In Situ Modification Strategy for Development of Room-Temperature Solid-State Lithium Batteries with High Rate Capability
2021-12-27JianghuiZhaoMaolingXieHaiyangZhangRuoweiYiChenjiHuTuoKangLeiZhengRuiguangCuiHongweiChenYanbinShenLiweiChen
Jianghui Zhao , Maoling Xie , Haiyang Zhang , Ruowei Yi , Chenji Hu ,4, Tuo Kang ,Lei Zheng 1,, Ruiguang Cui , Hongwei Chen , Yanbin Shen 1,,*, Liwei Chen ,4
1 School of Nano-Tech and Nano-Bionics, University of Science and Technology of China, Hefei 230026, China.
2 i-Lab, CAS Center for Excellence in Nanoscience, Suzhou Institute of Nano-Tech and Nano-Bionics, Chinese Academy of Sciences, Suzhou 215123, Jiangsu Province, China.
3 College of Materials Science and Engineering, Huaqiao University, Xiamen 361021, Fujian Province, China.
4 School of Chemistry and Chemical Engineering, Shanghai Jiaotong University, Shanghai 200240, China.
Abstract: The increasing development of society has resulted in the evergrowing demand for energy storage devices.To satisfy this demand, both energy density and safety performance of lithium batteries must be improved, which is challenging.Solid-state lithium batteries are promising in this regard because of their safe operation and high electrochemical performance.In recent years,intense effort has been devoted toward the exploration of materials with high ionic conductivity for room-temperature solid-state batteries.Among several types of solid-state electrolytes, Li1.5Al0.5Ge1.5(PO4)3 (LAGP), an inorganic NASICON-type electrolyte, has drawn considerable attention because of its high ionic conductivity,wide electrochemical window, and environmental stability.However, the formation of lithium-ion-conducting networks within the electrode and between the electrode-LAGP interface is limited because of high interfacial resistance caused by the direct contact and volume expansion between the electrode and electrolyte.Thus,the application of LAGP in the fabrication of solid-state batteries is limited.Moreover, the occurrence of the unavoidable side reaction because of the direct contact of LAGP with the lithium metal anode shortens battery life.In addition, the rigid brittle nature of the LAGP electrolyte leads to the limits the facile fabrication of solid-state batteries.To overcome these limitations, herein, a novel strategy based on in situ polymerization of a vinylene carbonate solid polymer electrolyte (PVCSPE) was proposed.The in situ formed PVC-SPE can effectively construct ion-conducting pathways within the cathode and on the interfaces of the LAGP electrolyte and electrodes.Furthermore, the PVC-SPE can significantly inhibit the side reaction between the lithium anode and LAGP electrolyte.The electrochemical performances of Li | LAGP | Li and Li |LAGP | Li with in situ PVC-SPE modified interface symmetrical solid-state batteries were compared.The in situ modified Li | LAGP | Li symmetrical solid-state battery exhibited stability toward plating and stripping for over 2700 h and a low overpotential (34 mV) at room temperature.Moreover, a Li | LAGP | LiFePO4 solid-state battery exhibited a capacity retention of 94% at 0.2 C after 200 cycles with a capacity of 158 mAh·g-1.In addition, high rate capability (72.4% capacity retention at 3 C) was achieved at room temperature.Therefore, the proposed in situ modification strategy was found to resolve the interface-related problem and facilitated the construction of the ion-conducting network within the electrode;thus, it can be a promising approach for the fabrication of high-performance solid batteries.
