Toll-like receptors and hepatitis C virus infection
2022-01-07YangGaoNarayanNepalShiZhuJin
Yang Gao, Narayan Nepal, Shi-Zhu Jin
Department of Gastroenterology and Hepatology, The Second Affiliated Hospital of Harbin Medical University, Harbin 150086, China
Keywords:Hepatitis C Toll-like receptors Interferons Inflammation Immune evasion
ABSTRACT
keywords:hepatitis C, toll-like receptors, interferons, inflammation, and immune evasion. We also used terms such as single-nucleotide polymorphisms (SNPs), susceptibility, fibrosis, cirrhosis, direct-acting antiviral agents,agonists, and antagonists to supplement the query results. We reviewed relevant publications analyzing the correlation between hepatitis C and TLRs and the role of TLRs in HCV infection.
Introduction
Hepatitis C virus (HCV) infection is a common epidemic disease. According to statistics, 71.1 million people are infected with HCV worldwide [1] . HCV causes liver fibrosis, cirrhosis, and hepatocellular carcinoma (HCC), imposing a great financial burden on patients. HCV is a positive-sense, single-stranded RNA virus approximately 9.4 kb in length. The HCV genome is highly variable due to various factors, including the following: environmental factors; the shaping of the host genome; the lack of an HCV proofreading mechanism, as an RNA virus, during replication; and more complex factors that constantly influence its evolution [ 2 , 3 ]. Seven genotypes and more than 90 subgenotypes of HCV have been identified thus far [ 4 , 5 ], and globally, the major HCV genotypes vary geographically by region [ 6 , 7 ]. Furthermore, the generation of quasispecies in infected individuals, especially those caused by errorprone RNA polymerase, allows HCV to escape the host immune response and complicates vaccine development [8] . HCV infection is often accompanied by chronic inflammation caused by innate immunity, which involves many pattern recognition receptors.The major pattern recognition receptors include toll-like receptors(TLRs), RIG-I-like receptors (RLRs), NOD-like receptors (NLRs), Ctype lectin receptors (CLRs) and cytosolic DNA sensors (CDs) [8] .Among them, TLR can establish antiviral response rapidly by recognizing pathogen-associated molecular patterns (PAMPs) and binding to various structures of HCV and is therefore an important molecule in the process of HCV infection.
TLRs are important members of liver immune system, which exist in liver parenchymal cells and various immune cells. TLRs act in mediating innate immunity and further induce the acquired immunity, improving the overall efficiency of the immune response.In the process of HCV infection, different TLRs have different biological functions, and the subsequent reaction caused by the binding of TLRs to ligands will also have a positive or negative impact on the prognosis of hepatitis C. HCV binds to the cell surface and enters the cell through receptor-mediated endocytosis. The HCV core and nonstructural proteins can be recognized by TLR family to encourage production of multiple inflammatory factors. HCV induces TLRs to maintain this inflammatory state in the body, whichmay lead to long-term chronic liver injury. Moreover, activation of some TLR-induced signaling pathways facilitates the cellular production of interferon (IFN) and other substances to inhibit viral replication, and HCV can selectively inhibit such TLR signaling [9] .Therefore, TLR-related signaling pathways can regulate the progression of hepatitis C. Exploring the role of TLRs in hepatitis C may help us to better understand the disease process and find more effective means to treat hepatitis C. In addition, a growing number of studies have shown that single-nucleotide polymorphisms (SNPs)inTLRgenes, especially TLR3, TLR4, TLR7, TLR8, and TLR9, may help in predicting HCV susceptibility [10] . In patients with HCV infection, SNP variants of TLRs appear to modulate immune responses,influencing inflammatory cytokines and IFN levels [11] , and therefore may predict the severity of liver fibrosis, whether cirrhosis or liver cancer will occur, and the choice of treatment strategy [ 12 , 13 ].
