Progress in hepatitis B virus-related acute-on-chronic liver failure treatment in China: A large, multicenter, retrospective cohort study using a propensity score matching analysis ✩
2022-01-07LanLanXiaoXiaoXinWuJiaJiaChenDongYanDongYanShiJianRongHuangXiaoWeiXuLanJuanLi
Lan-Lan Xiao, Xiao-Xin Wu, Jia-Jia Chen, Dong Yan, Dong-Yan Shi, Jian-Rong Huang,Xiao-Wei Xu , Lan-Juan Li
State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Center for Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, the First Affiliated Hospital, Zhejiang University, Hangzhou 310 0 03, China
Keywords:Hepatitis B virus-related acute-on-chronic liver failure Propensity score matching analysis Short-term survival rate Standard medical therapy Artificial liver support system
ABSTRACT
Introduction
Hepatitis B virus (HBV) infection is a global public health threat that causes considerable liver-related morbidity and mortality [1] .In China, 78 million people are currently estimated to be infected with the HBV; of these, 28 million have active hepatitis and account for nearly one-third of all chronic infections worldwide [ 2 , 3 ].HBV-related acute hepatitis and the reactivation of HBV are the leading causes of liver failure in Asia [4] . HBV-related acute-onchronic liver failure (HBV-ACLF) is a common syndrome with high mortality in the Asia-Pacific and African regions [5] . HBV-ACLF is most commonly caused by acute and severe exacerbation of chronic hepatitis B (CHB) [6] , which is in contrast to Western countries where drugs, alcohol, and hepatitis C are the major causes of liver failure [7] .
The mortality is extremely high (30% to 70%) in patients with ACLF unless patients promptly receive liver transplantation(LT). However, LT is often not available due to the shortage of donors [8] . The treatment of ACLF has been improving with an aim of increasing the survival or maintaining the condition of the patient until a donor is available. Over the past few decades, a variety of artificial liver support systems (ALSSs), including the molecular adsorbent recirculating system (MARS), Prometheus, and other methods, have been employed to treat liver failure [9-12] .MARS [ 13 , 14 ] and Prometheus [9] are the most widely used in countries outside of China. The plasma exchange (PE)-based ALSS is widely used in China, which innovatively uses plasma separators with an aperture of approximately 1/10 (membrane pore size = 0.03 micron) of that of a normal plasma separator for direct PE. This can remove toxic substances effectively in patients with liver failure, retain important plasma components, and reduce plasma dosage [15] .
The attitudes towards nucleos(t)ide analogs are controversial.Some studies have reported that nucleos(t)ide analogs were not able to generate an obvious biochemical effect or slow down the progression of liver failure in patients with HBV-ACLF [ 16 , 17 ]. However, several studies have noted that nucleos(t)ide analogs effectively improved the status of patients with severe decompensated chronic liver disease and HBV-related liver failure [18-21] . More evidence-based data are still needed to come to a consensus.
Treatment progression for HBV-ACLF in China in the past decade has not been well characterized. In this study, we assessed two different nationwide cohorts (from 2012 to 2015 and 2008 to 2011) to examine whether the treatment for HBV-ACLF has significantly improved during the past decade.
Methods
Study design
The present investigation was a multicenter, nationwide, retrospective cohort study. Between September 2008 and February 2011,we screened 1592 adults with HBV-ACLF at 10 Liver Disease Research Centers in China and enrolled patients in the cohort I. The patients in the cohort II were enrolled between December 2012 and March 2015 from 11 Liver Disease Research Centers. The patients in the cohorts I and II were assigned to the standard medical therapy (SMT) group (cohort I-SMT and cohort II-SMT) or the ALSS group (cohort I-ALSS and cohort II-ALSS) ( Fig. 1 ).
The study was performed in accordance with theDeclarationof Helsinkiand approved by the Clinical Research Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine(20 08-0 012 and 2011-0013). Consent was obtained from the patients or their legal guardians before enrollment.