Key Words: Ion network; Interface; In situ polymerization; Solid-state battery
1 Introduction
Nowadays commercial lithium batteries are widely used in a variety of fields due to their high energy density and other unique advantages1.However, the conventional liquid organic electrolyte used in commercial batteries still presents some safety issues, particularly the thermal runaway that limits their application in electric vehicles2.Such concerns have raised great expectations for solid-state batteries with no combustible solvents to improve safety characteristics3,4.Since the ionic conductivity of solids is usually lower than liquid electrolytes and most of them need to be operated at high temperature (40-60 °C) to achieve acceptable performance, people have devoted great efforts to the development of solid electrolytes with high ionic conductivity (~10-4S·cm-1) at room temperature recently5-7.Among various types of solid electrolytes,NASICON-type electrolyte Li1.5Al0.5Ge1.5(PO4)3(LAGP) has drawn much attention due to its high conductivity (10-3- 10-4S·cm-1), wide electrochemical windows, and environmental stability8.However, other key problems,i.e., the lithium-ion conducting network within the electrode and between the electrode-electrolyte interfacial issues still impede the application of LAGP electrolyte in the solid-state battery9,10, and the former one is usually more challenging11.Furthermore, it is especially difficult to design operational solid-state batteries due to the side reaction between the LAGP solid electrolyte and the Li metal, as well as the large interfacial resistance caused by the poor contact and the volume expansion issue between the electrode and the electrolyte12,13.Besides, the rigid brittle nature of LAGP electrolyte leads to the hard fabrication of solid batteries14.
Some works have been devoted to developing the ion conducting network in the porous cathodeviamany approaches15-19.The classic strategy is coating polymer or inorganic electrolyte on the active particles to ameliorate the contact and mitigate polarization20.Another option is infiltrating solvent mixture contained polymer or sulfide electrolyte into the tortuous porous cathode network21,22.Recently, Huetal.23developed a 3D electrode by constructing a ceramic textile network, which increased the contact area with electrolyte and enhanced the tolerance for electrode volume change.Note that the construction of an internal ionic network should not affect other functional components such as electron conduct additives24.It is also important to construct an intimate solid-solid contact on the interface25.Hanetal.26developed a method to ameliorate the wettability of the Li metal by atomic-layer depositing a thin layer of Al2O3coating on the electrolyte.Chenetal.27proposed to solve the poor interfacial contact and reduce the interface impedance by coating a layer of curable glue electrolyte between the electrolyte and electrodes.Solving the above two problems simultaneously in a facile way is desirable to realize highperformance solid-state batteries25,28.
As a potential organic solid electrolyte, poly(vinylene carbonate) (PVC) has drawn growing attention during recent years.Shriveretal.first proved that PVC can deliver favorable ionic conductivity and satisfactory mechanical strength29.Itohetal.30demonstrated a copolymer composed of vinylene carbonate (VC) monomer can exhibit higher ionic conductivity.More importantly, Cui’s group31showed VC can be directly applied in solid batteries by a facileinsitupolymerization.However, the ionic conductivity of PVC is not enough to satisfy the room temperature operation for a solid-state battery.On the contrary, the LAGP inorganic electrolyte shows higher conductivity of 10-4S·cm-132,33.Nevertheless, the rigid contact between the LAGP electrolyte and electrode is poor.Besides, the unavoidable side reaction will occur during the direct contact of LAGP with anode lithium metal12.Therefore, we herein developed a simpleinsitupolymerization strategy to fabricate the ionic conductive network within electrodes and the favorable electrode-LAGP electrolyte interface in order to address the abovementioned issues.
2 Experimental section
2.1 Preparation of the PVC-SPE
A homogeneous and transparent liquid solution (lithium bistrifluoromethanesulfonimide (LiTFSI) in VC) was pre-prepare by adding 1.43 g LiTFSI (Sigma (Shanghai)) in 5 mL monomer VC (Sigma) with stirring continuously for a few minutes.Then 10 mg azobisisobutyronitrile (AIBN) (Adamas (Shanghai)) was added in 5 mL of the as-prepared VC-LiTFSI solution.After the complete dissolution of AIBN, the transparent mixture solution was cast on the plate glass and heated at 60 °C and 80 °C for 24 h in a hot box for a few hours to obtain the white solid polymer electrolyte.To avoid the effect of moisture and oxygen, the above-mentioned procedure of fabricating the PVC-SPE film was carried out in an argon-filled glove box.
