Melatonin attenuates hepatic ischemia-reperfusion injury in rats by inhibiting NF- κB signaling pathway
2022-01-07YaoGaoZhiTaoLiLiJinJieLinZhengLeiFanZhongZengHanFeiHuang
Yao Gao, Zhi-Tao Li, Li Jin, Jie Lin, Zheng-Lei Fan, Zhong Zeng, Han-Fei Huang
Organ Transplantation Center, the First Affiliated Hospital of Kunming Medical University, Kunming 650032, China
Keywords:Melatonin Ischemia-reperfusion injury Inflammation NF- κB Liver
ABSTRACT
Introduction
Hepatic ischemia-reperfusion injury (HIRI) is commonly encountered in liver transplantation and hepatobiliary surgery in clinical practice. In the early stage of HIRI, the pathological changes and mechanisms of HIRI primarily include oxidative damage, inflammatory response, and apoptosis, which provide the theoretical basis for strategies using drug pretreatment to mitigate HIRI [1-4] . With deepening of the investigation into HIRI mechanisms, numerous non-drug pretreatment approaches have been used to treat HIRI. These include ischemic preconditioning, genetargeted therapy, hyperbaric oxygen chamber therapy, laser therapy, and autophagy-induced hepatic protective effects [4-8] . The inflammatory cascade can be elicited by oxidative stress injury, aggravating oxidative damage in hepatocytes [1] . These cells release a series of inflammatory cytokines, which ultimately contribute to hepatocyte necroptosis [ 2 , 9 ].
The NF-κB signaling pathway regulates inflammatory cytokines which participate in the aseptic inflammatory responses of organisms [10-12] . NF-κB/Rel proteins mainly include NF-κB2(p52/p100), NF-κB1 (p50/p105), c-Rel, RelA/p65, and RelB, which are proteins involved in dimerization processes to form NF-κB complexes with a regulatory function [ 13 , 14 ]. When hepatocytes experience harmful stimulatory signaling induced by extracellular signals, the “inhibitory effect” of transcriptional inhibitors on NFκB signaling pathway activity can be removed, which leads to activation of the NF-κB signaling pathway and efficaciously induces the expression of downstream inflammatory genes, such asTNF-α,IL-1,IL-6,IL-8, andcyclooxygenase-2[ 11 , 12 , 15 , 16 ]. NF-κB signaling pathway activation in late HIRI indirectly induces the expression of endogenous anti-apoptotic genes and the production of antioxidant enzymes, which exert protective effects against HIRI. More studies [17-19] reported the inactivation of the NF-κB signaling pathway aggravated late HIRI. There are a myriad of targeted signaling molecules involved in the activation of the NF-κB signaling pathway. Studies have shown that in comparison with other targeted signaling pathways, NF-κB signaling pathway activity can be significantly affected by the activity of phosphorylated transcriptional inhibitors (p-IκB-α) and phosphorylated transcriptional activators(p-NF-κBp65) [ 15 , 20 , 21 ]. Therefore, finding drugs with significant protective effects against HIRI is imperative and has important clinical significance for the treatment of HIRI.
Melatonin is an indole hormone that is mainly synthesized and secreted by the pineal gland, and its biological function is mainly involved in regulating the circadian rhythm of organisms [22] .Melatonin can directly bind to excess reactive oxygen species(ROS) in the liver and protect the liver from injury [23-26] . However, the regulatory effects of melatonin on inflammation have been controversial. Studies have indicated that melatonin induces a pro-inflammatory response by activating the NF-κB signaling pathway [27-29] . However, a large number of studies have documented that melatonin can prevent early I/R injury in the liver by inhibiting the NF-κB signaling pathway [30-34] . The present study was to establish an HIRI animal model with Sprague-Dawley rats which mimic HIRI in human. The protective effects and potential mechanisms of melatonin during different periods of HIRI were investigated.
Materials and methods
Reagents and instruments
Aninsitucell death detection kit (TMR red) (12156792910) was purchased from Sigma-Aldrich (St. Louis, MO, USA). A streptavidinperoxidase (SP) detection kit (SP0 041), melatonin (IM0 080) and a hematoxylin-eosin (HE) staining kit (G1120) were purchased from Solarbio Inc. (Beijing, China). Serum alanine aminotransferase (ALT) assay kit (C009-2-1) and lactate dehydrogenase (LDH)assay kit (A020-2-2) were purchased from Jiancheng Bioengineering Institute (Nanjing, China). An upright biological microscope and an upright fluorescence microscope were purchased from Olympus (Tokyo, Japan). A multi-mode microplate reader was purchased from Biotek (Winooski, Vermont, USA). The following antibodies were purchased from Bioss (Beijing, China):βactin (bs-0061R), HRP-conjugated goat anti-rabbit IgG (bs-0295GHRP), HRP-conjugated goat anti-mouse IgG (bs-0368G-HRP) and Alexa Fluor488-conjugated goat anti-rabbit IgG (bs-0295G-AF488).The following antibodies were obtained from Abcam (Cambridge,UK): t-NF-κB inhibitor-α(IκB-α) (ab7217), p-NF-κB activatorp65 (NF-κBp65) (S536) (ab86299), t-NF-κB activator-p65 (NFκBp65) (ab16502), IL-6 (ab9324) and TNF-α(ab270264). p-NF-κB inhibitor-α(IκB-α) (Ser32) (2859) was purchased from Cell Signaling Technology (Boston, MA, USA).