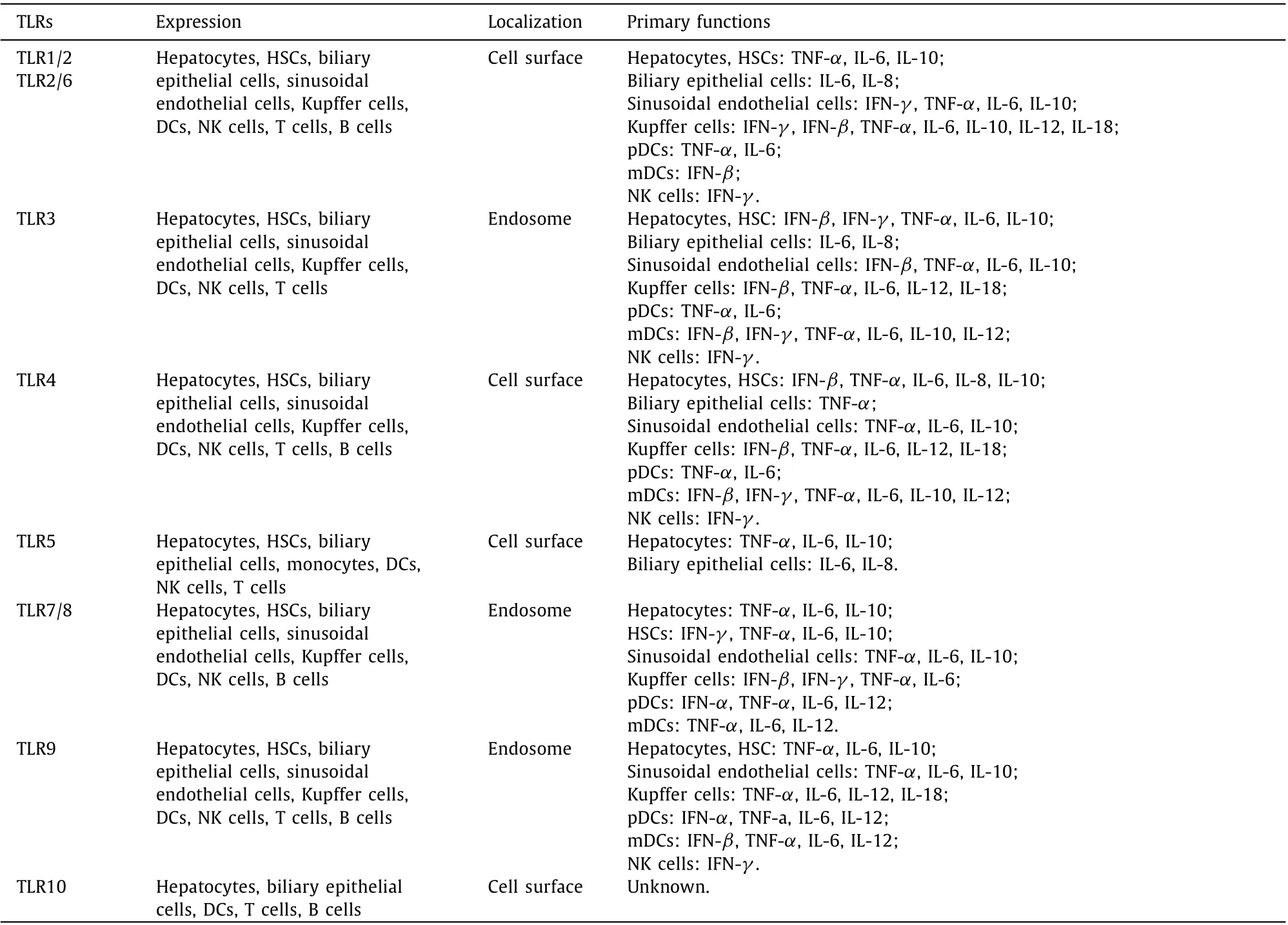
Table 1 Expression and cellular localization of TLRs and their primary functions in different cells.
Treatment for hepatitis C has evolved from the early use of IFN combined with ribavirin to antiviral drugs that act directly on HCV. Although direct-acting antiviral agents (DAAs) have been used worldwide for 10 years [14] , with a cure rate of more than 95%, DAAs are expensive and difficult to obtain in underserved and economically disadvantaged populations. Additionally, drug resistance and disease recurrence occur in some patients. Furthermore,DAAs cannot slow disease progression in the late stage of hepatitis C, when patients develop decompensated cirrhosis [15] . More importantly, 80% of those infected with HCV are asymptomatic [1] ;hence, prevention and treatment of hepatitis C are equally important. Due to the high heterogeneity between HCV genotypes,live-attenuated vaccines are not universally applicable, and HCV vaccines against nonliving antigens have poor immunogenicity [8] .Therefore, new therapeutic strategies focusing on TLRs and the development of TLR-related vaccine adjuvants may be a new strategy to further improve the treatment and prevention of HCV. We herein summarize the role of different TLRs in the process of HCV infection, hoping to find a new strategy of TLR-related HCV treatment.
TLRs and related signaling pathways
TLRs belong to the family of pattern recognition receptors,which can specifically recognize various pathogenic microorganisms and their products and promote the establishment of organismic barriers against these microorganisms. Humans are known to express 11 different TLRs, with TLRs 1-10 being best characterized.TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10 are located on the extracellular surface, whereas TLR3, TLR7, TLR8 and TLR9 are inside of the cells [ 16 , 17 ] ( Table 1 ). All TLRs consist of extramembrane,transmembrane and intramembrane signaling domains. The extramembrane domain contains multiple leucine repeat sequences with high variability that are conducive to specific ligand binding. The TLR transmembrane domain is rich in cysteine and is the basis of the subcellular localization of TLRs. The intracellular tollinterleukin-1 (IL-1) receptor (TIR) domain of TLRs triggers downstream signaling. SNPs in the extracellular or intramembrane domain of TLRs affect the immune response to infection [17] .