2.2 Preparation of the LAGP electrolyte
Precursors (Li2CO3, Al2O3, GeO2, and NH4H2PO4) (Aladdin(Shanghai)) were mixed in stoichiometric and ball-milled for 30 min.Then the powder mixture was heated to 1400 °C for 2-3 h.Later, the LAGP glass liquid was immediately poured into the crucible.The formed cooling LAGP glass was put into another muffle furnace and heated at 850 °C for 24 h.After that, the obtained opalescent LAGP ceramic block is cut to ceramic sheets for further use.
2.3 Preparation of in situ PVC-SPE in batteries and electrochemical characterization
The LiFePO4cathode prepared by the conventional casting method was prepared by mixing 85% (mass fraction) LiFePO4(Sinlion Battery Tech, Co., Ltd.(Suzhou)), 7.5% acetylene black, and 7.5% poly(vinylidene fluoride) (PVDF) (Aladdin)together.Then the as-prepared LiFePO4electrode was uniformly drop-added with a small amount of 7 μL PVC-SPE precursor,then the LAGP film with a diameter of 16 mm was covered on the wet electrode until the electrode was fully infiltrated by PVCSPE precursor.After that, 5 μL of the PVC-SPE precursor was further added on the opposite side of the LAGP film and a 200 μm-thick lithium metal disc (China Energy Lithium Co.Ltd.(Tianjin)) was covered on the wet LAGP.Then the sealed batteries were kept at 60 °C for 20 h and 80 °C for 10 h in a hot oven.There materials, LiCoO2(LCO), Li2TiO3(LTO), and LiNi0.5Mn1.5O4(LNMO), were used for preparing solid state cathode electrode.Galvanostatic charge-discharge test of solid batteries was recorded on a battery test system (NEWARE Technology Ltd., Shenzhen, China).The electrochemical stability of PVC-SPE was measured by the linear sweep voltammetry (LSV).The ionic conductivities were investigated by electrochemical impedance spectroscopy (EIS) with an amplitude of 10 mV in a frequency range between 7 MHz and 0.1 Hz.The LSV experiment and EIS measurements were obtained by an electrochemical workstation (Bio-Logic VMP300).
2.4 Physical characterization
Fourier transform infrared (FTIR) spectra of samples were recorded on a Thermo Scientific Nicolet 6700 spectrometer.The cross-section of the electrode was cut by a GATAN cross-section processing system.The 1H nuclear magnetic resonance (NMR)Spectra of the VC monomer and PVC was recorded on a Bruker Advance 400 and 600 Spectrometer in D2O.X-ray diffraction pattern of LAGP and PVC-SPE were recorded on a Bruker D8-advance X-ray diffractometer using Cu-Kα radiation.The morphologies of samples were investigated by a scanning electron microscope (SEM) (FEI Quanta 400 FEG equipped with Energy Dispersive X-ray Spectrometric Microanalysis(EDX) (Apollo 40 SDD) operated at 10 kV).The X-ray photoelectron spectroscopy (XPS) measurements were recorded on a PHI-5000 Versa Probe equipment employing AlKα radiation.
3 Results and discussion
The polymerization of the liquid VC can be initiated by AIBN easily under heating34.As illustrated in Fig.1a, the VC-LiTFSI solution with thermo-initiator is infiltrated into the electrode and it wet the LAGP-lithium metal interface at the same time, which is beneficial to ionically connecting active particles and other components.After the solidification of the PVC-SPE, the solid contact of the network and intimate interface in the battery is built and preserved, providing a lithium-ion pathway through the PVC-SPE in the solid state battery during the charge and discharge process.