Animals
Male Sprague-Dawley rats (weighing 220-250 g) were purchased from the Experimental Animal Center of Kunming Medical University. Rats were housed in the Biomedical Engineering Center of Kunming Medical University under standard conditions with a temperature of 24 ± 2 °C, humidity of 52% ± 5%, and a 12 h light/dark cycle and were fedadlibitum. All experimental protocols and animal handling procedures were performed with the permission of the Institutional Animal Care and Use Committee of the First Affiliated Hospital of Kunming Medical University (KMMU2020189). Rats were fasted but allowed to drink water freely 12 h before the experiments.
Animal model of HIRI
Sixty Sprague-Dawley rats were randomly divided into a sham group (n= 20), ischemia-reperfusion (I/R) group (n= 20), and melatonin-treated (M + I/R) group (n= 20); according to the time period of reperfusion, the I/R and M + I/R groups were divided into two subgroups including the I/R-5 h group (n= 10) and I/R-24 h group (n= 10) and M + I/R-5 h group (n= 10) and M + I/R-24 h group (n= 10), respectively. After anesthetized via intraperitoneal administration of a 5% chloral hydrate solution, the rats in the sham group underwent only surgery with cutting open and suturing the abdomen as well as separation of the peripheral ligaments of the liver. The 70% hepatic I/R rat models were established using previously described methods with slight modifications [35] .Briefly, the first porta hepatis was occluded for 45 min with nontraumatic microvascular clamps to induce an ischemic insult. Successful occlusion of the first porta hepatis was confirmed by observing a color change in the liver. The rats in the sham group and I/R group were injected with vehicle (5% DMSO in saline) 15 min prior to the onset of ischemia, and the M + I/R group were injected with melatonin (10 mg/kg). Melatonin was dissolved in 5%DMSO saline. The rats (n= 6 per group) from the above-described groups were sacrificed 5 or 24 h after reperfusion, and blood and samples of ischemic liver lobes were taken for future analysis.
Survival rate
Twenty rats were randomly divided into two groups, namely,the vehicle treatment (I/R) group (n= 10) and the melatonin treatment (M + I/R) group (n= 10). Experimental modeling was performed as described above. The 7-day survival rate was assessed after hepatic I/R surgery.
Liver damage
The serum ALT and LDH levels were measured using a microplate reader. The specific detection methods were performed according to the kit instructions.
Histopathological examination of the liver
Fresh liver tissue was fixed in a 10% neutral formalin solution for 24 h, rinsed under running water overnight, dehydrated with gradient alcohol (75%, 80%, 85%, 90%, 95%, and 100%) for a total of 180 min, immersed in wax for 2 h, embedded in a copper embedding frame, and sliced at 5μm after gradually cooling at room temperature. After paraffin sections were successfully prepared, HE staining was performed based on the kit instructions with slight modifications. The degree of liver tissue damage was assessed using Suzuki’s criteria [36] .
Immunohistochemical analysis of liver tissue samples
The relevant experimental procedures were based on a SP kit and the instructions for primary antibodies (anti-IL-6 and anti-TNF-α) with slight modifications. After sections were preheated,deparaffinized, and hydrated, endogenous peroxidase activity was inhibited with 3% H 2 O 2 for 10 min at room temperature, and antigen was retrieved with a pressure cooker for 2 min. The sections were treated with Immuno-Block reagent (Solarbio Inc., Beijing,China) for 30 min at room temperature. After the sections were incubated with primary antibodies [anti-IL-6 (1:250) and anti-TNF-α(1: 200)] at 4 °C overnight, they were incubated with a biotin-conjugated goat anti-mouse IgG secondary antibody (1:100)for 30 min at room temperature, followed by incubation with SP concentrates for 30 min at room temperature. The reactions in sections were visualized with diaminobenzidine, and the sections were counterstained using 50% Harris hematoxylin. The expression levels of inflammatory cytokines (IL-6 and TNF-α) in liver tissue were evaluated using previously described methods [30] .