Definitions and treatments
HBV-ACLF was defined according to the 2014 Asian Pacific Association for the Study of the Liver (APASL): jaundice [serum bilirubin ≥5 mg/dL (85 μmol/L)] and coagulopathy [international normalized ratio (INR) ≥1.5 or prothrombin activity<40%] complicated within 4 weeks by clinical ascites and/or encephalopathy in a patient with previously diagnosed or undiagnosed CHB or HBV-related liver cirrhosis [4] . The diagnosis of CHB corresponded to the criteria of the 2009 American Association for the Study of Liver Diseases (AASLD) guidelines, which were as follows: positive for the hepatitis B surface antigen (HBeAg) ≥6 months; serum HBV-DNA ≥20 0 0 0 IU/mL; persistent or intermittent elevated alanine aminotransferase (ALT)/aspartate aminotransferase (AST) levels; and a liver biopsy showing chronic hepatitis [22] . Patients with one or more of the following criteria were excluded: age<18 years or>80 years; had already received LT; diagnosed with HCC or other tumors; died within 1 day of admission; or had severe comorbidities that affected survival ( Fig. 1 ).
The SMT group received integrative treatment only, whereas the ALSS group received both integrative and ALSS treatments. Except for the nucleos(t)ide analogs, most therapeutic drugs are similar. In the cohort I-ALSS group, PE was used for most patients, whereas PE combined with other modes of ALSS (hybrid ALSS) was used for patients with hepatic encephalopathy. In the cohort II-ALSS group, hybrid ALSS was used according to the patient’s condition,including PE + continuous blood dialysis, PE + plasma diafiltration,PE + plasma bilirubin absorption, etc.
Clinical data collection and follow-up
We collected the following clinical data: demographic data,admission causes, laboratory measurements (e.g., ALT, AST, total bilirubin, creatinine levels and INR), HBV DNA levels, antiviral treatment for HBV [nucleos(t)ide analogs, including lamivudine(LAM), adefovir (ADV), entecavir (ETV), and telbivudine (LdT)], organ failure events, and prognosis. Detailed clinical data and routine investigations were performed daily during hospitalization. After discharge, the patients were followed up at least bi-weekly for 8 weeks. The survival rates at 28 and 56 days were considered the primary endpoints.
Statistical analysis
Categorical data were expressed as number (percentage) and compared usingχ2test or Fisher’s exact test. Continuous variables were expressed as mean ± standard deviation (SD) or median (interquartile range) and compared using Student’st-test or Wilcoxon rank-sum test. The effects of selection bias and potential confounding between the two groups were reduced using propensity score matching analysis. Propensity scores were computed using the following variables: age, sex, body mass index (BMI), baseline HBeAg positivity, serum levels of HBV DNA, ALT, total bilirubin, albumin, and creatinine levels, INR, model for end-stage liver disease (MELD) score, and Chinese Group on the Study of Severe Hepatitis B-ACLF (COSSH-ACLF) grade. For propensity score matching, a nearest-neighbor 1:1 matching scheme with a caliper size of 0.02 was used. The short-term (28/56 days) survival rates were calculated using Kaplan-Meier analysis and compared using log-rank test. Multivariate logistic regression model was used to analyze independent baseline risk factors associated with 28-day mortality.
All statistical analyses were performed using IBM SPSS Statistics 24.0 (IBM Corp., Armonk, NY, USA), and R statistical software, version 3.3.1 (R Foundation Inc; http://cran.r-project.org/ ). Significance was defined as two-tailedP<0.05.
Results
Description of cohorts I and II

Fig. 1. Flowchart of the patient selection process. ALSS: artificial liver support system; STM: standard medical therapy; HBV-ACLF: hepatitis B virus-related acute-on-chronic liver failure; HCC: hepatocellular carcinoma.
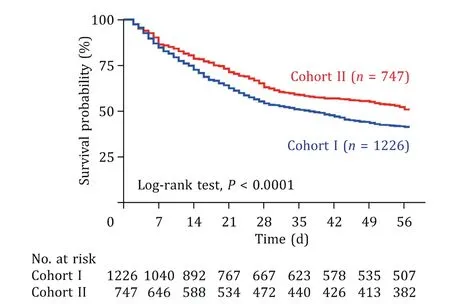
Fig. 2. The 56-day survival curve of patients with HBV-related liver failure in the cohort I vs. cohort II. HBV: hepatitis B virus.