As shown in the digital image in Fig.1b, the VC-LiTFSI precursor solution with radical thermal initiator AIBN can be polymerized as the PVC-SPE in a white solid-state.The FTIR spectra of VC monomer, PVC, LiTFSI, and PVC-SPE are shown in Fig.1c.Characteristic peaks of the C=C―H bond (3175 cm-1symmetric stretching) are only found in VC monomer samples,while they disappear in the PVC and PVC-SPE spectra35.Peaks of the C―C―H (2987 cm-1for symmetric stretching) are observed in both PVC and PVC-SPE spectra, suggesting that the VC monomer is completely polymerized and the structure is well preserved in the PVC-SPE, which agrees with previous report31.Besides, the characteristic peaks of C=O (1801 cm-1for stretching vibration) are slightly shifted to 1818 cm-1in the PVC-SPE spectrum, which is due to the intermolecular interaction between the lithium-ion and oxygen atom in C=O.The results show that the LiTFSI lithium salt can be dissociated in PVC-SPE, which is beneficial for lithium-ion conduction.
XRD results of the PVC-SPE are depicted in Fig.1d.Only one prominent hump peak is found in the PVC-SPE pattern,revealing the amorphous feature of PVC-SPE.This feature is beneficial for lithium-ion transportation in PVC-SPE36.The RD pattern of LAGP pellets sintered at 850 °C well corresponds to the standard spectrum of the rhombohedral NASICON structure LiGe2(PO4)3(PDF 41-0034)37.XRD spectra of LiTFSI and PVC, comparing to PVC-SPE can be found at Fig.S1(Supporting Information).SEM image confirms that the cuboid crystalized LAGP particles are about 500 nm in size and all particles are tightly packed together (Fig.S2).Above mentioned results indicate that the LAGP solid electrolyte was successfully prepared.

Fig.1 (a) The schematic illustration for the interface and the cathode ion-conducting pathway design of an in situ PVC-SPE polymerization procedure; (b) the digital optical photographs of the precursor solution and PVC-SPE solid electrolyte after thermal polymerization;(c) FTIR spectra of VC, PVC, LiTFSI, and in situ formed PVC-SPE; (d) XRD spectra of the pristine LAGP pellet and the PVC-SPE.
The PVC-SPE was coated andinsitupolymerized between symmetric stainless steels (SS) in blocking-type cells to evaluate the ionic conductivity.Fig.S3 shows the temperature dependence of the ionic conductivity of PVC-SPE in the range of 25-80 °C.Fig.2a clearly shows the relationship between 1000/T and logarithm of ionic value at different temperatures and the ionic conductivity of PVC-SPE is 1 × 10-5S·cm-1at 25 °C according to the equation 1 calculation in supporting information.Besides, the impedance of PVC-SPE decreases gradually as the temperature increases.As seen in Fig.2b, the ionic conductivity of the as-prepared LAGP is 4 × 10-4S·cm-1at 25 °C, which is comparable to reported values13.The electrochemical stability of the PVC-SPE was evaluated in a Li| electrolyte | SS sandwich cell by LSV measurement at room temperature.Fig.S4 shows that the lithium transfer number (tLi+)of PVC-SPE is 0.36, which is much higher than that of the PEO-based polymer electrolyte (0.2)38.Fig.2c reveals the electrochemical window curves of VC-LiTFSI and PVC-SPE.A strong oxidation current is observed at 4.3 V in the LSV curve of Li | VC-LiTFSI | SS cell, while the surge of oxidation current density is found at 4.65 V for the Li | PVC-SPE | SS cell.This result guarantees the electrochemical stability of PVC-SPE to be operated at a voltage lower than 4.6 V.

Fig.2 The ionic conductivity against the temperature of the PVC-SPE (a) and the Nyquist plot of the LAGP electrolyte (b);linear sweep voltammetry profiles of VC/LiTFSI and PVC-SPE at 0.5 mV·s-1 at 25 °C (c); voltage profiles of Li | LAGP | Li and in situ PVC-SPE modified Li | LAGP | Li batteries at a current density of 0.05 mA cm-2 at room temperature (d).