Hepatocyte apoptosis analysis
The procedures and protocols of the experiment were based on the kit instructions and previous methods with appropriate adjustments. Briefly, after sections were preheated, deparaffinized, and hydrated, they were incubated with proteinase K (20 mg/mL) at 37 °C for 30 min in a humidified chamber and then washed in PBS buffer for 30 min at room temperature. Section permeabilization was performed with an immunostaining permeabilization buffer for 8 min, and then the sections were washed with PBS buffer for 20 min at room temperature, subsequently incubated with TdT labelling buffer at 37 °C for 1 h in a humidified chamber, washed with the PBS buffer for 30 min at room temperature, and finally counterstained with DAPI. TUNEL-positive cells are shown in red,and nuclei are shown in blue. For quantification, three randomly selected high-power fields ( × 200) were analyzed in each section.Five positive visual fields were randomly selected for observation and quantification to measure the apoptotic index (%): (apoptotic cells/total hepatocytes) × 100%. Data from 5 fields were averaged to obtain the final results.
Immunofluorescence analysis of the liver
A liver immunofluorescence assay was carried out according to the methods of a previous report [30] and instructions for p-NF-κBp65. Briefly, after sections were preheated, deparaffinized,and hydrated, antigens were retrieved with a pressure cooker for 3 min. The sections were washed with PBS buffer, permeabilized with an immunostaining permeabilization buffer for 10 min, treated with 5% BSA at room temperature for 30 min, and then incubated with a primary anti-p-NF-κBp65 antibody (1:200)at 4 °C overnight. After being washed with PBS buffer, the sections were incubated with a goat anti-rabbit IgG Alexa Fluor 488-conjugated secondary antibody (1:200) at room temperature for 45 min, washed with PBS buffer again, and finally counterstained with DAPI. p-NF-κBp65-positive cells are shown in green, and nuclei are shown in blue. The nuclei number of p-NF-κBp65-positive cells (numerator) and the total number of cellular nuclei (denominator) were counted using previously described methods [30] .
Western blotting analysis
Western blotting analysis was performed based on previously described methods and antibody instructions with slight modifications. Briefly, fresh liver tissue samples were lysed, and total protein was extracted. Then the protein concentration (5 mg/mL) was determined with the BCA Protein Assay Kit (Jiancheng Bioengineering Institute, Nanjing, China). Total protein (40 μg) was separated on a 5%-12% Tris-Glycine resolving gel, and then transferred to a 0.45μm PVDF membrane. Then, the membranes were blocked with 5% BSA for 2 h at room temperature, incubated with primary antibodies [anti-t-IκB-α(1:20 0 0), anti-p-IκB-α(1:10 0 0), anti-t-NF-κBp65 (1:20 0 0), anti-p-NF-κBp65 (1:10 0 0), anti-IL-6 (1:20 0 0)and anti-β-actin (1:20 0 0)] at 4 °C overnight, washed with TBST for 1 h at room temperature, incubated with HRP-conjugated secondary antibodies [goat anti-mouse (1:20 0 0) and goat anti-rabbit(1:20 0 0)] for 2 h at room temperature, and washed with TBST for 45 min at room temperature. The membranes were then visualized with Millipore ECL Western Blotting Substrate (Millipore Inc.,Billerica, MA, USA) using a GenoSens 1880 luminescence imaging workstation (Qinxiang Scientific Instrument Inc., Shanghai, China),and the blots were analyzed using Image-Pro Plus 6.0 software(Media Cybernetics Inc., Rockville, MD, USA). Band densities were normalized toβ-actin densities, and evaluation of each protein was performed three times.
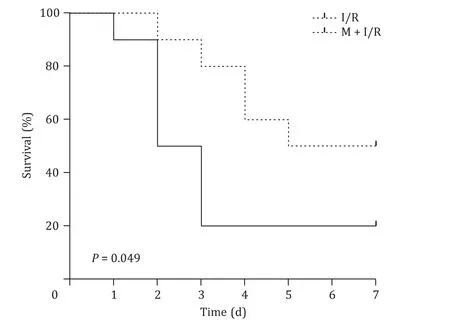
Fig. 1. Melatonin attenuates mortality of rats after hepatic ischemia-reperfusion injury ( n = 10 per group).
Statistical analysis
Quantitative data were expressed as mean ± standard deviation(SD). Statistical analyses were performed using GraphPad Prism software v8.0 (GraphPad Inc., San Diego, CA, USA). For comparisons of multiple groups, one-way analysis of variance (ANOVA) followed by Bonferroni’sposthoctest was used. Nonparametric analyses were utilized for the variables included in liver histopathology and injury scores. APvalue<0.05 was considered statistically significant.