Fig. 3. The 56-day survival curve of patients with HBV-related liver failure in hybrid ALSS group vs. plasma exchange group. HBV: hepatitis B virus; ALSS: artificial liver support systems.
In the cohort I, 1226 patients with HBV-ACLF were enrolled;533 (43.5%) and 693 (56.5%) of them received SMT and ALSS treatments, respectively. In the cohort II, 747 patients were enrolled; 410 (54.9%) and 337 (45.1%) of them received SMT and ALSS treatments, respectively ( Fig. 1 ). The MELD score and COSSH-ACLF grade were not significantly different between the two cohorts(P= 0.801,P= 0.766, respectively; Table S1). The study revealed that the short-term (28/56 days) survival rates were significantly higher in the cohort II than those in the cohort I (63.2% vs. 54.4%,51.1% vs. 41.4%,P<0.001, respectively; Fig. 2 ). We compared the baseline characteristics between cohorts I and II before the propensity score matching analysis, and the results showed that the baseline parameters were different between the two cohorts ( Tables 1 and 2 ). We also found that the short-term (28/56 days) survival rates were higher in the ALSS group than those in the SMT group(60.4% vs. 54.8%, 47.3% vs. 42.6%,P<0.05, respectively; Table S2).Additionally, different modalities of ALSS were associated with different prognoses. In particular, patients receiving hybrid ALSS had higher short-term (28/56 days) survival rates than those in the PE group (66.8% vs. 56.6%, 53.7% vs. 43.5%,P<0.001, Fig. 3 ).
Therefore, we considered that the short-term (28/56 days) survival rates would be affected by the treatment method and baseline parameters; thus, the patients were grouped according to whether they were treated with ALSS, and we compared the shortterm (28/56 days) survival rates after the propensity score matching analysis.
Baseline characteristics of the SMT groups
The patients’ characteristics at baseline and after propensity score matching analysis are shown in Table 1 . Before matching,age, BMI, baseline ALT level, baseline HBV DNA level, and other clinical data significantly differed between the cohort I-SMT andcohort II-SMT groups. To allow reliable comparisons between the two groups, we used propensity score matching method with selected key characteristics. Propensity score matching analysis generated 394 pairs, and the characteristics of pairs were balanced, withP>0.05 for all baseline variables. In the matched cohort I-SMT group, the mean age was 43.0 ± 11.4 years, and 319 patients (81.0%) were male. In the matched cohort II-SMT group,the mean age was 42.0 ± 11.2 years, and 333 patients (84.5%)were male ( Table 1 ). The COSSH-ACLF grade and MELD score were similar between the cohort I-SMT and cohort II-SMT groups. In the cohort I-SMT group, 57 patients did not receive a nucleos(t)ide analog, 124 received 100-mg/day LAM, 18 received 10-mg/day ADV, 46 received 100-mg/day LAM plus 10-mg/day ADV, 14 received 600-mg/day LdT, and 135 received 0.5-mg/day ETV. In the cohort II-SMT group, all patients received antiviral treatmentwith nucleos(t)ide analogs. Among them, 175 patients received 100-mg/day LAM and 219 patients received 0.5-mg/day ETV( Table 3 ).

Table 1 Characteristics and demographics of the SMT groups.

Table 2 Characteristics and demographics of the ALSS groups.
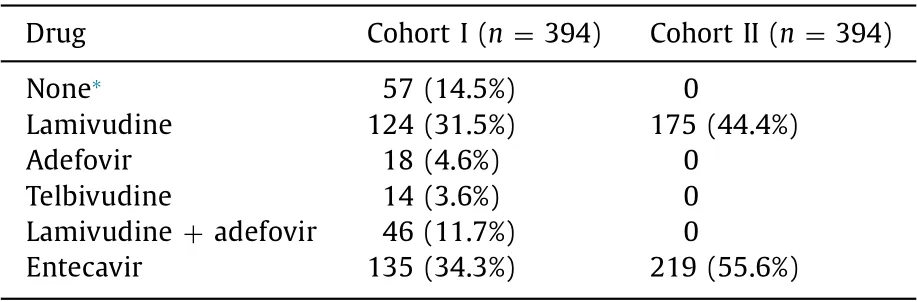
Table 3 Nucleos(t)ide analogs for patients in the SMT groups after propensity score matching analysis.