Fig. 1. TLRs and related signaling pathways. In addition to TLR3, the downstream signaling pathways of TLRs can be stimulated by MyD88, which induces production of the cytokines TNF- α, IL-1 β, IL-6, IL-8, IL-12, IL-18 and IFN- α. The MyD88-independent signaling pathway is induced only by TLR3 and TLR4 and is transmitted by TRIF, which promotes the production of IFN- β in cells. In addition, MyD88-dependent and MyD88-independent signal transduction pathways interact. TRIF in the MyD88-independent pathway activates NF- κB through RIP1, TRAF6 or activates AP-1 through MAPKs, which eventually leads to the production of IFN and inflammatory factors and promotes the progression of HCV infection.
The subsequent events that occur after TLRs bind to HCV entering the host are as follows. TLR-mediated signal transduction pathways are divided into myeloid differentiation factor (MyD88)-dependent and -independent pathways based on their dependence on the downstream signaling molecule MyD88. All TLRs except TLR3 utilize MyD88. After ligand recognition, the intracellular domain of TLR5, TLR7, TLR8 and TLR9 directly interacts with MyD88. Conversely, TLR1/2, TLR2/6 and TLR4 first bind to the junction molecule Mal (MyD88 adapter-like), which acts as a bridging adapter between TLRs and MyD88 [5] , after which MyD88 binds to IL-1 receptor-associated kinase (IRAK). Activated IRAK interacts with tumor necrosis factor (TNF) receptor-associated family 6 (TRAF6) to form a complex, and transformation growth factorβ(TGF-β)-activated kinase (TAK1), inhibitor of nuclear factor-κB(NF-κB) kinase (IKK) complex, mitogen-activated protein kinases(MAPKs), activated protein-1 (AP-1), nuclear translocation of NFκB, and IFN regulatory factors 5 and 7 (IRF5 and IRF7) are successively activated. A series of signaling pathways promote the production of inflammatory factors and initiate IFN regulation [ 18 , 19 ].
The MyD88-independent pathway is only associated with TLR3 and TLR4. Toll/IL-1 receptor domain-containing adaptor inducing IFN-β(TRIF) binds to the intracellular domain of TLRs. Then, activated TRAF family member-associated NF-κB activator (TANK)binding kinase 1 (TBK1) and IKK-εpromote the activation and nuclear translocation of IRF3, which eventually causes IFN-βproduction. Of course, MyD88-dependent and -independent pathway activity can merge. For example, TRIF can activate NF-κB and promote its nuclear translocation by stimulating receptor-interacting protein 1 (RIP1) or TRAF6 [19] , or it can activate MAPKs to induce AP-1 and thus trigger a proinflammatory response [20] ( Fig. 1 ).
Role of TLRs in HCV infection
As TLRs function by recognizing PAMPs, the structures of HCV that are related to TLRs should be highlighted. HCV encodes three structural proteins (core, E1, and E2) and seven nonstructural proteins (P7, NS2, NS3, NS4A, NS4B, NS5A and NS5B) [4] . The HCV core, NS3, NS4A and NS5A proteins are closely related to TLRs. For example, the core, NS3 and NS5 proteins activate cellular immune functions through TLR1, TLR2, TLR4, and TLR6. NS3 and NS4A proteins derail downstream pathways of IFN production by interacting with downstream molecules TBK1 and TRIF. NS5A can interact with death domain of MyD88 inhibiting the production of downstream inflammatory factors. Ultimately, these effects are responsible for the inflammatory response caused by HCV coexisting with the antiviral response ( Fig. 2 ). The relationship between different TLRs and HCV is described as follows. It should be noted that TLR5 and TLR10 have rarely been mentioned in HCV infection. The ligand of TLR5 is bacterial flagellin, and the specific ligands and functions of TLR10 have not been thoroughly explored. However, in patients with HCV infection, it was found that the mRNA levels of TLR5 and TLR10 are increased in monocytes. TLR10 expression is also upregulated in lymphocytes [21] , but the internal mechanism has not been revealed. Although a specific ligand for TLR10 has not yet been identified, TLR10 is expressed in a variety of immune cells and may be involved in inflammation [22] .Invitro, TLR10 inhibits the production of a series of inflammatory cytokines induced by other TLR agonists [23] .

Fig. 2. HCV-encoded proteins and their effects on TLRs and related downstream molecules. The core and NS3 proteins of HCV activate innate immunity through TLR2 and TLR4. The NS5A protein binds to TLR4 and participates in the inflammatory response. Intermediate products of HCV RNA replication can bind to TLR3. However, there are immune evasion mechanisms involving the degradation of TRIF by the HCV NS3 and NS4A, the combination of NS3 and TBK1 to destroy subsequent reactions. The NS5A protein also inhibits the MyD88-dependent pathway, affecting the signaling of the related TLRs, which may decrease the production of IFN and inflammatory cytokines.NS4A, NS4B, NS5A can disrupt antiviral response, in which NS5B synthesizes TLR3 ligand to promote IFN- β production. Green arrow: bind, activate. Red arrow: inhibit,destroy.