Results
Survivals
The success rate of surgery was 100% in the sham group, 70%and 60% in hepatic I/R-5 h group and the I/R-24 h group, respectively. The 7-day survival rates were 20% in the I/R group and 50%in the M + I/R group (P<0.05, Fig. 1 ).
Liver damage
The effects of melatonin on rat liver damage after 45 min of warm ischemia followed by 5 or 24 h reperfusion are shown in Fig. 2 . As expected, I/R significantly increased the levels of ALT and LDH in the I/R-5 h and I/R-24 h groups compared with those of the sham group (P<0.01), while the ALT and LDH levels were significantly reduced in the M + I/R group compared with those of the I/R group (P<0.05).
Liver histopathological examination
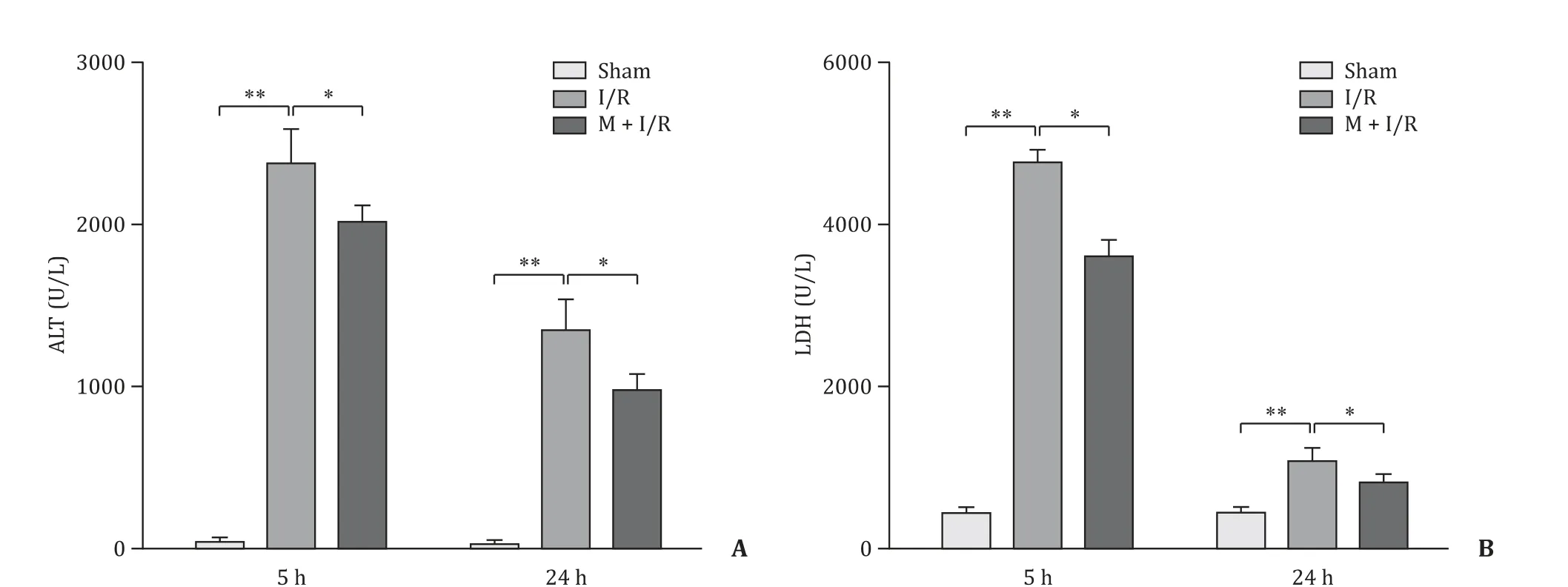
Fig. 2. Melatonin alleviated hepatocyte injury. A: ALT; B: LDH. n = 6 per group; * P < 0.05; ** P < 0.01.

Fig. 3. Histopathological finding of liver injury score (arrows indicating degenerative hepatocytes) (original magnification × 200). A-C: Microscopic finding of rat liver after 45 min of warm ischemia followed by 5 h of reperfusion. D-F: Microscopic finding of rat liver after 45 min of warm ischemia followed by 24 h of reperfusion. G: Histological injury scores based on the Suzuki’s criteria were quantified at different time points of reperfusion. n = 4 per group; * P < 0.05; ** P < 0.01.