The electrochemical compatibility of the interface between the LAGP electrolyte and Li metal anode is one of the determining factors to successfully operate solid-state lithium metal batteries.Thus, the Li | LAGP | Li symmetrical solid batteries are used to investigate the lithium plating/stripping and dendrite growth behaviors.Both sides of the LAGP surface are wetted by the liquid VC-LiTFSI precursor solution and then heated to conduct the polymerization.A long-term lithium plating/striping cycling test is conducted afterward.The unmodified Li | LAGP | Li cells were assembled as the control group.Fig.2d shows that the distinct performance of different symmetric cells at a current density of 0.05 mA·cm-2.The cell with unmodified LAGP electrolyte shows very large overpotential and get short circuit after 7 cycles (Fig.S5).The large interfacial resistance of the cell could be explained as the accumulated side reaction products that with poor Li-ion conductivity in the interlayer between Li metal and LAGP,which was detrimental to the Li-ion transportation39.And the short circuit phenomenon occurs after the initial 7 cycles could be caused by the formation of Li dendrites along with LAGP grain boundary, which is commonly observed in the previous research40.In contrast, theinsitumodified Li | LAGP | Li symmetrical solid battery shows steady plating and stripping curve with long stable cycling for 2700 h and a low overpotential(34 mV) (Figs.2d and S6).Besides, the symmetricinsitumodified Li | LAGP | Li cells is further evaluated under continuous different current densities of 0.05, 0.1 and 0.2 mA·cm-2for 10 cycles of stripping and plating.As seen in Fig.S7,the polarization voltage was gradually increased with the ascending current density.The cycling performance of Li |LAGP | Li symmetrical cells is compared with previously reported results in Table S1.Compared with other modification methods, theinsituPVC-SPE modified method achieves a longer cycling lifespan of Li | LAGP | Li symmetrical cells.These results show that the PVC-SPE could greatly improve the interface compatibility and Li-ions could uniformly plat/strip on the lithium metal through the PVC-SPE protective layer.
The morphologies of lithium foil from the cycled symmetric cells were intuitively observed by SEM.As shown in Fig.3a,compared to the pristine Li metal anodes in Fig.S8, Li metal anode after 50 cycles in theinsitumodified Li | LAGP | Li cell exhibits uniform and flat morphology, which is mainly attributed to the protective effect of the PVC-SPE on lithium metals.In sharp contrast, a distinctly nonuniform morphology of Li anode from the unmodified Li | LAGP | Li cell is shown in Fig.3b, due to the uneven deposition of lithium-ions on Li metal surface and the deleterious reaction between LAGP electrolyte and lithium metal.
To further investigate the effect ofinsituPVC-SPE modification on the chemical components of the Li | LAGP interface, X-ray photoelectron spectroscopy (XPS) was conducted on the surface of the cycled Li metal anode and LAGP of symmetric cells.For the unmodified Li | LAGP | Li cell, the Li-related species on the cycled Li metal anode mainly include Li2CO3(55.5 eV), ROCO2Li (54.2 eV), and LiOH (54.8 eV)(Fig.3d).The inorganic components are mainly originated from side reactions between the LAGP pellet and lithium anode41, and the interface layer is continuously destroyed and rebuilt during cycles, which is not conducive to the uniform deposition of lithium-ions31.As a comparison, for the PVC-SPE modified Li| LAGP | Li cell, Li-F (~685.0 eV) and C―F (688.6 eV)components are found in the F 1s spectrum (Fig.S9), Li―F(~56.2 eV) and LiOCO2R (~55.1 eV) can be found in the Li 1s spectrum (Fig 3c)42.C-F peak could be originated from LiTFSI and LiF is the decomposition product of LiTFSI43.LiOCO2R could come from the reaction between the PVC and Li anode44.Besides, the in-depth analysis shows that the content of C-F and LiOCO2R decreases with the depth, while the content of inorganic LiF increases.LiF is with high mechanical strength,considerable ionic conductivity, and ability to block electron conduction45.Therefore, enriched LiF in the inner layer of the SEI is beneficial to the uniform lithium deposition.Comprehensively, interface contains inorganic LiF with high mechanical strength and elastic organic LiOCO2R avoid repeated destruction and reconstruction during cycling46.
