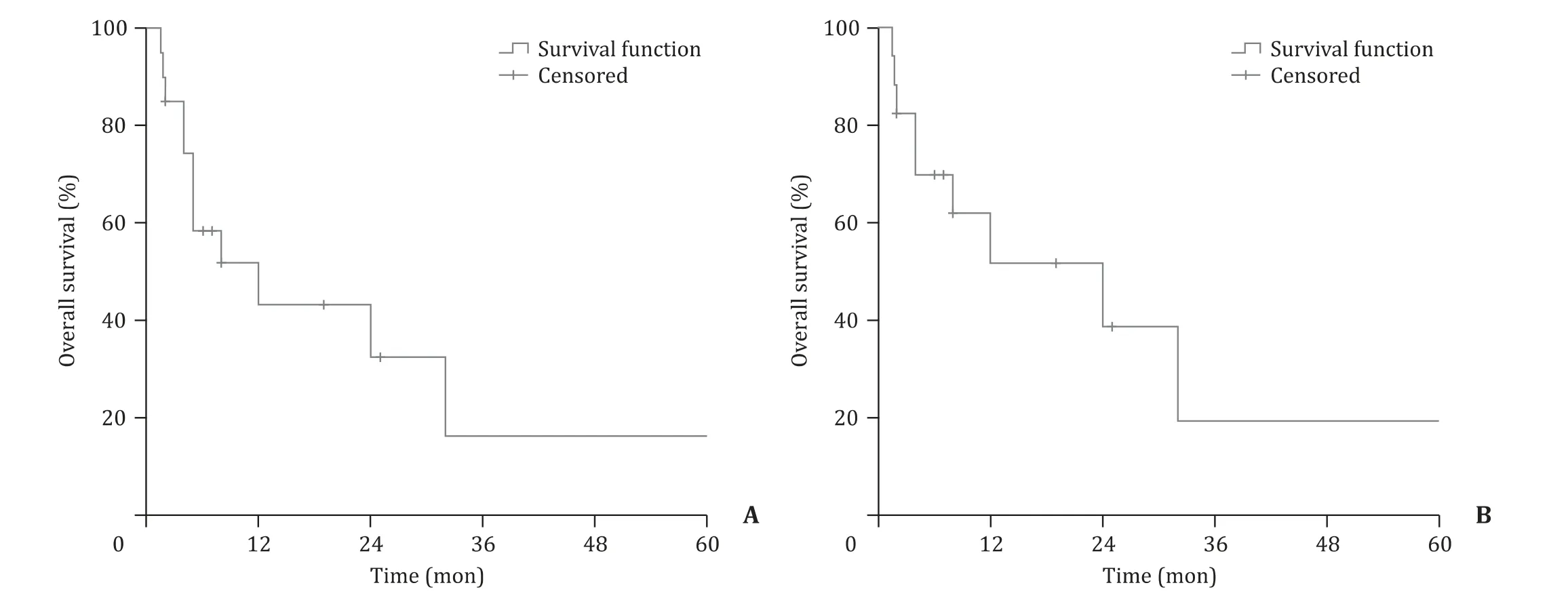
Fig. 2. A: The survival curves of all available cases (9 cases not available). B: The survival curves of all available cases who underwent hepatectomy or transplantation.
Currently, surgical resection is the preferred treatment for sarcomatoid cHCC-CCA, but the prognosis remains poor even with R0 resection. Liver transplantation seems to be a reliable treatment for a better survival. Two patients who underwent liver transplantation (one is from our case, and the other one is from Korea [7] ) were still alive (25 months and 8 months until submitting). TACE/TAE might be a more palliative option for those whose lesions could not be removed surgically and it can also be an adjuvant therapy after hepatectomy or transplantation. More prognostic data are needed to assess the effectiveness.
The results might imply that surgical procedures may improve long-term survival. The sarcomatous components showed higher Ki-67 labeling index than the definite HCC or CCA components and higher invasive and metastatic potential in clinical practice(eg. portal vein and central venous system, diaphragm, bone and peritoneal cavity) [18] . The first patient of our cohort had intraand extra-hepatic metastases 2 months postoperatively, while bone metastases were detected in the second patient. These results suggest that the presence of the sarcomatous component may worsen the prognosis of cHCC-CCA, and that the prognosis may be related to the physical constitution, tumor size and the existence of metastasis. The prognosis for sarcomatoid cHCC-CCA faces conflicting evidence because of its rarity. Extended follow-up and more reported cases are necessary.
In summary, we described 3 cases and summarized 26 patients from published literatures on sarcomatoid cHCC-CCA, of which data about clinicopathological features and prognosis have been reported. This type of tumor shows an unfavorable prognosis with frequent intra- and extra-hepatic metastases. Further investigations are needed to fully identify the clinicopathological features and the prognosis of sarcomatoid cHCC-CCA.
Acknowledgments
None.
CRediT authorship contribution statement
Ye-Rong Qian: Data curation, Formal analysis, Writing - original draft. Na Jiang: Data curation, Resources. Peng Liu: Conceptualization, Formal analysis. Xu-Feng Zhang: Methodology, Project administration. Xue-Min Liu: Data curation, Validation. Yi Lv:Conceptualization, Funding acquisition. Jun-Xi Xiang: Supervision,Writing - review & editing.
Funding
This study was supported by a grant from Institution Foundation of the First Affiliated Hospital of Xi’an Jiaotong University (No.2019QN-09).
Ethical approval
This study was approved by the Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University. Written informed consent was obtained from all participants.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Successful treatment of complete traumatic transection of the suprahepatic inferior vena cava with veno-venous and cardiopulmonary bypass with hypothermic circulatory arrest ✩
- Successful withdrawal of antiviral treatment in two HBV-related liver transplant recipients after hepatitis B vaccination with long-term follow-up
- High perioperative lactate levels and decreased lactate clearance are associated with increased incidence of posthepatectomy liver failure
- An NSQIP survey of outcomes after resection of choledochal cysts in adults
- Glucagonoma syndrome with necrolytic migratory erythema as initial manifestation
- A rare type of choledochal cysts of Todani type IV-B with typical pancreaticobiliary maljunction