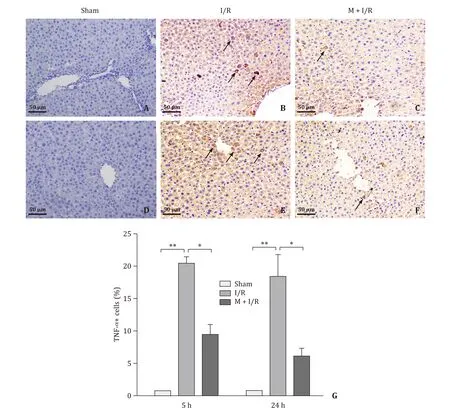
Fig. 4. Immunohistochemical findings for TNF- α (arrows indicating TNF- α+ cells) (original magnification × 200). A-C: Microscopic finding of rat liver after 45 min of warm ischemia followed by 5 h of reperfusion. D-F: Microscopic finding of rat liver after 45 min of warm ischemia followed by 24 h of reperfusion. G: The number of TNF- α+cells were quantified at different time points of reperfusion. n = 4 per group; * P < 0.05; ** P < 0.01.
The histopathological findings for the rats treated with ischemia for 45 min followed by 5 or 24 h of reperfusion are presented in Fig. 3 . The liver sections from the sham rats displayed a normal structure. In contrast, liver tissue samples obtained from the rats in the I/R group showed severe ballooning, hepatocyte necrosis,and abnormalities in the structure of hepatic lobules. Melatonin treatment significantly attenuated the severity of liver tissue injury. Suzuki’s scores were higher in the I/R group than those in the sham group (P<0.01) but was decreased after melatonin treatment (P<0.05).
Immunohistochemical staining
As shown in Figs. 4 and 5 , there were no obvious expression of TNF-αand mild expression of IL-6 in liver tissue from sham rats.Expressions of TNF-αand IL-6 were significantly increased in the I/R group (P<0.01), but the expression levels of these inflammatory cytokines did not significantly differ between the I/R-5 h and I/R-24 h groups (P>0.05). However, these levels were decreased in melatonin treatment group (P<0.05).
Hepatocyte apoptosis
TUNEL staining showed that there was no obvious hepatocyte apoptosis in the rats in the sham group ( Fig. 6 ). The number of apoptotic hepatocytes in the I/R group was significantly increased compared with that of sham rats (P<0.01). There were no significant differences between the I/R-5 h and I/R-24 h groups in the number of apoptotic hepatocytes (P>0.05). Compared with that in the rats in the I/R group, the number of apoptotic hepatocytes in the melatonin treatment group was significantly reduced(P<0.05).
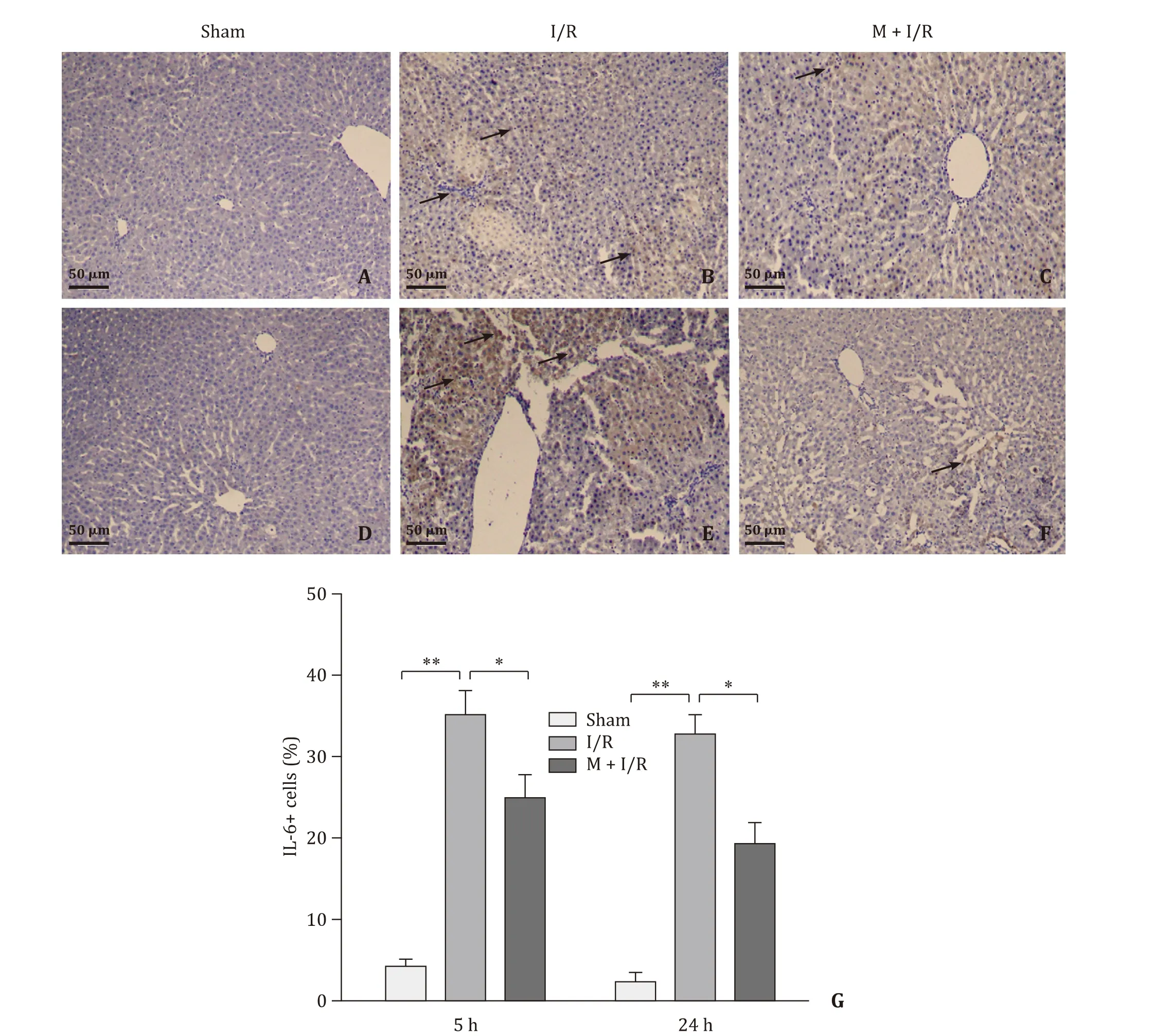
Fig. 5. Immunohistochemical findings for IL-6 (arrows indicating IL-6 + cells) (original magnification × 200). A-C: Microscopic finding of rat liver after 45 min of warm ischemia followed by 5 h of reperfusion. D-F: Microscopic finding of rat liver after 45 min of warm ischemia followed by 24 h of reperfusion. G: The numbers of IL-6 + cells were quantified at different time points of reperfusion. n = 4 per group; * P < 0.05; ** P < 0.01.
p-NF- κBp65 expression
The number of p-NF-κBp65-positive cells between the I/R-5 h and I/R-24 h groups showed no significant difference (P>0.05,Fig. 7 ). Compared with the I/R group, the number of p-NF-κBp65-positive cells in the melatonin-treated group was significantly reduced (P<0.05).
Expression of key NF- κB signaling pathway proteins in the liver