Meanwhile, the XPS measurement on the LAGP surface was conducted to verify the inhibition of side reaction between the PVC-SPE modified LAGP and lithium.For the Ge 3dspectrum of the cycled unmodified LAGP, it shows a dominating peak of GeO (30.8 eV), which means that the Ge4+in the pristine LAGP pellet is reduced by contacting with lithium anode (Fig.3f)47.In contrast, the deconvolved Ge 3dspectrum obtained from the PVC-SPE modified LAGP cell confirms the strong Ge4+peak and much depressed GeO peak.This is benefited from the PVCSPE which effectively blocks the direct contact between lithium anode and LAGP electrolyte and restrains the reduction of Ge4+(Fig.3e).
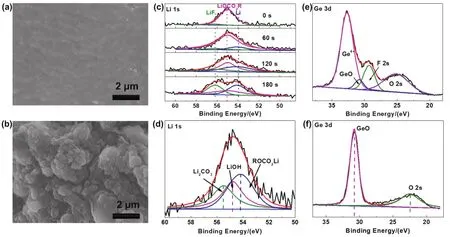
Fig.3 The SEM images showing the morphology of the Li metal anode obtained from in situ PVC-SPE modified Li | LAGP | Li cells (a) and the unmodified Li | LAGP | Li cell (b) after 50 cycles; the in-depth XPS spectra of Li 1s on the surface of Li metal anodes obtained from in situ PVC-SPE modified Li | LAGP | Li (c) and unmodified Li | LAGP | Li (d) cell after 20 cycles; the XPS spectrum of Ge 3d on the surface of LAGP pellets obtained from the in-situ PVC-SPE modified Li | LAGP | Li (e) and unmodified Li | LAGP | Li (f) cell after 20 cycles.
Solid-state Li | LAGP | LiFePO4 batteries were also investigated by EIS measurement.As shown in Fig.S10b, two semicircles are observed in spectra.The first semicircle at high frequencies is attributed to the bulk LAGP electrolyte resistance and the second one at medium frequencies is from the electrodeelectrolyte interfacial resistance.The interface resistance of the battery before polymerization is about 200 Ω, and it increases to 1100 Ω after theinsitupolymerization process, which is due to the transition from the liquid to the solid.In contrast, theexsituoptimization battery and the untreated battery show much larger resistance values.As shown in Fig.S10c-e, the unmodified Li |LAGP | LiFePO4battery shows an extremely large interfacial resistance (~400 thousand Ω) before cycling due to the rough electrolyte-cathode interface and non-optimized conductive network within the electrode, which is far larger than the standard of working batteries at room temperature.Besides, the impedance value of theexsituPVC-SPE optimized solid-state battery is 25000 Ω, which is greater than that of theinsituoptimized solid-state battery (1100 Ω).This may be ascribed to that the lithium-ion transport network in electrode built by theex situapproach cannot provide intimate contact as theinsituone does, which increases the difficulty of ion conduction inside the battery.Impedances of Li | LAGP | LiFePO4 battery at different conditions are summarized in Fig.S10a.
The morphology of the LiFePO4cathode with and withoutin situformed PVC-SPE was observed by SEM.As shown in Fig.4a,b, particles in the pristine LiFePO4cathode are isolated from each other, while the electrode withinsituformed PVC-SPE looks very compact, in which particles are connected to each other, compared with the pristine cathode electrode.
As shown in Fig.4c, TheinsituPVC-SPE modified Li | LAGP| LiFePO4solid battery cycle stably at room temperature.It shows a discharge capacity of 158 mAh·g-1at 0.2Cand the capacity retention after 200 cycles is 94%.In contrast, the Li |LAGP | LiFePO4 cell that withoutinsituPVC-SPE modification can hardly be operated at room temperature, likely due to the large electrode | electrolyte interfacial resistance and the harsh ionic transmission inside the cathode electrode.The cycling performance of the Li | LAGP | LiFePO4solid battery at 1Cis also investigated (Fig.S11).At a larger rate of 1C, a steady cycling performance can be obtained with a capacity of 148 mAh·g-1.The capacity retention after 200 cycles reaches 98%.