TLR2, TLR1 and TLR6
TLR2 is necessary for recognition of the multiprotein components of HCV. The HCV core and NS3 proteins activate innate immune cells in a TLR2-dependent manner and induce activation of inflammatory cells through a TLR2-specific intracellular pathway [24] . The specific downstream signaling of TLR2 depends on the presence of MyD88, and under stimulation, MyD88 recruits IRAK. Eventually, TLR2 causes activation of the NF-κB and AP-1 transcription factors as well as c-Jun N-terminal kinase (JNK)phosphorylation, which may contribute to hepatocyte damage in chronic HCV infection [25] . Indeed, the TLR-activated MAPK and JNK are involved in hepatocyte injury, metabolism, inflammation and fibrosis [26] . The transduction of a series of signals has been confirmed to eventually result in production of TNF-α, IL-6 and IL-8 in human monocytes and the pro- and anti-inflammatory factors TNF-αand IL-10 in human macrophages [ 24 , 27 ]. TNF-αand IL-10 are ubiquitous in patients with high levels of HCV.IL-10 inhibits activation of T lymphocytes and maturation of plasmacytoid dendritic cells (pDCs). IL-10 also induces apoptosis in pDCs, that are the most powerful antigen-presenting DCs. DCs can be divided into myeloid DCs (mDCs) and pDCs based on the cell surface phenotype. The main function of mDCs is antigen presentation, and pDCs produce large amounts of IFN to help clear the virus [28] . DCs infected with HCV are also thought to affect the production of TNF-α. These factors cause gradual and chronic infection in the body [29] .
TLR2 may also recognize the HCV core protein at a later stage of HCV infection. In fact, TLR2 fails to effectively recognize the HCV core protein monomer in the early stage of HCV infection. It may be that the conformation of the HCV core protein monomer is different from that of the complete HCV core protein or that the envelope protein of HCV impairs TLR2 recognition of the core protein, promoting immune evasion by HCV [30] . It should be mentioned that the HCV core and NS3 proteins are recognized by heterodimers formed by TLR2 and its coreceptors TLR1/TLR6. The formation of such heterodimers derives from the selection pressure accompanying the diversity of PAMPs in the process of evolution,which allows TLRs to recognize various ligands efficiently [31] .Therefore, among many TLRs, ligands of TLR2 have the most diverse structures, and the dimer structure of TLR2 can mediate a variety of reactions.
TLR3
TLR3 detects and binds to HCV RNA in cells. However, the RNA to which TLR3 binds may not be the HCV RNA that has recently entered the body but rather the intermediate product of RNA replication after a period of time [32] . TLR3-ligand interaction activates the TRIF pathway, and IFN-βis upregulated through expression of IFN-stimulating genes (ISGs), which are promoted by TBK1/IKK-εand IRF3. Conversely, HCV inhibits upregulation of IFN-βby the TLR3 pathway. Firstly, HCV NS3/NS4A proteolytically degrade the TLR3 adaptor TRIF, which can lower the activity of IRF3 and hinder the production of IFN induced by IRF3. Secondly, NS3 binds to TBK1 directly, which impairs activation of IRF3 and upregulation of IFN-β. These two effects im pede clearance of HCV [ 33 , 34 ].Interestingly, studies have suggested that in human and murine hepatic stellate cells (HSCs), stimulation by TLR3 agonists may exert antiviral effects to control the replication of HCV [ 35 , 36 ]. HSCs are nonparenchymal cells of the liver, and when the liver is injured by virus infection, HSCs change from a resting state to an activated state to perform immunomodulatory functions and participate in liver repair. In particular, TLR3 agonists mainly exert antiviral effects in murine HSCs by stimulating the production of IFN-β[37] . Furthermore, NS5B may produce a nonspecific doublestranded RNA (dsRNA) as a ligand of TLR3, thus promoting the production of IFN-β. Nevertheless, NS4A, NS4B, and NS5A can defuse this reaction chain by affecting the synthesis of dsRNA or recognition between TLR3 and dsRNA [38] . We conclude that the immune evasion mechanisms of HCV are diverse, which may explain why HCV infection easily develops into chronic infection.
TLR4
TLR4 signals through the MyD88-independent pathway and activates downstream molecules through TRIF-related pathways. The classic TLR4 ligand is lipopolysaccharide (LPS), which is the main component of endotoxin. With increased levels of endotoxin in the blood, TLR4 binds LPS and activates TLR4-induced signaling pathways. The serum level of endotoxin in patients with HCV infection is higher than that in healthy volunteers [39] ; it cannot be ruled out that this increase in endotoxin is due to increased intestinal permeability caused by hepatitis infection. Kupffer cells are specialized macrophages in the liver that maintain immune homeostasis. After TLR4 binds to a corresponding ligand, Kupffer cells produce several inflammatory factors, such as IL-1β, IL-6, IL-12, IL-18, and TNF-α[40] . Expression of IL-12 in macrophages after TLR4 activation contributes to the release of IFN-γ, which activates lymphocytes to attack HCV [41] .