The expressions of p-IκB-α/t-IκB-αand p-NF-κBp65/t-NFκBp65 were significantly increased in the I/R group compared with those of the sham group (P<0.05, Fig. 8 ). These molecules showed no significant change between the I/R-5 h and I/R-24 h groups(P>0.05). However, melatonin treatment significantly decreased the expressions of these molecules (P<0.05).
Discussion
HIRI can induce an inflammatory cascade, leading to necrotic apoptosis of hepatocytes. A plethora of studies have shown that melatonin can protect against early HIRI by inhibiting NF-κB signaling pathway activity and downregulating the release of inflammatory cytokines [30-34] . However, in late HIRI, the mechanisms by which melatonin regulates NF-κB signaling pathway activity and the protective effects on the liver have not yet been clarified.
Herein, hepatocyte injury was assessed by detecting liver enzyme levels. Melatonin significantly prevented I/R injury in hepatocytes via antioxidative effect. Previous studies showed that melatonin scavenges excess ROS to mitigate oxidative damage to hepatocytes [ 23 , 37 ]. Melatonin decreased the activity but elevated the survival rate in rats treated with I/R, which may be associated with the sedative effect and hypnotic efficacy of melatonin [22] .Our results corroborated the safe doses and low toxicity of melatonin seen in other studies [ 38 , 39 ], and the benefits of melatonin on HIRI were further evidenced by the liver histopathology.Studies [ 40 , 41 ] have shown that melatonin can preserve the integrity of the glomerular ultrastructure, stabilize the cell membrane structure, and enhance a cell’s refractoriness to oxidative stress injury. However, whether melatonin can also have this protective effect on hepatocytes needs to be verified.
The concentration of exogenous melatonin is highest in hepatocyte membranes, followed by the mitochondria and nucleus. Melatonin has good lipophilicity, and hepatocyte membranes are rich in transport proteins for melatonin, which contributes to melatonin uptake by hepatocytes [42] . Intraperitoneal injection of 10 mg/kg melatonin in rats produces a half-life of 30 min and a bioavailability of 74%, while intravenous injection of 5 mg/kg melatonin in rats results in full elimination after 90 min [43] . However, the longterm protective effects of melatonin were not significantly affected by the elimination of melatonin in the blood of rats, as metabolites of melatonin play key roles in preventing the liver from I/R injury.Melatonin can bind to ROS, generating cyclic 3-hydroxymelatonin(c3-OHM), 6-hydroxymelatonin (6-OHM), 4-hydroxymelatonin (4-OHM), 2-hydroxymelatonin (2-OHM), and so on, which can further generate beneficial metabolites to offer persistent protective effect on the liver [24] . Studies [ 24 , 44-46 ] have shown that melatonin metabolites have anti-inflammatory and antioxidant activities, such as N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK). This may explain why melatonin has overwhelming protective effects against I/R injury in the liver; however, the mechanisms by which melatonin mediates these protective effects remain to be elucidated.