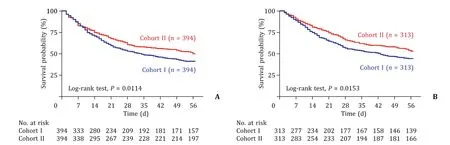
Fig. 4. The 56-day survival curve of patients with HBV-related liver failure after propensity score matching. A: 56-day survival curve of patients in the cohort I-SMT vs.cohort II-SMT; B: 56-day survival curve of patients in the cohort I-ALSS vs. cohort II-ALSS. SMT: standard medical therapy; ALSS: artificial liver support system.

Table 4 The 28/56 day survival rates for patients in the cohort I-SMT with or without nucleos(t)ide analogs treatment.
Baseline characteristics of the ALSS groups
The patient characteristics at baseline and after propensity score matching analysis are shown in Table 2 . Before matching,age, BMI, baseline HBV DNA level, baseline HBeAg positivity, and other clinical data differed significantly between the cohort I-ALSS and cohort II-ALSS groups. Propensity score matching analysis generated 313 pairs, and the characteristics of each pair were balanced, withP>0.05 for all baseline variables. The ALSS models were significantly different between the two cohorts. For patients in the cohort I-ALSS, 282 (90.1%) received PE treatment and 31(9.9%) received hybrid ALSS treatment. For patients in the cohort II-ALSS, 22 (7.0%) received PE treatment and 291 (93.0%) received hybrid ALSS treatment.
Survival rates
All patients were followed up for at least 56 days or until death. The short-term survival (28/56 days) rates were significantly higher in the cohort II-SMT group than those in the cohort I-SMT group (60.7% vs. 53.0%, 50.0% vs. 39.8%,P<0.05; Fig. 4 A). Similarly, the short-term survival (28/56 days) rates were also significantly higher in the cohort II-ALSS group than those in the cohort I-ALSS group (66.1% vs. 56.5%, 53.0% vs. 44.4%,P<0.05; Fig. 4 B).
Outcomes of patients with or without nucleos(t)ide analog treatment
Considering that nucleos(t)ide analogs might impact the outcomes of patients with HBV-ACLF, we compared the short-term survival rates between patients treated with nucleos(t)ide analogs and those without such treatments. The results showed that the short-term survival (28/56 days) rates were 54.6%/41.5% in patients treated with nucleos(t)ide analogs and 40.4%/29.8% in patients without such treatments. A higher 28-day survival rate was found in patients treated with nucleos(t)ide analogs (P= 0.046,Table 4 ). However, the 56-day survival rate did not differ between the two cohorts (P= 0.095, Table 4 ). Besides, the short-term survival (28/56 days) rates were similar in patients treated with LAM and those with ETV (Table S3).
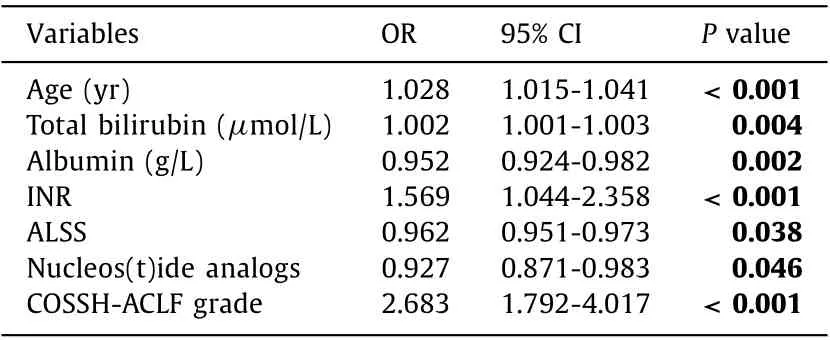
Table 5 Multivariate logistic regression model investigating independent factors for 28-day mortality.