Fig.4 The SEM images showing the morphology of the pristine LiFePO4 cathode (a) and the morphology of the in situ PVC-SPE modified LiFePO4 electrode (b); the long-term cycling performance of the in situ and ex situ PVC-SPE modified Li | LAGP | LiFePO4 solid-state batteries at 0.2C rate (c) and the corresponding galvanostatic charge-discharge profiles of the in-situ PVC-SPE modified Li | LAGP | LiFePO4 battery (d);rate performance of the in-situ PVC-SPE modified Li | LAGP | LiFePO4 battery (e) and the corresponding voltage profiles of the in situ PVC-SPE modified Li | LAGP | LiFePO4 battery at different C-rates (f).
The galvanostatic profiles of theinsituPVC-SPE modified Li| LAGP | LiFePO4battery at different cycles are shown in Fig.4d.As indicated by the plot, characteristic charge and discharge voltage plateaus of the olivine LiFePO4are observed with limited overpotential and the battery shows steady polarization with cycles.Besides, as shown in Fig.S12, the galvanostatic charge-discharge profile at the second cycle of theinsituPVCSPE modified Li | LAGP | LiFePO4battery andexsituPVC-SPE modified Li | LAGP | LiFePO4battery at the 2nd cycles is selected for comparison.Note that the first cycle at 0.1Cis operated for the activation of the battery.TheexsituPVC-SPE modified Li | LAGP | LiFePO4 presents a capacity of only 60 mAh·g-1with expanded voltage hysteresis.The failure to construct a benign lithium-ion transport network inside the electrode results in the poor performance of the battery byexsitumodified way, which is corresponding to the EIS data in Fig.S10.
The excellent cycling performance is attributed to the benign electrochemical interface compatibility and ionic conduct network in the electrode of theinsituPVC-SPE modified battery.These merits further prove their superiority on electrodes with different active mass loading.Fig.S13a shows the longterm performance of solid-state batteries with different active mass loading.When the active mass loading of the electrode is almost doubled (2.4 mg·cm-2), it offers barely affected capacity performance during 290 cycles, exhibiting an excellent capability of theinsituPVC-SPE modification on the thick electrode.Although the 4.0 mg·cm-2electrode presents a slightly depressed capacity performance for the initial 60 cycles and collapses afterward, its galvanostatic profile at the 2nd cycle(Fig.S13b) confirms the well-preserved plateau with small voltage hysteresis.This indicates the capacity degradation of the 4.0 mg·cm-2electrode may be limited by the anode issue, due to the surged Li consumption that increases the charge and discharge depth of the Li anode.Therefore, theinsituPVC-SPE modification method still proves its capability with high-loading electrodes.
Importantly, the rate performance of theinsituPVC-SPE modified Li | LAGP | LiFePO4 solid-state batteries were also evaluated at room temperature.As seen in Fig.4e, the battery shows excellent rate capability, and the discharge capacity at 0.2C, 0.3C, 0.5C, 1C, 2C, 3C, and 5Care 158.8, 157.1, 156.7,148.8, 133.9, 115.0, 80.2 mAh·g-1, respectively, corresponding to a capacity retention of 100%, 98.9%, 98.6%, 93.7%, 84.3%,72.4% and 50.5% of that at 0.2C.Impressively, the discharge specific capacity at 0.2Ccould be recovered when the current density switches back from 5Cto 0.2C, which shows good reversibility of theinsituPVC-SPE modified Li | LAGP |LiFePO4battery.Fig.4f displays the corresponding voltage profiles at different current densities.It is seen that the plateau hysteresis expands and the capacity shrinks as the current density increases, which is ascribed to the limited Li+diffusion rate at high current rates.The satisfactory rate capability is attributed to the synergy effect of the intimate electrode/electrolyte interfaces,the highly conductive LAGP electrolyte, as well as the benign lithium-ion conduct network within the electrode in theinsituPVC-SPE modified Li | LAGP | LiFePO4 solid-state battery.