Fig. 7. Immunofluorescence findings for p-NF- κBp65 + cells (arrows indicating p-NF- κBp65 + cells) (original magnification × 200). A-C: Microscopic finding of rat liver after 45 min of warm ischemia followed by 5 h of reperfusion. D-F: Microscopic finding of rat liver after 45 min of warm ischemia followed by 24 h of reperfusion. G: The numbers of p-NF- κBp65 + cells were quantified at different time points of reperfusion. n = 4 per group; * P < 0.05; ** P < 0.01.
A previous study has revealed that melatonin can alleviate colitis damage in the intestinal tract by inhibiting the expression of cyclooxygenase-2 and inducible nitric oxide synthase [16] . The roles of melatonin in regulating the inflammatory response are controversial. Studies [ 27 , 28 , 47 ] have shown that melatonin can inhibit tumor cell growth by enhancing the immune response of organisms, indicating that melatonin can upregulate the inflammatory response. However, some studies have found that melatonin exerts protective effects against HIRI by downregulating the expression of inflammatory factors and that this protective mechanism may be mainly related to the inhibition of NF-κB signaling pathway [ 31 , 4 8 , 4 9 ]. In our experiment, the expression levels of the inflammatory cytokines, IL-6 and TNF-α, in the melatonin treatment group were significantly downregulated, confirming that melatonin has inhibitory effects on the inflammatory response. Meanwhile, we also found that the levels of inflammatory cytokines in the early and late stages of HIRI were not notably different, suggesting that NF-κB pathway activity was upregulated at all times in the rat liver after 45 min of warm ischemia followed by 5 or 24 h of reperfusion. Additionally, we also found relatively low levels of IL-6 but nearly no expression of TNF-αin rats in the sham group, suggesting that low levels of the inflammatory cytokine IL-6 may have had protective effects on the rats in the sham group.A previous study has documented that IL-6 can promote the proliferation of hepatocytes by inducing the expression of intracellular STAT3 within hepatocytes [50] , which can be explained by the above findings. However, compared with that in the sham group,the level of the inflammatory cytokine IL-6 in the I/R group was significantly elevated, particularly in the I/R-24 h group, and liver pathological changes and enzymes were significantly exacerbated,indicating that the inflammatory cytokine IL-6 possibly performs dual roles in regulating HIRI, which needs to be corroborated in future investigations. Our study found that p-NF-κBp65/t-NF-κBp65 and p-IκB-α/t-IκB-αin the melatonin-treated group was significantly lower than those in the I/R group. The change of these ratios implied that melatonin treatment can inhibit the activity of NF-κB signaling pathway. Our data are consistent with other studies [ 30 , 31 ]. Inhibition of NF-κB signaling pathway activation exerts an anti-apoptotic effect, which was also found in our study.Compared with the I/R group, hepatocyte apoptosis was significantly reduced in the melatonin-treated group. However, we cannot rule out the positive effect of NF-κB upregulation on endogenous antioxidant enzyme, and further studies are needed to evaluate the antioxidative activity of melatonin in HIRI. The limitation of this study is that the optimal dose of melatonin has not been proposed.