Factors associated with 28-day mortality
Multivariate logistic regression analysis showed that the independent factors associated with 28-day mortality were ALSS(OR = 0.962, 95% CI: 0.951-0.973,P= 0.038), nucleos(t)ide analogs(OR = 0.927, 95% CI: 0.871-0.983,P= 0.046), age (OR = 1.028,95% CI: 1.015-1.041,P<0.001), total bilirubin (OR = 1.002, 95%CI: 1.001-1.003,P= 0.004), INR (OR = 1.569, 95% CI: 1.044-2.358,P<0.001), COSSH-ACLF grade (OR = 2.683, 95% CI: 1.792-4.017,P<0.001), and albumin (OR = 0.952, 95% CI: 0.924-0.982,P= 0.002) ( Table 5 ). However, the MELD score, cirrhosis, serum creatinine and ALT were not independent predictors of 28-day mortality.
Discussion
HBV-ACLF is a complex syndrome that develops in patients with CHB. Patients with HBV-ACLF have specific clinical characteristics, including high short-term (28/90 days) mortality, high prevalence of liver and coagulation failure, and low prevalence of renal failure [23-25] , which differ markedly from those of patients with non-HBV-related liver failure in the European Association for the Study of the Liver (EASL) and AASLD studies [ 23 , 24 ]. In this study,we explored the short-term survival rates of patients with HBVACLF in China over the past decade.
Although the severity of disease was similar between cohorts I and II (MELD score,P= 0.801; COSSH-ACLF criteria,P= 0.766), the short-term (28/56 days) survival rates were significantly higher in the cohort II than those in the cohort I. ALSS is a machine that provides transient liver function support for liver failure, which encompasses metabolic, detoxification, and synthetic and immune regulatory functions. Some studies have demonstrated that ALSS may improve the short-term survival in acute liver failure [ 7 , 26 ].In our study, after propensity score matching analysis, cohort II still had significantly higher survival rates than cohort I in both the SMT and ALSS groups. The underlying possible reasons may be the therapeutic improvement.
In chronically infected patients, an elevated serum HBV DNA level is the determining risk factor for disease progression, although there are other clinical and viral parameters that influence disease outcomes [27] . Therefore, available antiviral therapies are indispensable for HBV-ACLF patients. Oral nucleos(t)ide analog therapy, including LAM, ADV, LdT, ETV, and TFV, confers biochemical, virological, and serological improvement in CHB patients, and these are the main forms of anti-HBV treatment worldwide [27] .Recently, nucleos(t)ide analogs have been proven effective on improving the prognosis and survival rate of patients with severe decompensated chronic liver disease and HBV-related liver failure [17-20] . We found that the prescribing behavior towards nucleos(t)ide analog therapy for HBV-ACLF patients was different in the two cohorts. All patients in the cohort II received nucleos(t)ide analog therapy after admission, whereas only 85.5% of the patients in the cohort I received nucleos(t)ide analog therapy. We also found that the 28-day survival rate was lower in patients without nucleos(t)ide analog treatment. The increased use of nucleos(t)ide analogs may be one of the reasons of the observed treatment improvement in the cohort II. Early-generation nucleos(t)ide analogs, such as LAM, ADV, and LdT, can easily result in drug resistance [23] . By contrast, ETV and TFV, recommended as firstline therapeutics for CHB, achieve high virological suppression and are less likely to develop resistance [28-30] . Compared to 65.7%of the patients in the cohort I-SMT group who received earlygeneration nucleos(t)ide analogs, only 44.4% of the patients in the cohort II-SMT received early-generation nucleos(t)ide analogs.However, the results in our study revealed that LAM and ETV were equally effective in treating HBV-ACLF.