Although LPS accelerates hepatitis C progression, experiments have indicated that HCV NS5A can bind directly to TLR4 on monocytes, without LPS stimulation. This process increases IL-10 production and decreases IL-12 production by monocytes, resulting in an imbalance of inflammatory factors. IL-10 triggers the secretion of TGF-β, and both IL-10 and TGF-βare anti-inflammatory cytokines [42] . TGF-βdownregulates natural killer group 2 member D (NKG2D) expression in natural killer (NK) cells and impairs NK cell function. NKG2D, the major histocompatibility complex (MHC)class I-related chain (MIC) molecule, is an activating receptor on NK cells [30] . Similarly, expression of NKG2D on the surface of NK T cells, a kind of T cell subtype with NKG2D receptors, is also downregulated under the influence of HCV, and thus, attack of the virus by NK T cells is weakened [43] . These effects of NS5A ultimately inhibit the virus-killing effect of NK cells, accounting for another immune evasion mechanism.
NS5A upregulates expression of TLR4 in peripheral blood mononuclear cells and B cells to augment TLR4 signaling, which in turn enhances production of IFN-βand IL-6. In addition, NS5A has been suggested to increase TLR4 transcription in hepatocytes [44] ,yet there is a view that HCV NS5A downregulates TLR4 expression in hepatocytes. Because LPS can activate the TLR4 pathway, downregulation of TLR4 expression may reduce the harmful effect of LPS on hepatocytes as well as apoptosis in these cells [45] . Moreover,NS5A might affect the progression of hepatitis C and activate NFκB via oxidative stress by altering calcium homeostasis [46] . This finding also indicates that HCV does not aim to destroy hepatocytes but rather strives for long-term survival in hepatocytes to perpetuate the state of chronic infection.
TLR7 and TLR8
TLR7/8 recognize single-stranded RNA, which occurs in a manner different from viral dsRNA detection by TLR3 [17] . However, as receptors that recognize viral RNA, expression of TLR3 and TLR7 is similarly reduced in peripheral blood monocytes of HCV-infected patients [47] . During the transcription and regulation of HCVin vivo, theTLR7gene is disturbed, and TLR7 mRNA becomes unstable. Inhibition of protein synthesis occurs in many virus-infected host cells via viral-mediated degradation of RNA, which is a common mechanism of immune evasion [48] . Other studies have proposed that viral proteins are associated with exosomesinvivoand affect RNA by using these exosomes as carriers [ 49 , 50 ]. Nevertheless, the mechanism by which HCV affects the stability of TLR7 mRNA remains uncertain. Other studies have confirmed that activation of IRF7 is increased in HCV-infected cells in which HCV is being replicated [ 51 , 52 ]. However, this activation does not compensate for the reduced expression of TLR7. It is possible that activation of the IRF7 pathway is induced by other pattern recognition receptors or TLRs but that the nuclear translocation of IRF7 induced by TLR7 is significantly decreased [51] . Although HCV possesses an immunological evasion mechanism and invades a host by attacking the immune system, TLR7 is a pivotal molecule in adaptive immunity. It has been confirmed that a selective agonist of TLR7 can enhance the function of pDCs and other immune cells to eliminate HCV [53] .
In addition, studies have demonstrated that the NS5A protein can inhibit the MyD88 signaling pathway by interacting with MyD88 directly to further suppress TLR7/8 signaling [ 54 , 55 ] and affect other pathways downstream of TLRs that are also dependent on MyD88. Moreover, activation of CD4 T cells is impaired in patients infected with HCV, resulting in a decrease in IFN produced by pDCs and mediated by TLR7-ligand binding [39] .
TLR9
TLR9 only binds to DNA and effectively identifies unmethylated cytosine-phosphate-guanine (CpG) dinucleotide motifs, which is a DNA sequence rich in CpG dinucleotide that regulates gene expression under the sequence-specific background in bacteria or viruses. Therefore, HCV RNA does not seem to be directly bound by TLR9 [56] . TLR9 appears to facilitate the process of liver fibrosis,and it is well known that hepatocyte apoptosis can induce fibrosis.TLR9 in the endosome of HSCs can bind DNA from apoptotic cells to promote the differentiation of HSCs, which are the main cells that contribute to liver fibrosis [57] . Although TLR9 cannot bind to HCV RNA directly, it can indirectly affect HCV infection through apoptotic cell DNA. pDCs are the main cells expressing TLR9, and TLR9 binds ligands to achieve IRF7 activation and nuclear translocation through the MyD88-dependent pathway, resulting in IFN-αproduction. However, the combination of virus particles and pDCs might downregulate expression of TLR9 [58] . Similarly, a previous study has shown that TLR9 mRNA and protein levels in peripheral blood mononuclear cells from patients infected with HCV are lower than those of control groups and correlate negatively with serum antiviral antibodies [59] . Moreover, the use of CpG as a TLR9 agonist can enhance TNF-α, IL-12, and IFN secretion by pDCs [53] . B cells also express TLR9. Experiments have shown that expression of TLR9 increases under CpG stimulation of B cellsinvitro[60] .Therefore, the use of TLR9 agonists may promote virus clearance by effector cells.