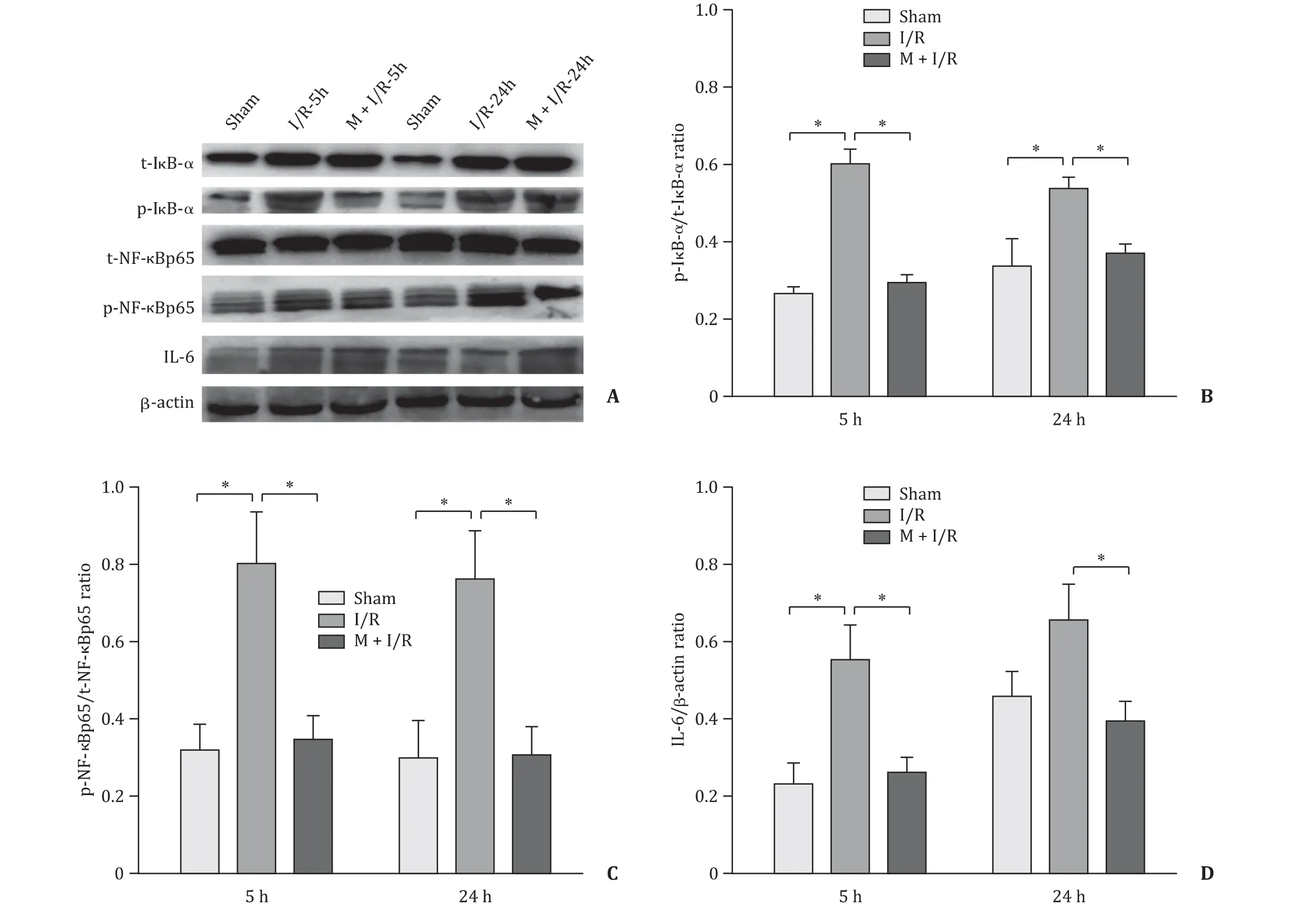
Fig. 8. Effects of melatonin on NF- κB signaling pathway activity. A: Findings of Western blotting. B: Quantification analysis of the p-I κB- α/t-I κB- α ratio. C: Quantification analysis of the p-NF- κBp65/t-NF- κBp65 ratio. D: Quantification analysis of the IL-6/ β-actin ratio. n = 4 per group; * P < 0.05.
In conclusion, melatonin downregulated the activity of the NFκB signaling pathway in the early and late stages of HIRI, alleviating the inflammatory response in HIRI.
Acknowledgments
None.
CRediT authorship contribution statement
Yao Gao: Data curtion, Formal analysis, Investigation, Methodology, Writing - original draft. Zhi-Tao Li: Formal analysis, Methodology, Writing - original draft. Li Jin: Methodology, Writing - review & editing. Jie Lin: Methodology, Writing - review & editing.Zheng-Lei Fan: Methodology, Writing - review & editing. Zhong Zeng: Data curtion, Funding acquisition, Writing - review & editing.Han-Fei Huang: Conceptualization, Funding acquisition, Resources,Supervision, Writing - review & editing.
Funding
This study was supported by grants from the National Natural Science Foundation of China ( 81960123 and 81760119 ) and Yunnan Provincial Science and Technology Department and Kunming Medical University Collaborative Fund ( 2019FE001-037 ).
Ethical approval
This study was approved by the Institutional Animal Care and Use Committee of the First Affiliated Hospital of Kunming Medical University (KMMU2020189).
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Hepatocellular-cholangiocarcinoma with sarcomatous change:Clinicopathological features and outcomes
- Recent advances in immunotherapy for hepatocellular carcinoma
- Toll-like receptors and hepatitis C virus infection
- Involvement of the circular RNA/microRNA/glucose-6-phosphate dehydrogenase axis in the pathological mechanism of hepatocellular carcinoma
- Progress in hepatitis B virus-related acute-on-chronic liver failure treatment in China: A large, multicenter, retrospective cohort study using a propensity score matching analysis ✩
- From conventional two-stage hepatectomy to ALPPS: Fifteen years of experience in a hepatobiliary surgery unit