The evaluation of Li || LTO, Li || LCO solid batteries was conducted to demonstrate the versatility of thisinsitumodification with various cathodes.Fig.S14 shows the galvanostatic profile and cycling performance of the Li || LTO solid-state battery, which exhibits high reversibility and slow capacity fading over 70 cycles.Besides, the Li || LCO solid battery is also demonstrated to operate successfully at room temperature (Fig.S15).However, the capacity of LCO only remains steady for 10 cycles and fast fades in the following cycles.Such poor performance is mainly caused by the interfacial reaction between electrolyte and cathode, due to the higher operating voltage of the cathode48.Finally, the electrochemical performance of the Li || LNMO battery was also evaluated to explore the adaptability of PVC-SPE at the high oxidation potential.Unfortunately, as seen in Fig.S16, the batteries cannot charge up to 4.7 V at the first cycle, which is corresponding to the electrochemical window of PVC-SPE.Besides, the full battery based on graphite || LiFePO4was tested(Fig.S17).It exhibits 141 mAh·g-1in the first cycle and high reversibility in 40 cycles.All the above-mentioned results show that it is a common method to synchronously fabricate the lithium-ion network within the electrode and improve the electrode-inorganic electrolyte interfacial contact in solid batteries by theinsituPVC-SPE modification.
Furthermore, the interfacial stability of PVC-SPE with the electrode was also evaluated by the EIS measurement before and after cycles.The alternating impedance variation spectra are illustrated in Figs.5a and S18.The pristine interfacial resistance value decreases constantly from 1100 Ω to 700 Ω after 30 cycles and keeps steady until the 100th cycle, which is similar to previous reports ofinsitumodification of LAGP49.ExsituSEM observation was conducted to character the polymer ion conduction network morphology after the LiFePO4electrode cycling.Morphology demonstrates the PVC-SPE polymer electrolyte is uniformly distributed in the LiFePO4electrode as it is in the pristine electrode (Fig.5b) and is in close contact with active particles.These results manifest theinsituPVC-SPE modification strategy could improve the ion transport network in the electrode and maintains stability during cycling.
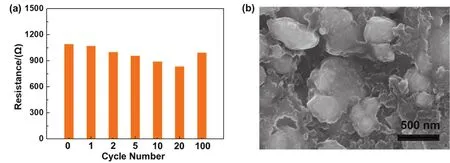
Fig.5 EIS resistance histogram of the Li | LAGP | LiFePO4 cell after different cycles at room temperature(a); the SEM image showing the morphology of the in situ PVC-SPE modified LiFePO4 cathode after 100 cycles (b).
4 Conclusions
In summary, aninsitupolymerization strategy of PVC-SPE was demonstrated to synchronously fabricate the lithium-ion network within the electrode and improve the electrodeinorganic electrolyte interfacial contact in solid-state lithium metal batteries.Theinsitumodified method not only reduces the interface impedance in inorganic electrolyte solid-state batteries but also inhibits harmful interfacial side reactions between lithium anode and LAGP electrolyte.More importantly, the PVC-SPE infiltrated in porous cathodes ensures the benign ion conduct and intimate contact between active particles and the polymer electrolyte during charge/discharge cycles.As a result,insituPVC-SPE modified Li | LAGP | Li symmetrical cell could be steadily cycled for over 2700 h at a current density of 0.05 mA·cm-2with a capacity of 0.1 mAh·cm-2.Adopting this strategy, the solid-state Li | LAGP | LiFePO4battery shows stable cycling performance at 0.2Cand 1Crate and good rate capability at room temperature.It is anticipated that theinsituPVC-SPE modification will be an effective and facile strategy to synergy address the interfacial problem and constructing the ion conduction network within the electrode to fabricate solid batteries.
Supporting Information:available free of chargeviathe internet at http://www.whxb.pku.edu.cn.