Effect of TLR SNPs
Susceptibility and clearance of HCV differ among individuals due to TLR SNPs, resulting in different genotypes of HCV-infected patients with different degrees of progression, liver fibrosis, cirrhosis and even HCC susceptibility. Moreover, different SNPs may be able to predict the progression and prognosis of liver fibrosis after HCV infection. SNPs of TLR2, TLR3, TLR4, TLR7 and TLR8 all affect the occurrence and development of HCV to different degrees.For instance,TLR2gene polymorphisms are related to HCC susceptibility [61] ; minor allele variants of TLR2 are also associated with chronic HCV status and HCC [62] . In patients with hepatitis C infection, SNPs of TLR3 were found to differ between those who had cleared the virus and those who developed chronic hepatitis C. In addition, significantly greater frequencies of the TLR3 rs3775290, rs3775291, and rs5743312 C allele in the group with spontaneous virus clearance has been reported [10] . The TLR4 SNPs rs4986790 (A/G) and rs4986791 (C/T) also show significant differences between HCV-infected and uninfected control subjects, indicating that SNPs of TLR4 may affect susceptibility of HCV [63] .It has been proven that SNPs of TLR4 can change the risk of hepatocarcinogenesis [64] . One study compared TLR4 gene differences among healthy people, HCV-infected people and HCV-related HCC patients and found that the TLR4 rs2148356 T allele was a protective factor against chronic hepatitis caused by HCV infection and related to a low risk of HCC [65] . The genes encoding TLR7 and TLR8 are located on the X chromosome, and their effect on HCV patients varies according to sex [66] . In Moroccan subjects, the TLR7 rs179008 A allele and rs179009 A allele are mostly found in patients who spontaneously eliminate HCV. Among patients with the rs179009 A allele, the virus clearance rate of females was higher than that of males; TLR8 rs3764879 C and TLR8 rs3764880 A alleles in males were closely related to advanced liver disease [67] . RegardingTLR9gene polymorphisms, researchers performing a study with HCV patients in Egypt found that TLR9 rs352140 polymorphisms may affect the pathological stage of liver cirrhosis in those with genotype 4 HCV infection [68] . Regardless,in more studies onTLR9gene polymorphisms, it was shown that

Fig. 3. Antiviral and inflammatory reactions. After HCV infection, TLRs bind their corresponding ligands, and the body produces IFN to inhibit viral replication and propagation. In addition, the TLR signaling pathway causes various effector cells to produce the inflammatory cytokines TNF- α, IL-1 β, IL-6, IL-8, IL-12, and IL-18, which activate immune functions and can also damage organs. It can be summarized as a dynamic balance between the functions of IFN and inflammatory cytokines.
TLR9 gene may not play a major role in patients with HCV infection [ 10 , 69 ]. Indeed, conclusions differ in many studies on the effect of TLR SNPs on the progression of hepatitis C disease, which may be due to the diversity between samples or insufficient sample sizes.
Effect of activated TLR signaling pathways
Effector cells can produce numerous inflammatory cytokines and IFN after TLRs bind to their corresponding ligands. With regard to monitoring inflammatory cytokines and IFN in patients infected with HCV, determining which TLR is responsible for altering the level of one particular inflammatory cytokine or IFN or whether the interaction of multiple TLRs is responsible for this is often difficult. Nonetheless, assessing overall changes in inflammatory factors is a valuable research aim; most studies have revealed that the level of inflammatory cytokines increases in patients infected with HCV [ 24 , 27 , 38 , 39 ]. Many inflammatory cytokines exert significant action on the process of HCV infection. Notably, changes in cytokines during HCV infection are not attributed to the function of TLRs alone. T-helper lymphocytes also regulate the serum level of cytokines [70] . From another perspective, IFN inhibits the replication of HCV. In addition to type I IFNs (IFN-αand IFN-β)and type II IFN (IFN-γ), HCV infection stimulates the production of type III IFN (IFN-λ/IL-28/29), which requires the participation of IRF3, IRF7 and NF-κB [71] . Therefore, we can consider that IFN and inflammatory cytokines are balanced. If the function of IFN is more significant than that of inflammatory cytokines, the virus will be cleared quickly. In contrast, if the function of inflammatory cytokines is more significant than that of IFN, the liver will be seriously damaged, and the virus will persist for a long time ( Fig. 3 ).