It is notable that model for end-stage liver disease-sodium(MELD-Na) score was not a predictor of short-term mortality in our cohort. The ACLF grade 3 (ACLF-3), defined as the development of 3 or more organ failures, portends extremely high mortality without transplantation (approaching 80% at 28 days and>90% at 1 year), which may not be reflected by the MELD-Na score [ 31 , 32 ]. The LT treatment reported a reduced survival probability for individuals with ACLF-3. However, the 1-year post-LT survival after LT remains lower than the expected outcomes (ranging from 50% to 80%) [33] . As the outcomes of patients with LT were completely different and complicated, we excluded the patients who underwent LT in this study. Nearly 90 patients with ACLF-3 were included in this study. Given the markedly poor prognosis of ACLF-3, which may not be fully accounted for by the MELD, we use both MELD score and COSSH-ACLF grade to assess the risk of mortality.
In view of the shortage of organs for transplantation, ALSS is one of the main treatments for HBV-ACLF in China. The use of ALSS has shown a variable range of safety, tolerance, and efficiency in several clinical trials, and it may help to sustain life until LT or recovery in selected patients with liver failure [34] . Most patients in the cohort I-ALSS group were treated with PE only (89.8%),whereas patients in the cohort II-ALSS group were usually treated with hybrid ALSS (92.3%), which is more targeted and efficient. Furthermore, there were some differences in the ALSS treatment process between the two cohorts. In the cohort II-ALSS group, patients received ALSS treatment at day 2 after admission, whereas, in the cohort I-ALSS group, patients usually received ALSS treatment at day 3-4 after admission. In addition, the cohort II-ALSS group generally received 2-3 routine treatments with an interval of 1 day,and extra treatments were offered according to the patient’s condition. The cohort I-ALSS group received three ALSS treatments (one ALSS every 3-4 days). The cohort II-ALSS group received high frequency of ALSS treatments in the early period of hospitalization.The therapeutic difference may explain the better therapeutic effects in the cohort II.
There may be other reasons for the improved treatment effects in the more recent cohort, including improvements in the national economy, patients more in compliance with doctors, increased recognition of ALSS treatment, early diagnosis and treatment, and optimal clinical management, which may have led to significant improvement in HBV-ACLF treatment.
Owing to the limited follow-up time, we were unable to compare the difference in the 90-day and long-term survival rates between the two cohorts. The collection of long-term follow-up data of a larger cohort would help to further define the treatment progress of HBV-ACLF.
In conclusion, there has been considerable improvement in HBV-ACLF treatment over the past decade. However, the shortterm survival rate remains very low in patients with HBV-ACLF.More effective medications for liver failure should be investigated in the future.
Acknowledgments
The authors thank Shao-Jie Xin, Zhi-Liang Gao, Zhong-Ping Duan, Tao Han, Yu-Ming Wang, Jian-He Gan, Chen Pan, Yong-Ping Chen, Qing Xie, Shu-Mei Lin for data collection.
CRediT authorship contribution statement
Lan-Lan Xiao: Formal analysis, Methodology, Writing - original draft. Xiao-Xin Wu: Formal analysis, Writing - original draft. Jia-Jia Chen: Data curation, Investigation, Project administration. Dong Yan: Data curation, Investigation, Project administration. Dong-Yan Shi: Data curation, Investigation, Project administration. Jian-Rong Huang: Conceptualization, Data curation, Funding acquisition, Investigation, Project administration, Supervision, Writing - review& editing. Xiao-Wei Xu: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing - original draft, Writing - review & editing. Lan-Juan Li: Conceptualization, Funding acquisition, Supervision, Writing - review & editing.
Funding
This work was supported by grants from the Science & Technology Key Program of Zhejiang China (2017C03051) and the National Science & Technology Major Project of China (2017ZX10203201).
Ethical approval
This study was approved by the Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine (2008-0012 and 2011-0013). Written informed consent was obtained from all participants.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Hepatocellular-cholangiocarcinoma with sarcomatous change:Clinicopathological features and outcomes
- Recent advances in immunotherapy for hepatocellular carcinoma
- Toll-like receptors and hepatitis C virus infection
- Involvement of the circular RNA/microRNA/glucose-6-phosphate dehydrogenase axis in the pathological mechanism of hepatocellular carcinoma
- From conventional two-stage hepatectomy to ALPPS: Fifteen years of experience in a hepatobiliary surgery unit
- Melatonin attenuates hepatic ischemia-reperfusion injury in rats by inhibiting NF- κB signaling pathway