Potential of TLRs in antiviral therapy
Approximately 85% of those infected with HCV develop chronic disease [72] . Moreover, HCV possesses complex immune evasion mechanisms, and actively treating the infection is thus necessary. Infection with HCV effectively activates innate immunity and causes the body to produce IFN to inhibit viral replication and resist the infection. Therefore, IFN combined with ribavirin is often employed for the treatment of HCV [33] . Although IFN has a considerable therapeutic effect on patients with acute HCV infection,its therapeutic effect on patients with chronic HCV infection is not ideal. Moreover, IFN alone has limited effects. In fact, the severity of liver damage depends mainly on the severity of cellular immunity caused by the virus and not on the virus itself because the immune response can damage cells. As viral-related components bind to TLRs of various immune cells, causing these cells to produce cytokines and damaging the body, we consider inhibiting the main immune effector cells that mediate inflammation, such as DCs, to be a valuable therapeutic strategy.
As mentioned in this article, the various proteins expressed by HCV can not only promote the occurrence of immunity but also assist in immune evasion. Protease inhibitors targeting HCV NS3/4A are currently being applied in clinical practice. NS5A and NS5B polymerase inhibitors are also being developed. These kinds of drugs that affect HCV replication directly are called DAAs [73] .DAAs can result in an over 95% sustained virological response(SVR) in patients with compensatory hepatitis C cirrhosis [15] , but there are still many shortcomings. DAAs have a tendency to induce resistance, though efforts are being made to counteract this. Some DAAs have side effects, such as headache, nausea, fatigue, and even symptoms of diarrhea and skin rash, which are difficult to resolve.Overall, the combination of DAAs and other drugs is complex and requires great caution [14] .
In consideration of the important role of the TLR superfamily in the process of HCV infection, TLRs act as the main targets of current treatment strategies. TLR agonists and antagonists are in development: both can bind to the receptor, but the former stimulates the receptor to produce subsequent biochemical reactions,whereas the latter does not activate relevant signaling pathways and does not produce biological effects. Furthermore, enhancement of TLR activity is conducive to the elimination of viruses, but some TLRs can cause cells to produce various cytokines that damage the liver. For this reason, activation of TLRs should be selective and partial. To date, TLR3, TLR7 and TLR9 agonists are the most promising antiviral therapies. Despite few studies on TLR3 agonists in HCV treatment in the past five years, the potential of TLR3 agonists remains. Polyriboinosinic:polyribocytidylic acid [poly(I:C)], a synthetic analog of dsRNA, is the most studied TLR3 agonist and stimulates the production of IFN-βand inflammatory cytokines such as IL-6 and IL-8invitro[74] . Poly(I:C) can also promote DC maturationinvivo[75] . The TLR7 agonist human immunodeficiency virus aldrithiol-2 promotes the production of IFN-αin pDCs and changes pDCs to killer pDCs, which lyse infected cells [53] . Additionally, it has been proven that the TLR9 agonists class C CpG oligodeoxynucleotides (CpG ODNs) promote robust production of IFN-αin pDCs [76] . Notably, some studies have found that the application of TLR9 agonists leads to liver fibrosis but that interruption of TLR9 signaling significantly attenuates fibrosis [ 77 , 78 ]. Current data also support the notion that inhibiting TLR pathways associated with virus eradication and activating TLR pathways associated with inflammation likely promote the development of fibrosis and cirrhosis. These findings suggest that liver function index measurements are necessary when utilizing TLR agonists and that TLR agonist treatment should be combined with concurrent traditional antiviral drugs to achieve a better curative effect. TLR2 agonists have also been found to significantly enhance the antiviral immune response in mice as an adjuvant to genotype 1a HCV vaccine [79] . In general, TLR agonists are promising vaccine adjuvants.Due to the high mutation rate of HCV during replication, it is diffi-cult for the vaccine against HCV to completely prevent infection.Therefore, vaccine adjuvants should be developed to strengthen the antigen and antibody reaction generated by the vaccine and strengthen vaccine activity [80] .
TLR antagonists are active in research. At present, a wide range of TLR antagonists serve as analogs of TLR agonists. Although they are bound by TLRs, there is no biological effect that impacts the immune system. In particular, antagonists of TLR4 are widely studied. These agents prevent the binding of LPS by TLR4, reduce the inflammatory damage caused by LPS, and are beneficial in HCV infection therapy [81] . Expression of C-X-C motif chemokine ligand 10 (CXCL10) is closely related to the severity of HCV. The receptor for CXCL10 is TLR4, and higher CXCL10 expression leads to a greater degree of hepatocyte apoptosis [82] . Additionally,CXCL1gene polymorphism is an independent risk factor in patients with HCV infection and cirrhosis, whereas CXCL1 expression in HCVinfected patients is mediated by TLR2 activation [83] . Therefore,TLR2 and TLR4 antagonists may constitute a therapeutic option to reduce the progression of hepatitis C.
Liver transplantation due to HCV infection also deserves attention. Severe hepatitis C or progression of hepatitis C to decompensated cirrhosis are indications for liver transplantation. However,the recurrence rate of hepatitis C is high, and fibrosis progresses rapidly; based on peripheral blood collected after liver transplantation, the researchers proposed that these findings may be related to a decreased ability of monocyte TLR3 to invoke IL-6 production and TLR7/8 in peripheral blood mononuclear cells to invoke IFN-αproduction. In addition, production of IFN-γby NK cells mediated by TLR8 was decreased, precluding activation of HSCs [84] . Furthermore, the TLR3 leu412phe wild-type genotype was found to be an independent factor related to the severity of HCV recurrence after liver transplantation [85] . Hence, regulation of TLR expression or function is a new approach to improve the quality of life of patients after liver transplantation.
In view of the great influence of TLR SNPs on HCV infection,a large number of experiments conducted using patient samples have shown that TLR SNPs have a certain predictive ability regarding the occurrence and progression of disease [ 10 , 62 , 63 , 65 , 67 , 68 ].Therefore, detection of the TLR genotype and classification of patients before treatment may improve treatment efficiency and help predict prognosis.
Drugs that target TLRs still have some drawbacks. At present,IFN and DAA therapy can reverse HCV-induced liver fibrosis in some patients [ 79 , 86 ], but prognosis has no significant correlation with the HCV virus clearance rate in those with persistent liver fibrosis and decompensated cirrhosis [15] . In this case, the use of TLRs may be helpful in clearing the virus, but it is unable to alleviate severe liver fibrosis or cirrhosis. Despite a lack of studies on the combination of these TLRs and other antiviral agents for patients with such advanced cirrhosis, we suggest that TLR-related agents should be used as early as possible for treatment of HCV infection. When HCV enters the body, it quickly activates the innate immune response, triggering liver parenchymal cells and various immune cells to produce inflammatory factors and IFN; thus, it is helpful for the treatment of acute HCV infection [ 87 , 88 ]. Nevertheless, there are no studies comparing the effectiveness of TLRs in acute or chronic HCV infection, and most animal studies using TLR agonists or antagonists have been conducted in chronic hepatitis C models [ 87 , 89 ]. As for patients with persistent liver fibrosis despite clearance of HCV, TLR-related therapy can also be considered. TLR2-, TLR4-, TLR9-deficient mice are protected from liver fibrosis and further liver damage [90] . Therefore, TLR antagonists may be helpful in the treatment of liver fibrosis. Although many studies have used TLRs as a new target for the treatment of hepatitis C, there are few clinical trials. The TLR7 agonist GS-9620 has been applied in patients with chronic HCV infection, and an increase in plasma mRNA levels of ISG was observed within two days [91] , though the decrease in HCV mRNA was not significant, possibly due to the low dosage and short time frame. In another clinical trial using the TLR7 agonist ANA773, plasma HCV mRNA levels were significantly reduced in patients compared with those of patients with placebo [92] . The TLR7/8 agonist resiquimod and TLR9 agonist CPG 10101 also decrease HCV mRNA levels in patients [ 93 , 94 ]. These clinical trials support further development of drugs that target TLRs. Regardless, no TLR-related drugs have been used in combination with classical DAAs in clinical practice.
Conclusions
The TLR superfamily is an important part of the immune system and can have both positive and negative effects on HCV infection. Activation of TLRs enhances the body’s defensive functions and contributes to the clearance of viruses, thus exerting a positive effect. However, TLRs can have a negative effect, as their activation damages the liver itself and leads to long-term cirrhosis. Effective antiviral strategies involving TLRs have been developed, and meaningful preliminary results have been achieved. TLRrelated drugs also exhibit synergism with other antiviral therapies,and we predict that the regulation of TLRs will be a novel therapeutic approach for HCV infection. To be objective, when TLRrelated drugs are applied in clinical practice in conjunction with traditional drugs, it is necessary to control dosages, assess the pros and cons, and monitor the effect.
Acknowledgments
None.
CRediT authorship contribution statement
Yang Gao: Data curation, Writing - original draft, Writing -review & editing. Narayan Nepal: Validation, Writing - review& editing. Shi-Zhu Jin: Conceptualization, Supervision, Validation,Writing - review & editing.
Funding
None.
Ethical approval
Not needed.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Hepatocellular-cholangiocarcinoma with sarcomatous change:Clinicopathological features and outcomes
- Recent advances in immunotherapy for hepatocellular carcinoma
- Involvement of the circular RNA/microRNA/glucose-6-phosphate dehydrogenase axis in the pathological mechanism of hepatocellular carcinoma
- Progress in hepatitis B virus-related acute-on-chronic liver failure treatment in China: A large, multicenter, retrospective cohort study using a propensity score matching analysis ✩
- From conventional two-stage hepatectomy to ALPPS: Fifteen years of experience in a hepatobiliary surgery unit
- Melatonin attenuates hepatic ischemia-reperfusion injury in rats by inhibiting NF- κB signaling pathway
